Abstract
Background
Although several studies have provided evidence for the therapeutic potential of bone marrow-derived mononuclear cells (MNCs) in animal models of stroke, the mechanisms underlying their benefits remain largely unknown. We have determined the neuroprotective potential of MNCs in primary neuronal cultures exposed to various injuries in vitro.
Methods
Cortical neurons in culture were exposed to oxygen-glucose deprivation, hypoxia, or hydrogen peroxide and cell death was assayed by MTT, caspase-3 activation or TUNEL labelling at 24 hrs. Cultures were randomized to co-treatment with MNC-derived supernatants or media before injury exposure. In separate experiments, macrophage or microglial cultures were exposed to lipopolypolysacharide (LPS) in the presence and absence of MNC-derived supernatants. Neuronal cultures were then exposed to conditioned media derived from activated macrophages or microglia. Cytokines from the supernantants of MNC cultures exposed to normoxia or hypoxia were also estimated by enzyme-linked immunosorbant assay (ELISA).
Results
MNC-derived supernatants attenuated neuronal death induced by OGD, hypoxia, hydrogen peroxide, and conditioned macrophage/microglial media and contain a number of trophic factors including IL-10, IGF-1, VEGF, and SDF-1.
Conclusion
MNCs provide broad neuroprotection against a variety of injuries relevant to stroke.
Keywords: Neurons, microglia, stroke, mononuclear cells, neuroprotection
Introduction
Cellular therapy represents a new approach to enhance recovery after stroke (Zhang and Chopp 2009). Various types of cells are under investigation for a range of neurological disorders. Recent studies suggest the therapeutic potential of bone marrow-derived mononuclear cells (MNCs) for ischemic stroke (Baker et al. 2007; Brenneman et al. 2009; Giraldi-Guimaraes et al. 2009). However the underlying mechanisms are largely unknown but may include reducing pro-inflammatory responses (Brenneman et al. 2009) and angiogenesis (Baker et al. 2007). Our previous study (Brenneman et al. 2009) showed that MNCs when administered at 24 hrs after stroke reduce infarct maturation at 7 days in a rodent model and in a differet study MNCs have been shown to protect neurons in the peri-infarct area within the same time frame after stroke (Giraldi-Guimaraes et al. 2009). These results together with others (Iihoshi et al. 2004) collectively suggest that MNCs exert neuroprotective effects within the first few days after stroke. We therefore investigated in the present study whether MNCs directly protect neurons in cell culture injury models relevant to ischemia/reperfusion.
Several studies have also suggested that ischemia-activated microglia play an adverse role in the pathogenesis of stroke. Microglia may endanger neuronal survival through the release of free-radicals or pro-inflammatory cytokines and other neurotoxins(Stoll et al. 1998). Thus, modulation of microglial activation might be a potential therapeutic avenue for stroke and other neurodegenerative disorders. Therefore, we also explored here whether MNCs protect neurons from injury by modulating activated microglia in cell culture models.
Materials & Methods
Animals
The experimental protocols were approved by local Animal Care Committee at the University of Houston. The experiments were conducted under the auspices of Research Animal Resources, a facility approved by the American Association for the Accreditation of Laboratory Animal Care.
Preparation of Bone Marrow-Derived Mononuclear Cells (MNCs)
Bone marrow was extracted from the tibia of Sprague-Dawley rats under anesthetized conditions and mononuclear cells were prepared as previously decribed (Brenneman et al. 2009). Freshly collected cells were suspended at a density of 5.0 × 106 cells/ml in DMEM supplemented with 10% fetal calf serum and 1% penicillin/streptomycin. Cells were seeded on a 24-well culture plate at various concentrations and incubated under normoxia or hypoxia at 37°C. After 24 h of cultivation, the total number of surviving cells was counted after staining with 0.4% trypan blue solution (Sigma), and the supernatant was used for the studies below.
