Abstract
Adipose Derived Stromal/Stem Cells (ASCs) have been gaining recognition as extremely versatile cell source in tissue engineering. The usefulness of ASCs in biofabrication is further enhanced by our demonstration of unique properties of these cells when they are cultured as three dimensional cellular aggregates or spheroids. As described herein, three-dimensional formulations or self-assembling ASC spheroids develop their own extracellular matrix that serves to increase the robustness of the cells to mechanical stresses. The composition of the extracellular matrix can be altered based on the external environment of the spheroids and these constructs can be grown in a reproducible manner and to a consistent size. The spheroid formulation helps preserve the viability and developmental plasticity of ASCs even in defined, serum-free media conditions. For the first time, we show that multiple generations of adherent ASCs produced from these spheroids retain their developmental plasticity and their ability to differentiate into multiple cell/tissue types. These demonstrated properties support the fact that culture expanded ASCs are an excellent candidate cellular material for “organ printing” – the approach of developing complex tissue structures from a standardized cell “ink” or cell formulation.
1. Introduction
A major challenge for regenerative medicine is to develop clinically viable methods of generating cells, tissue constructs and/or organs that are scalable and reproducible. One general approach, now drawing increasing attention, involves the fabrication and use of self-assembling 3-dimensional cellular ‘aggregates’. There are many different strategies for growing cells in a three-dimensional architecture, as well as a great number of candidate cell types that are being studied with the intent to translate therapies to the clinic.
Methodologies for growing and studying cells in 3-D ex vivo systems can be broadly categorized as those that use exogenous scaffolds on which cells are grown and those that develop a scaffold free 3-D structure by self-assembly [1]. Scaffolds have been developed with the intention of serving as a substrate for cell attachment, providing an architecture for the anticipated three-dimensional structure being grown and a source of cell signals to direct cell growth [2–4]. While they achieved some of these intended objectives, persistent challenges include minimal, or delayed vascular in-growth and increased antigenicity of the scaffolds leading to exuberant inflammatory responses [5,6]. There are also difficulties in achieving accurate and uniform placement of cells onto the scaffold and in achieving intended cell density within the scaffold structure. Additionally, the scaffolds often do not necessarily provide a genuine 3-dimensional environment, because cells growing on the scaffold surface still largely interact with each other in a two dimensional manner [7–9].
The use of gravity-mediated 3-D cell self assembly has been gaining attention as a method for generating micro- and macro- networks of cells, or ‘organoids’, with the potential for the engineering of tissue constructs [7]. This method enables cells to assemble and interact under natural forces, and permits them to generate their own extracellular matrix. One of the driving forces behind scaffold free self-assembly has been the fact that embryological development of organs does not rely on a predetermined scaffold. The self assembly of these organoids can be understood as an application of the differential adhesion hypothesis. This theory was advanced in the 1960s and proposed that differential surface tensions of liquid like particles gave rise to segregation and self assembly behavior [10,11]. Furthermore, if the appropriate cellular material is available, tissue fabrication might be possible by printing ‘pre-assembled’ modular cell aggregates onto hydrogel or similar substrates using computer aided robotic printers. The patterned “printing” of such organoids can facilitate further self-assembly into macro-structures, such as tissues or organs [7]. This technology has been applied to create “bioink” particles to rapidly print scaffold-free multilayered tubular grafts [12]. Clearly, one critical aspect of this strategy is the availability of large numbers of durable, standardizable and manipulatable 3-dimensional cellular aggregates that represent the “ink” for this ”biofabrication” approach [13,14].
Adipose tissue is gaining wide interest as an abundant and expendable source of therapeutic cells (ASCs) [15]. Adipose-derived stem/stromal cells are traditionally considered anchorage dependent cells and routinely cultured as adherent monolayers on tissue culture plastic. Recently, however, the use of gravity mediated spheroid assembly and culture has been described for ASCs. For example, Amos et al. demonstrated that 3-D suspension culture of culture-expanded human ASCs significantly and positively impacts gene expression and secretome activity both in vitro and in vivo [16]. However, they did not explore the developmental plasticity of cells grown in this manner. In contrast, Dromard et al. describe spheroid formation and suspension culture of fresh, uncultured adipose-derived stromal vascular fraction (SVF) cells, and the developmental plasticity of subsequent spheroid-derived cells after plating on gelatin-coated plates [17]. This growing interest in 3-D self-assembly of adipose-derived cells led us to further explore the growth, composition and behavior of culture expanded ASCs grown in suspension as spheroids, and to investigate whether ASCs within such constructs maintain multilineage developmental plasticity while maintained in suspension as well as after plating and expansion as adherent monolayers.