Cell Cultures
Neuronal cultures were prepared from embryos of Sprague-Dawley rats as previously described in our laboratory (Zhao et al. 2006), using Neurobasal medium (Gibco-BRL, Gaithersburg, MD) with B27. The purity of the neuronal cultures was confirmed by MAP-2 staining showing the cultures were >95% neurons. Microglial cultures were prepared as previously described in our laboratory (Zhao et al. 2007) using postnatal (day 1–2) pups and were confirmed by OX-42 staining. Peritoneal macrophages were prepared as previously described (Yawata et al. 2008), elicited with 2 ml of 4% thioglycolate (Difco) injected intraperitoneally into Sprague-Dawley rats 60 hr earlier, were washed with PBS, purified by adherence to tissue culture plastic plate for 2 h and then cultured with the same medium as microglia. Microglia and peritoneal macrophages were cultured at a concentration of 5 × 104 cells/well in 24-well culture plates (BD Falcon, Lincoln Park, NJ) in Nerve-Cell Culture Medium (Sumitomo Bakelite) with 1 μg/ml LPS. LPS was chosen because it is well-known to activate pro-inflammatory responses of microglia/macrophages. For experiments involving transfer of microglial or macrophage-conditioned media (MCM) to neuronal cultures, we followed a previous protocol (Yawata et al. 2008) with some modifications: the microglial or macrophage cultures were first treated with LPS or LPS with media derived from MNCs for 24 hrs, washed, re-cultured for 24 hrs and then media from these cultures were transferred to neuronal cultures (1:1 ratio).
MTT Cell Survival Assay
The viability of the cells in neuronal monocultures was assessed by their ability to uptake thiazolyl blue tetrazolium bromide (MTT). The cells were incubated with MTT for 1 h, then lysed with dimethyl sulfoxide (DMSO) and left at room temperature in the dark overnight. The lysates were then read on a plate reader (PowerWave X, Bio-Tek) at the absorbance wavelength of 540 nm.
Caspase-3 Activation
Primary neurons were cultured in 24-well plates on glass coverslips. Cells were fixed in 4% paraformaldehyde in PBS, incubated for 5 min in 0.2% Triton X-100 (Sigma), and then incubated overnight at 4°C with an antibody against cleaved caspase-3. After washing once with PBS, immune complexes were detected by incubating for 1 h at RT with an Alexa Fluor 488-conjugated goat anti-rabbit IgG antibody (Invitrogen, Barcelona, Spain) in PBS containing 1% goat serum. The cells were counterstained with Hoechst dye to label nuclei. Samples were viewed and photographed using a Zeiss Axiophot microscope (Zeiss, Oberkochen, Germany). Numbers of neurons exhibiting cleaved caspase-3 staining were quantified (n = 5 cultures per group).
TUNEL Staining
Primary neurons were cultured in 24-well plates on glass coverslips. Cells were fixed in 4% paraformaldehyde in PBS, incubated for 5 min in 0.2% Triton X-100 (Sigma) in PBS, and then ApoTag Fluorescein In-Situ Apoptosis Detection Assay kit (Chemicon International Cat: S-7110 Green: FITC) was used as per manufacturer’s recommendations.
Hypoxia or oxygen glucose deprivation
Neurons at 13 days in vitro (DIV) were exposed to combined oxygen and glucose deprivation (OGD). In brief, cultures were replaced with serum- and glucose-free medium then placed in a Billups-Rothenberg modular incubator chamber (Del Mar, San Diego, CA, U.S.A.), which was flushed for 5 minutes with 95% nitrogen and 5% CO2 and then sealed (hypoxia). The chamber was placed in a water-jacketed incubator (Forma scientific USA) at 37°C for 90 minutes. Control glucose-containing normoxic cultures were incubated for the same periods of time at 37°C in humidified 95% air and 5% CO2. In separate experiments, neuronal cultures were subjected to hypoxic conditions using similar experimental conditions as above; however, the cultures were maintained in normoglucemic media for 24 hrs and a set of corresponding control cultures were maintained in normoxic conditions using normoglucemic media. OGD was chosen to simulate the conditions of acute ischemic stroke while hypoxia was chosen to simulate conditions of milder injury that may be occurring in the areas around the infarct in the first few days after stroke.
Cytokine Analysis
Microtiter colorimetric sandwich ELISA was performed to quantitatively estimate cytokine levels as per Manufacturer’s recommendations: IGF-1(Cat # MG-100: R& D Systems, Minneapolis, MN); IL-10 (Prod # ERIL-10: Thermo-Scientific, Rockford, IL, USA); VEGF (Cat # ELR-VEGF-001: RayBiotech, Norcross, GA, USA); SDF-1 α: (Cat # ELM-SDF1alpha-001: RayBiotech, Norcross, GA, USA). Fifty to hundred μl samples were run in triplicate using Molecular Devices (Emax) Precision Microplate Reader.