2. Materials and Methods
2.1. Adipose Stem Cell Isolation and Culture
Subcutaneous adipose tissue was obtained from patients undergoing elective surgical procedures in the Department of Plastic Surgery, University of Virginia in accordance with the University’s Human Investigation Committee. Discarded excisional abdominoplasty specimens and/or liposuction aspirates from 58 patients (average age 42.4 years, range 24–70 years; average BMI of 30.14, range of 18.4–63.6) were processed as described previously [18,19]. During the described studies, cells were cultured at 37 C, 5% CO2 in one of the following medias (table 1). All the studies described in this manuscript were performed a minimum of two times unless otherwise specifically mentioned.
Table 1.
Different culture media used in the study.
| Medium | Serum | Other Additives |
|---|---|---|
| D-10 | 10% FBSa | (−)b |
| HGFMc-1% | 1% HSd | L- glutamine, dexamethasone, ascorbic acid 2-phosphate, ITS+3, fatty acid supplement, NEAA, estradiol, progesterone, hydrocortisone, EGF, PDGF, SCGF-beta, TNF-alpha, IL-1beta |
| HGFM-SFe | (−) Serum Free | L- glutamine, dexamethasone, ascorbic acid 2-phosphate, ITS+3, fatty acid supplement, NEAA, estradiol, progesterone, hydrocortisone, EGF, PDGF, SCGF-beta, TNF-alpha, IL-1beta |
| LGFMf,g-1% | 1% HS | L- glutamine, dexamethasone, ascorbic acid 2-phosphate, ITS+3, fatty acid supplement, NEAA, estradiol, progesterone, hydrocortisone, EGF, PDGF, SCGF-beta, TNF-alpha, IL-1beta |
| LGFM-SF | (−) Serum Free | L- glutamine, dexamethasone, ascorbic acid 2-phosphate, ITS+3, fatty acid supplement, NEAA, estradiol, progesterone, hydrocortisone, EGF, PDGF, SCGF-beta, TNF-alpha, IL-1beta |
| D-0 | (−) | (−) |
Fetal Bovine Serum
(−) all contain same base medium (DMEM/F12) and antibiotic-antimycotic (ABAM)
HGFM: High Growth Factor Medium
Human Serum
Serum Free
LGFM: Low Growth Factor Medium
LGFM contains 1/10th the concentration of “other additives” relative to HGFM.
Freshly isolated cells were plated into monolayer culture in traditional culture-ware (Nunclon, 100 × 15mm) in serum-containing media (either D-10, HGFM-1%HS, or LGFM-1%HS). The initial plating was designated as passage 0 (P0). At confluence, P0 cells were lifted using the fungal derived enzyme TrypLE (Gibco Cat No. 12604-013) and counted on a hemocytometer using trypan blue dye exclusion. Cells were then used for study or re-plated at 2,000 cells/cm2 for continued expansion. In all studies described, ASCs from passage 5 or less were used. For some studies, adherent monolayer ASCs were fluorescently labeled with DiI or DiO (Molecular Probes) per manufacturer’s instructions. In some experiments, ASC-spheroids were labeled with Hoechst 33342 (Molecular Probes Cat#H1399) to label cell nuclei.
2.2. Formation and Culture of ASC-Spheroids
Self-assembling ASC spheroids were formed using previously described methods [13]. After inversion as hanging droplets for 24–48 hours, resulting spheroids were transferred to either Ultra Low Attachment (ULA) culture-ware (Corning) for suspension culture, and/or were subsequently placed into standard culture-ware for adherent culture. For serial passaging of spheroids in adherent culture, spheroids were physically dislodged with forceps under loupe magnification within 72 hours of plating, and placed into a new culture well.
2.3. Spheroid Size, Growth and Growth Factor Production
Using a hanging drop method, 10k, 20k, 30k, and 50k-cell spheroids were formed and then suspension cultured in low attachment 24-well plates. Digital microscopic images of spheroids were taken on day 0, day 3, and day 7 and image pro software was used to measure size. Conditioned media was collected on day0, day3 and day7 and evaluated with ELISA for quantification of HGF and VEGF secretion (Invitrogen, Inc.). Spheroids were also processed for H&E and Sirius Red staining for collagen.