Drug treatment
(+)-5-methyl-10,11-dihydro-5H-dibenzo[a, d] cyclohepten-5, 10-imine maleate (MK-801) was dissolved in sterile purified water prior to dilution into Neurobasal medium.
Statistical Analyses
The data were presented as Mean ± SD. Data were analysed by GraphPad Prism software (GraphPadSoftware, San Diego, CA) by either Student’s t-test or ANOVA followed by post-hoc analysis with Bonferroni's test.
Results
MNC supernatants protect neurons against oxygen glucose deprivation
Cultured neurons exposed to 90 min of OGD showed morphological changes indicative of injury or death at 24 hrs after the insult. Pre-treatment of neurons with media derived from MNCs (103 – 107 cells) led to a concentration dependent neuroprotection against OGD (Fig 1A). An approximately 25% increase of cell survival was observed when media from the highest concentration of MNCs was employed. Interestingly, media from MNCs that were heat denatured (HD) prior to its transfer had no effect on neuronal cell viability in this injury model (Fig 1A). In agreement with previous studies, the NMDA recepror antagoninst, MK-801 during OGD provided robust (80%) neuroprotection, indicating the importance of excitotoxic damage in our injury model.
Fig 1.
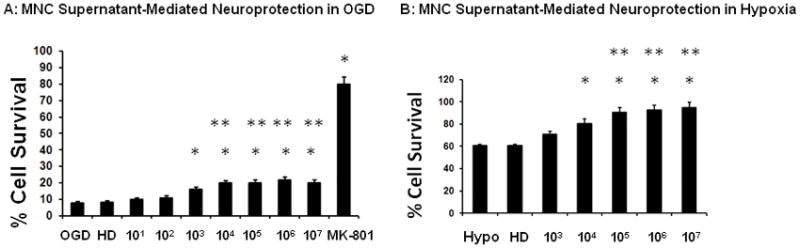
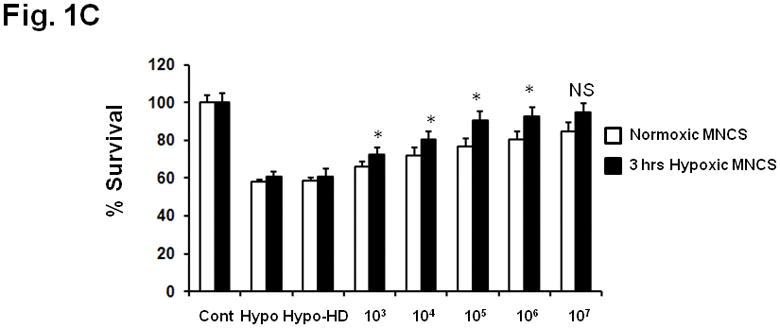
MNC-derived supernatants protect neurons from OGD or hypoxia. MNCs at increasing concentrations (0 to 1×107) were cultured in DMEM for 24 hrs. Equal volume (100μL) of supernatants of these cultures were then transferred into wells containing primary cultured neurons just prior to exposure to either (A) OGD for 90 minutes and then 24 hrs of re-oxygenation and glucose re-supplementation for 22.5 hrs or (B) 24 hrs of hypoxia. At 24 hrs, MTT cell survival assay was performed in triplicate to determine the level of neuronal injury. The data were normalized to normoxic controls and by subtracting the background optical densities taken at 590 nm spectral wavelength. The group labeled OGD or Hypo were neurons exposed to DMEM media collected from plates cultured for 24 hrs that contained no MNCs. As a different control, supernatant from MNCs (1×107) was heat denatured (HD) before transferring to neuronal cultures and exposure to OGD (A) or hypoxia (B);this group was labeled HD MK-801 (1μM) was added to neuronal cultures before exposure to OGD as a positive control (A). In C, the experimental conditions in B were repeated except that prior to the media transfer, MNCs were exposed to 3 hrs of hypoxia and then 21 hrs of normoxia. The supernatants of these cultures were transferred to wells containing neurons and subjected to hypoxia for 24 hrs. Drugs, vehicles, or MNC-derived media were present throughout the exposure and recovery periods. N= 8 per group. *p<0.05 compared to OGD or hypoxia conditions alone.. **p<0.05 compared to OGD or hypoxia conditions co-treated with media derived from 103 MNCs. Hypo = hypoxia. OGD = oxygen glucose deprivation. MNCS = supernatant derived from cultures of MNCs.
MNC supernatants protect neurons against hypoxia
Exposure of neurons in culture to 24 hrs of hypoxia increased the index of injury by 40%. Addition of media derived from MNCs led to a concentration-dependent alleviation of hypoxia-induced injury (Fig 1B). Hypoxia is known to condition MNCs and increase their secretion of cytokines (Takahashi et al. 2006). Interestingly, further reduction in neuronal death was seen when using media from MNCs that were first exposed to 3 hrs of hypoxia followed by 21 hrs of normoxia (Fig 1C). Media from MNCs that were first heat denatured (HD) lost its effect on neuronal cell viability in this injury model (Fig 1B).
MNC supernatants reduce apoptosis
Previous studies have provided evidence that many neurons in the ischemic penumbra die by apoptosis; the protease caspase-3 plays a key role in ischemic neuronal apoptosis. In agreement with these studies, cultured neurons subjected to hypoxia showed increased caspase-3 activity as indicated by increased amounts of neurons immunoreactive for the caspase-3 cleavage product (Fig 2). Exposure to media derived from 10 million MNCs reduced the number of neurons exhibiting cleaved caspase 3 immunoreactivity, indicating that media from MNC could prevent caspase 3 activation and neuronal death. Similarly, media from 10 million MNCs also reduced the number of neurons with DNA fragementation, labeled by TUNEL (Fig 2).
Fig 2.
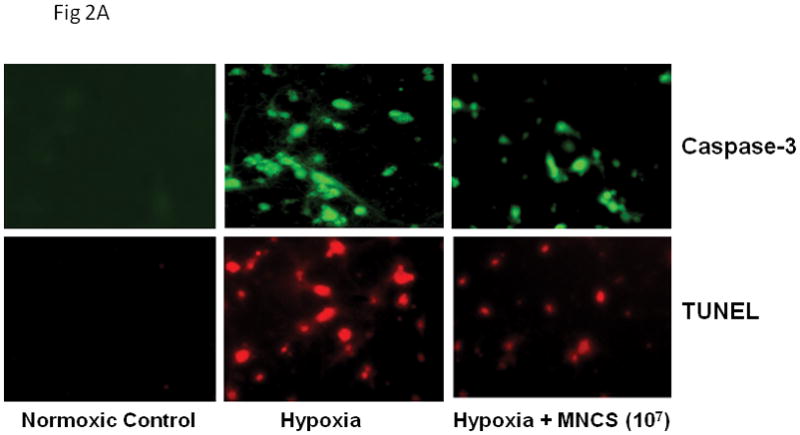
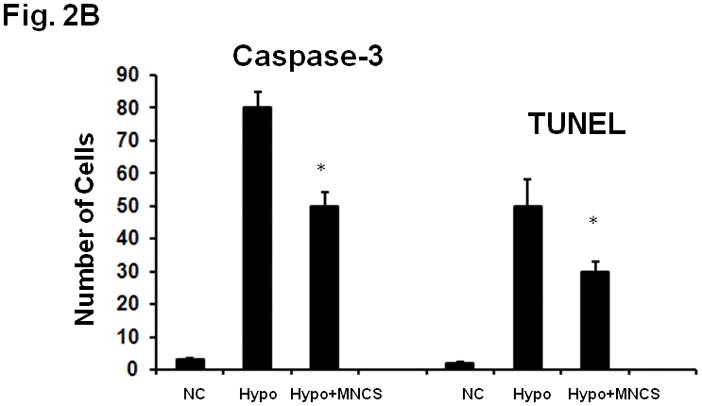
(A) Fluorescence microscopic pictures of activated caspase-3 and TUNEL staining in primary cultured neurons in response to 24 hrs of hypoxia in the absence or presence of MNC derived supernatants. (B) Quantitative analysis of activated caspase-3 and TUNEL positive neurons after 24 hrs of hypoxia in the absence of MNC derived supernatant. Hypoxia-induced caspase-3 activity and TUNEL staining was significantly reduced in response to treatment with MNC-supernatants. MNC-derived media was present throughout the exposure and recovery periods. *p<0.05 compared with media treated cultures. N=5 per group. MNCS = supernatant derived from cultures of MNCs. Hypo = hypoxia. NC = normoxic controls.