2.4. Cell Proliferation in Suspension-Cultured Spheroids
Spheroids in suspension culture were pulsed with 10μM BrdU (BD Biosciences) for one day and then collected for analysis. Aggregates were fixed in methanol for 10 minutes, denatured in 2N HCl for 1 hour and then neutralized with 0.1 M borate buffer. Sections were incubated with anti-bromodeoxyuridine antibody (Fitzgerald Industries, #RDI-PRO61015) for 1 hour (at room temperature) and then incubated with a secondary Biotin-SP-conjugated AffiniPure Goat Anti-Mouse IgG(H+L) antibody (Jackson ImmunoResearch). Positive staining was visualized using a Vectastain ABC kit and 3,3′-Diaminobenzidine (DAB) (Vector Laboratories, Burlingame, CA).
2.5. Differentiation Assays
The multilineage potential of ASC-spheroids was evaluated in both 3-D suspension culture and after placement into adherent culture. Induction medias used are listed in table 2. The control media for all the experiments were LGFM-SF (low growth factor medium-serum free)
Table 2.
Different induction media used in the study.
| Adipogenic Medium (AM) | DMEM/F12, 0.5mM isobutyl-methylxanthine, 1uM dexamethasone, 10uM insulin, 200uM indomethacin, 10% FBS, and 1% ABAM |
| Osteogenic Medium (OM) | DMEM/F12, 0.1uM dexamethasone, 50uM ascorbate-2-phosphate, 10mM beta- glycerophosphate, 1% ABAM, and 10% FBS |
| Chondrogenic Medium (CM) | DMEM/F12, 6.25 ug/ml insulin, 10ng/ml TGFB1, 50nM ascorbate-2-phosphate, 1% ABAM, and 1% FBS |
2.5.1. ASC-Spheroids in Suspension Culture
Human ASC-spheroids were grown in serum-free suspension culture in LGFM-SF for 2 weeks and then in either Adipogenic Media or Osteogenic Media for 3 weeks. Control spheroids were cultured in LGFM-SF for a total of 5 weeks. After the 5-week culture period, spheroids were frozen, cryosectioned, and stained with either Oil Red O to detect lipid or with Alizarin Red to detect calcified matrix.
2.5.2. Monolayer ASCs derived from Adherent Spheroids
ASC spheroids were suspension cultured for 4 weeks in serum-free conditions (LGFM-SF or D-0 media) and then placed into adherent culture. Within 3 days of plating, cells began to grow from the edge of the attached spheroids and these aggregate-derived cells were cultured for another 14 days in lineage-induction medium (AM, OM and Chondrogenic Media) or D-10 control medium. The cultures were then fixed and stained.
To evaluate the developmental plasticity of ASCs that were derived from spheroids that had been serially passaged in adherent culture, ASC spheroids were initially cultured in suspension for 12 days in serum free medium (LGFM-SF) and then placed into adherent culture in D-10 medium. After waiting 2–4 days to allow cells to spread from the attached spheroid, the adherent spheroids were manually lifted from the culture surface and transferred into new wells. After each new plating, cells that had spread from the spheroid were grown to confluence as monolayers. ASCs derived from the 10th transfer of a single spheroid were expanded to confluence in D-10 medium over 6 weeks time. The cells were then cultured in AM or OM for 2 weeks, at which time they were fixed and stained with Oil Red-O or Alizarin Red.
2.6. Histology
Spheroids were fixed for 3–12 hours in 10% formalin, followed by incubation in 30% sucrose in PBS, and then flash frozen in cold isopentane. A cryostat was used to prepare 10-micrometer sections for staining. Alternatively, MAs were embedded in paraffin by Tissue-Tek VIP automated instrument and sectioned at 6 micrometers for analysis. Sections were stained with H&E, Oil Red O, Alizarin Red, Alcian Blue, or Gomori’s Trichrome using standard methods [20–21].
2.7. PCR Analysis
After lineage induction in suspension culture, total RNA from spheroids was isolated by PureLink Micro-to-Midi Total RNA Purification System (Invitrogen Cat# 12183-018) according to the manufacturer’s protocol. Parallel control groups were maintained in LGFM-SF. cDNA reverse transcription was performed on 1 μg of total RNA isolated from each sample using iScript cDNA Synthesis Kit (Bio-Rad Cat# 170-8890). Real-time PCR analysis was then performed with the IQ SYBR Green Supermix (Bio Rad, Cat#170-8880). Standard curves were generated, and quantities of each transcript were normalized to 18S as an internal control. Probe sequences used are listed in table 3. Standard curves have been listed as supplementary tables 17, 18, 19 and have been included as supplemental figures 4, 5 and 6.
Table 3.
PCR induction primers.
| Gene | Forward Primer | Reverse Primer | Size(bp) |
|---|---|---|---|
| 18S | 5′-CGGCGACGACCCATTCGAAC-3′ | 5′-GAATCGAACCCTGATTCCCCGTC-3′ | 99 |
| LPL | 5′-ATTTGCCCTAAGGACCCC-3′ | 5′-ATGACAGGTAGCCACGGAC-3′ | 85 |
| PPAR2 | 5′-TCCATGCTGTTATGGGTG-3′ | 5′-AGGAGTGGGAGTGGTCTT-3′ | 189 |
| FABP4 | 5′-AAACTGGTGGTGGAATGC-3′ | 5′-GCTAAATCAGGGAAAACAA-3′ | 162 |
| RUNX2 | 5′-CTCTACTATGGCACTTCGTCA-3′ | 5′-CTTCCATCAGCGTCAACAC-3′ | 164 |
| AP | 5′-GACGCTGGGAAATCTGTG-3′ | 5′-GGCATCTCGTTGTCTGAGTA-3′ | 113 |
| AGGRECAN | 5′-CGCTACTCGCTGACCTTT-3′ | 5′-GCTCATAGCCTGCTTCGT-3′ | 106 |
| COLLAGEN I | 5′-GCCATCAAAGTCTTCTGC-3′ | 5′-ATCCATCGGTCATGCTCT-3′ | 145 |
| COLLAGEN II | 5′-TCCCAGAACATCACCTACC-3′ | 5′-AACCTGCTATTGCCCTCT-3′ | 131 |
2.8. Collagen Production
Cryopreserved human ASCs were thawed and plated using established protocols. Cells were cultured as adherent monolayers in LGFM-1%HS until confluency. Spheroids of 100k cells were fabricated and maintained in suspension culture in either LGFM-SF, LGFM-1%HS, or D0. Each of these study arms was further divided into parallel cultures with or without Ascorbic Acid-phosphate. Media was changed on culture day 3, and spheroids were harvested and analyzed on day 5.
2.8.1. Sirius Red F3B Dye Binding Assay
Sterile bovine acid-soluble Type I Collagen of 1mg/ml in 0.5M acetic acid solution (Biocolor Ltd, Belfast, Northern Ireland) was used to establish the standard curve. The known mass of the reference collagen (0, 19.5, 37.5, 75 ug) were mixed in 300ul of the extraction buffer and sonicated accordingly. 200ul of the standard collagen and the sample solutions were then mixed with 1ml of Sirius Red Dye solution (1mg/ml Direct Red 80, Sigma Aldrich, in 0.5 M acetic acid with 0.1% TWEEN 20) for 30 minutes. The samples were then centrifuged down at 13.2 kg for 10 minutes. Supernatants were removed without disrupting the pellet by decanting and tapping the tube on soft tissue paper, and the dye-bound sample pellets were resuspended in 1ml of 0.5 M sodium hydroxide solution. After 5 minutes, 200ul of the sample solutions were transferred to a 96 well plate and their absorbance was read at 540 nm.
2.8.2. Picrosirius Red Staining and Cross Polarization Microscopy
The spheroids were embedded and cryosectioned to 5 um thickness. The cryosections were washed with deionized water and stained with the picrosirius red solution (0.1% Sirius red in saturated picric acid) for 1 hr. The stained sample was then washed in two different 0.5 M acetic acid solutions, cleared in xylene, and mounted in synthetic resin. The pictures were captured with an Olympus BH-2 digital microscope camera with and without linear cross polarization.
3. Results
3.1. Self-Assembling Spheroid Formation, Characterization and Culture
Using a hanging-drop culture technique, human ASCs reproducibly self-assemble into 3-dimensional spheroids using varied numbers of early passage ASCs (ranging from 500 to 50,000). The spheroids form in a range of media volumes (15–30μL) as well as in a variety of media types (D-10, D-0, LGFM and HGFM) with and without serum. Spheroids ‘assemble’ within 24–72 hours and can be reliably transferred to suspension or adherent culture conditions thereafter without damage to, or loss of form. To test durability and structure, spheroids were subjected to a variety of enzymatic and mechanical strategies in efforts to (re)dissociate their cellular components. Aggregates that were more than several days old were exceptionally robust and durable, and resisted mechanical dissociation strategies. Enzymatic compounds (collagenase, trypsin, blendzyme, etc.) produced the best dissociation, reflecting the presence of an established extracellular matrix milieu within the spheroids (data not shown) [16].