MNC supernatants reduce oxidative stress
MNCs reduce neurological deficits when administered at 24 hrs after stroke (Brenneman et al. 2009). Since it has been shown that ischemia/reperfusion is associated with oxidataive stress that lasts for days after the injury, we determined whether MNCs protect neurons against insults relevant to this aspect of the pathogenesis of stroke. We used a cell culture model of oxidative stress in which cultured cortical neurons were exposed to hydrogen peroxide. Hydrogen peroxide induced significant neuronal injury while pre-treatment with media derived from 10 million cultured MNCs efficiently increased survival of neurons in the presence of hydrogen peroxide (Fig 3).
Fig 3.
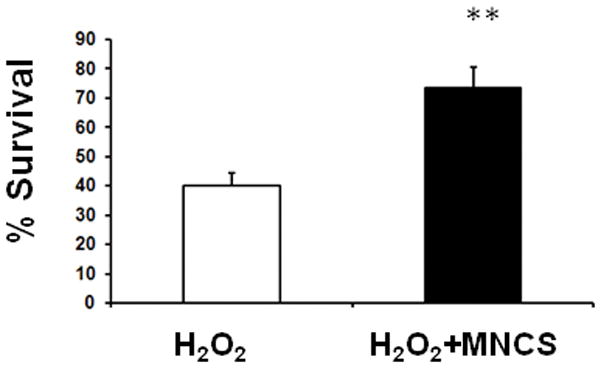
MNC-derived supernatants protect neurons from hydrogen peroxide. Pretreatment with supernatants derived from a culture of 10 million MNCs rescued neurons from hydrogen peroxide (100 uM)–mediated injury. The group labeled H202 were neurons exposed to hydrogen peroxide and pre-treated with DMEM media collected from plates cultured for 24 hrs that contained no MNCs. **p<0.05 compared with the hydrogen peroxide group. (n=8 per group). MNCS = supernatant derived from cultures of MNCs.
MNC supernantants reduce microglial and macrophage mediated toxicity
Activated microglia are a well-known source of oxidative stress that contributes to the injury of brain cells in the subacute stages of stroke when MNCs may have a beneficial effect (Brenneman et al. 2009; Giraldi-Guimaraes et al. 2009). We therefore tested whether MNCs modulate the detrimental effects of activated macrophages and microglia on cultured neurons. We used LPS to activate macrophages and microglia and then transferd conditioned media from these cultures to neurons in a well-known paradigm of macrophage-microglial mediated neurotoxicity(Yawata et al. 2008). As shown in Fig 4A, conditioned media of LPS-treated macrophages was neurotoxic (labeled Macrophage Sup), as established using MTT. Conditioned media from macrophages treated with LPS in the presence of MNC-derived media, however, was significantly less damaging to neurons (labeled Macrophage Sup+ MNCS) (Fig 4A). DMEM used to grow the macrophages had no effect on neuronal viability. Similar results were obtained using primary microglia rather than hematogenous macrophages (Fig 4B).
Fig 4.
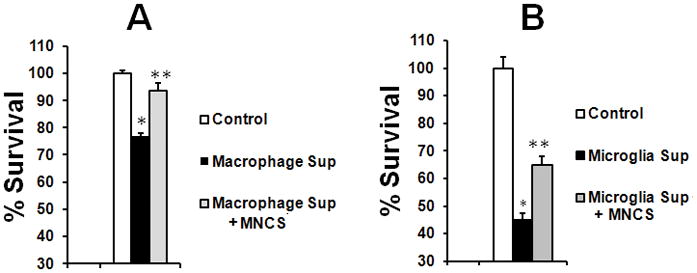
MNC-derived supernatants significantly (p<0.05) attenuated neurotoxicity of activated macrophages or microglia. Primary rat macrophages (A) or microglia (B) were treated with LPS (100 ng/ml) or LPS plus MNC-derived supernatant (MNCS) for 24 hrs, then washed with PBS and re-cultured for another 24 hrs. An aliquot of media from re-cultured cells was transferred into wells containing primary cortical neurons. Neurons in a third group were exposed only to DMEM used to grow macrophages or microglia (labeled Control). MTT assay was performed at 24 hrs after neurons were exposed to the conditions described above. N=6 per group. *p < 0.05 compared with control; **p<0.05 compared with the group treated with Macrophage Sup or Microglial Sup. Macrophage Sup or Microglial Sup represents an aliquot of media from re-cultured macrophages or microglia after treatment with LPS. Macrophage Sup or Microglia Sup + MNCS represents an aliquot of media from re-cultured macrophages or microglia after co-treatment with LPS and MNC-derived media.
Cytokines released by MNCs in culture
Numerous trophic factors including IGF1, SDF-1a, VEGF or IL-10 have been demonstrated to have direct neuroprotective effects and modulate microglia/macrophages activation (Kooijman et al. 2009; Sawada et al. 1999; Spera et al. 1998). The above data suggest that trophic factors released by MNCs may in fact lead to direct neuroprotection and microglial modulation. Accordingly, we found that VEGF, IL-10, IGF-1, and SDF-1 were indeed increased in the media, detected at 24 hrs after rat MNCs were placed in culture. The cytokine elevations were proportional to the number of MNCs in the media (Fig 5). The secretion of these 4 cytokines was also enhanced when MNCs were first exposed to 3hrs of hypoxia followed by 21 hrs of normoxia (Fig. 5).
Fig 5.
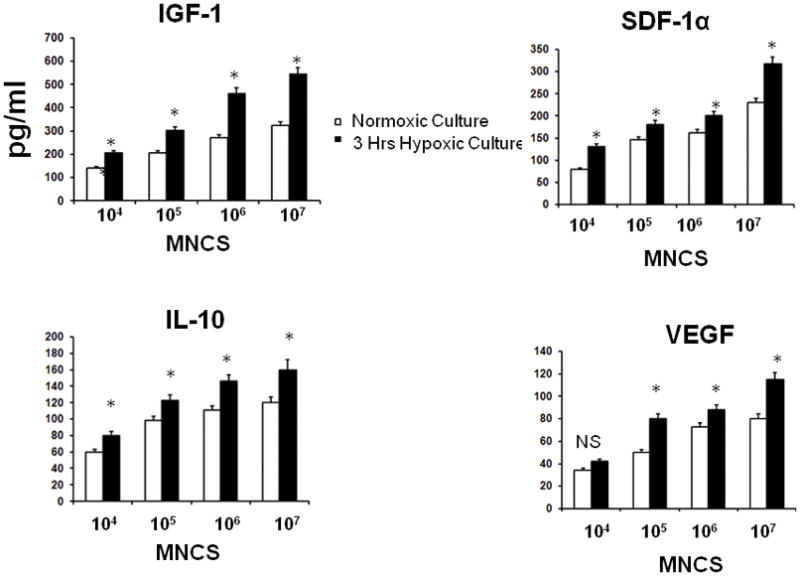
Cytokines in the supernatants of MNCs (102–107 cells) after 24 hrs in culture under conditions of normoxia for 24 hrs or 3 hrs of hypoxia followed by 21 hrs of normoxia. ELISA showed that the MNCs produced VEGF, IL-10, IGF-1, and SDF-1, all of which were enhanced when exposed to transient hypoxia. *p<0.05 compared with normoxic MNCs. N=6 per group. MNCS = supernatant derived from cultures of MNCs.
Discussion
We have determined the neuroprotective potential of MNCs in neuronal injury models relevant to the pathogenesis of ischemic stroke. The experimental studies of this report are focused on addressing important translational applications of MNCs as a potential new therapeutic approach for ischemic stroke. We found that MNCs only provide mild protection of neurons from OGD but lead to robust protection against hypoxia. Neuronal death in this OGD model is largely mediated by excitotoxic necrosis in which caspase mediated mechanisms of cell death may play a less prominent role (Gottron et al. 1997; Jones et al. 2004) and in which the NMDA antagonist, MK-801, is markedly neuroprotective as shown here and in prior studies(Jones et al. 2004). On the other hand, 24 hrs of hypoxia likely involves more caspase-mediated cell death (Jin et al. 2001). The differences in the extent of protection between the two models may reflect the severity of injury in which apoptosis plays a more prominent role in milder forms of damage. It is therefore possible that MNCs protect neurons through inhibition of apoptotic mechanisms. To support this hypothesis, we found that MNCs reduced the number of neurons positive for activated caspase-3 while simultaneously reducing the number of TUNEL labelled neurons in hypoxic cultures. The concentration of MNC's that showed the best (25%) protection after OGD is a feasible cell number that can be extracted from bone marrow in rats for in vivo autologous studies (Brenneman et al.).
Since MNCs in animals have been found to reduce neurological deficits when administered within the first few days after stroke (Brenneman et al. 2009; Giraldi-Guimaraes et al. 2009), in this study we used selected types of neuronal injury in vitro that simulate various aspects of pathogenic mechanisms against which MNCs might exert neuroprotection in the subacute stages of stroke. For example, we used hypoxia as a method to cause milder, pro-apoptotic stimuli and we also used peroxide-medaited injury as a model of oxidative stress, which contributes to progressive injury through various pro-inflammatory mechanisms in the peri-infarct region (Wang et al. 2007). Supernatants derived from MNCs reduced neuronal death in this model, suggesting that MNCs increase resistance to oxidative sterss. Microglia are a source of oxidative mediators (Zhao et al. 2007) that may contribute to injury during the subacute period after ischemic stroke. When activated, microglia can pose direct injury to neurons (Zhao et al. 2007). In agreement with these prior studies, media derived from primary cultures of microglia or macrophages, activated by LPS, were neurotoxic. When microglia or macrophages were co-treated with LPS and MNC-derived media, followed by a 24 hrs wash-out period, the toxicity of microglial or macrophage derived media was significantly attenuated.
Recent studies raise the hypothesis that MNCs might behave as minipumps producing cytokines and/or trophic factors that promote cell survival in the peri-infarct region and reduce infarct maturation (Brenneman et al. 2009; Giraldi-Guimaraes et al. 2009). Our present data support this hypothesis as the supernatants derived from MNCs grown in culture for 24 hrs protect neurons in in vitro models of injury and directly modulate activated microglia in culture. We furthermore found that the media of cultured MNCs after 24 hrs contains several cytokines such as IL-10, IGF-1, VEGF, SDF-1; these cytokines were chosen because they are known to be neuroprotective in animal models of stroke (Kooijman et al. 2009; Spera et al. 1998; Sun et al. 2003) and they may underlie, at least in part, the beneficial effects of MNCs in models of myocardial infarction (Takahashi et al. 2006) Interestingly, supernatants from heat inactivated media did not protect neurons in any of the injury paradigms tested, which suggests that factors within the media were denatured. In addition, a mild increase in neuroprotection was observed when cultured MNCs were first exposed to 3 hours of hypoxia. The enhanced protection was associated with increased IL-10, IGF-1, VEGF, and SDF-1, pointing toward the possibility of conditioning MNCs to exert greater beneficial effects.(Takahashi et al. 2006). Collectively, these findings suggest that MNCs release soluble factors that directly protect neurons and possibly modulate the pro-inflammatory or pro-oxidative phenotype of microglia. Further work is needed to ascertain which cytokines are the most important contributors to these effects. Identifying such factors has important translational implications and raise the possibility that MNCs could be primed to release a cocktail of cytokines that could be used for therapeutic purposes. Other types of cells may also exert therapeutic effects in the injured brain after stroke by releasing neurotrophic factors. For example, mesenchymal stem cells from bone marrow produce several different neuroptrohpic and growth supporting factors (Qu et al. 2007).
In conclusion, MNCs may provide direct neuroprotection through anti-apoptotic and anti-oxidative mechanisms relevant to stroke. MNCs may also indirectly protect neurons by altering the detrimental effects of activated microglia. Microglial modulation by MNCs might have therapeutic potential as a novel strategy for ischemic stroke.
Acknowledgments
We thank Drs. Kazim Sheik and Kobori for permitting us to use their fluorescence microscopy labs. This research work was supported by grants from the AHA, Howard Hughes Medical Institute, and NIH.
Supported by grants from the AHA, Howard Hughes Medical Institute, and NIH.
References
- Baker AH, Sica V, Work LM, Williams-Ignarro S, de Nigris F, Lerman LO, Casamassimi A, Lanza A, Schiano C, Rienzo M, Ignarro LJ, Napoli C. Brain protection using autologous bone marrow cell, metalloproteinase inhibitors, and metabolic treatment in cerebral ischemia. Proc Natl Acad Sci U S A. 2007;104(9):3597–3602. doi: 10.1073/pnas.0611112104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman M, Sharma S, Harting M, Strong R, Cox CS, Jr, Aronowski J, Grotta JC, Savitz SI. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab. 30(1):140–149. doi: 10.1038/jcbfm.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman M, Sharma S, Harting M, Strong R, Cox CS, Jr, Aronowski J, Grotta JC, Savitz SI. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab. 2009 doi: 10.1038/jcbfm.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldi-Guimaraes A, Rezende-Lima M, Bruno FP, Mendez-Otero R. Treatment with bone marrow mononuclear cells induces functional recovery and decreases neurodegeneration after sensorimotor cortical ischemia in rats. Brain Res. 2009 doi: 10.1016/j.brainres.2009.01.062. [DOI] [PubMed] [Google Scholar]
- Gottron FJ, Ying HS, Choi DW. Caspase inhibition selectively reduces the apoptotic component of oxygen-glucose deprivation-induced cortical neuronal cell death. Mol Cell Neurosci. 1997;9(3):159–169. doi: 10.1006/mcne.1997.0618. [DOI] [PubMed] [Google Scholar]
- Iihoshi S, Honmou O, Houkin K, Hashi K, Kocsis JD. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res. 2004;1007(1–2):1–9. doi: 10.1016/j.brainres.2003.09.084. [DOI] [PubMed] [Google Scholar]
- Jin K, Mao XO, Batteur SP, McEachron E, Leahy A, Greenberg DA. Caspase-3 and the regulation of hypoxic neuronal death by vascular endothelial growth factor. Neuroscience. 2001;108(2):351–358. doi: 10.1016/s0306-4522(01)00154-3. [DOI] [PubMed] [Google Scholar]
- Jones PA, May GR, McLuckie JA, Iwashita A, Sharkey J. Apoptosis is not an invariable component of in vitro models of cortical cerebral ischaemia. Cell Res. 2004;14(3):241–250. doi: 10.1038/sj.cr.7290225. [DOI] [PubMed] [Google Scholar]
- Kooijman R, Sarre S, Michotte Y, De Keyser J. Insulin-like growth factor I: a potential neuroprotective compound for the treatment of acute ischemic stroke? Stroke. 2009;40(4):e83–88. doi: 10.1161/STROKEAHA.108.528356. [DOI] [PubMed] [Google Scholar]
- Qu R, Li Y, Gao Q, Shen L, Zhang J, Liu Z, Chen X, Chopp M. Neurotrophic and growth factor gene expression profiling of mouse bone marrow stromal cells induced by ischemic brain extracts. Neuropathology. 2007;27(4):355–363. doi: 10.1111/j.1440-1789.2007.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M, Suzumura A, Hosoya H, Marunouchi T, Nagatsu T. Interleukin-10 inhibits both production of cytokines and expression of cytokine receptors in microglia. J Neurochem. 1999;72(4):1466–1471. doi: 10.1046/j.1471-4159.1999.721466.x. [DOI] [PubMed] [Google Scholar]
- Spera PA, Ellison JA, Feuerstein GZ, Barone FC. IL-10 reduces rat brain injury following focal stroke. Neurosci Lett. 1998;251(3):189–192. doi: 10.1016/s0304-3940(98)00537-0. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol. 1998;56(2):149–171. doi: 10.1016/s0301-0082(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111(12):1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Li TS, Suzuki R, Kobayashi T, Ito H, Ikeda Y, Matsuzaki M, Hamano K. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol. 2006;291(2):H886–893. doi: 10.1152/ajpheart.00142.2006. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184(1–2):53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawata I, Takeuchi H, Doi Y, Liang J, Mizuno T, Suzumura A. Macrophage-induced neurotoxicity is mediated by glutamate and attenuated by glutaminase inhibitors and gap junction inhibitors. Life Sci. 2008;82(21–22):1111–1116. doi: 10.1016/j.lfs.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8(5):491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Ou Z, Grotta JC, Waxham N, Aronowski J. Peroxisome-proliferator-activated receptor-gamma (PPARgamma) activation protects neurons from NMDA excitotoxicity. Brain Res. 2006;1073–1074:460–469. doi: 10.1016/j.brainres.2005.12.061. [DOI] [PubMed] [Google Scholar]
- Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, Grotta JC, Aronowski J. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol. 2007;61(4):352–362. doi: 10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]