Spheroids of consistent size form at high efficiency when larger numbers of cells are used (~5,000 and higher). Nuclear staining with Hoechst’s stain reveals extensive and relatively uniform cellularity on the outer surface of the spheroids (figure 1). H&E staining reveals uniform cell (i.e. nuclei) distribution within a hyaline-positive matrix. Trichrome stain reveals the presence of extensive collagen-based ECM (figure 2) This stain has been used in published literature to show the extent of collagen production within the extracellular matrix [22]. To further characterize and quantitate this self-generated collagen-based matrix, spheroids were grown under a variety of culture conditions and evaluated with Sirius Red binding. ASC-spheroids generated collagen in all media tested, including serum free, minimalist conditions (i.e. D0). The amount of collagen produced was significantly higher in LGFM-SF and LGFM-1%HS compared to D0 and the addition of Vitamin C further increased collagen production. On comparing the data there is a significantly higher amount of collagen produced by spheroids grown in LGFM-1%HS+Vitamin C compared to spheroids grown in D0 (p=0.006 by unpaired t-test). There is also a significantly higher amount of collagen produced by spheroids grown in LGFM+Vitamin C compared to spheroids grown in D0 (p=0.019) Spheroids growing in D0 medium did not significantly respond to the addition of Vitamin C (p=0.54) (table 4). Visualization of picro-sirius red stained sections under crossed-polarized bright field microscope reveal the presence of both Type I and Type III collagen (figure 3).
Figure 1. Formation and suspension culture of human ASC spheroids.
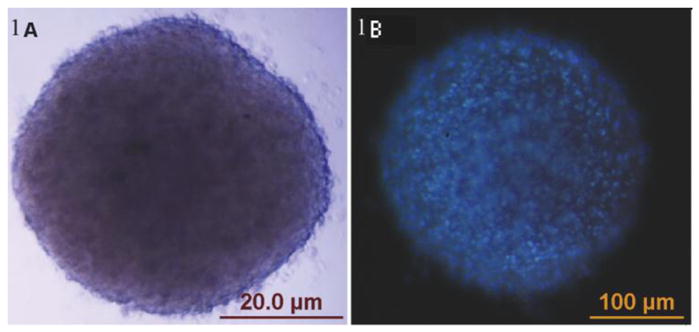
(A) ASC aggregates are reproducibly formed using a hanging drop technique. After 24–72 hours in a hanging drop, they can be reliably transferred to non-adherent culture-ware for suspension culture, (or to regular culture-ware for adherent culture). In image B, cell nuclei have been stained blue with Hoescht dye, providing additional perspective on the cellular topography of these spheroids.
Figure 2. ASC spheroids are composed of cells and cell-generated extracellular matrix.
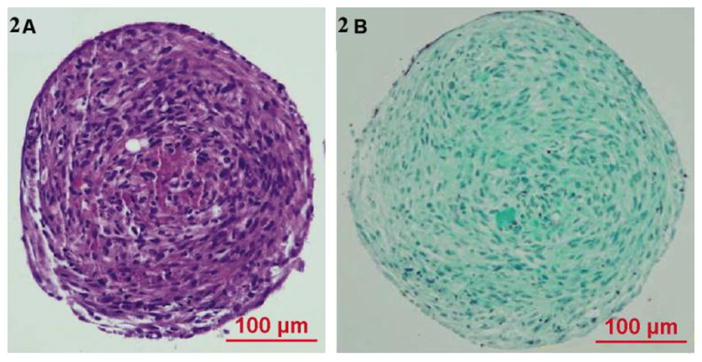
ASCs were prepared as spheroids and maintained in 3-D suspension culture. Histology of stained spheroid sections demonstrates the presence of abundant cells (ASCs) embedded within a self-generated extracellular matrix, composed of abundant collagen. (A) H & E staining, (B) Trichrome staining.
Table 4.
Collagen production by spheroids in different media conditions.
| Medium | Serum (1%HS) | Vitamin C | Collagen (ug/200k Sph) | Standard Deviation |
|---|---|---|---|---|
| LGFMa | + | + | 29.4 | 4.34 |
| LGFM | + | − | 25.27 | 4.47 |
| LGFM | − | + | 27.96 | 6.04 |
| LGFM | − | − | 23.07 | 2.47 |
| D-0 | − | + | 14.76 | 2.21 |
| D-0 | − | − | 13.38 | 2.86 |
LGFM: Low Growth Factor Medium
Figure 3. Vitamin C induces increased collagen production in ASC spheroid extracellular matrices.
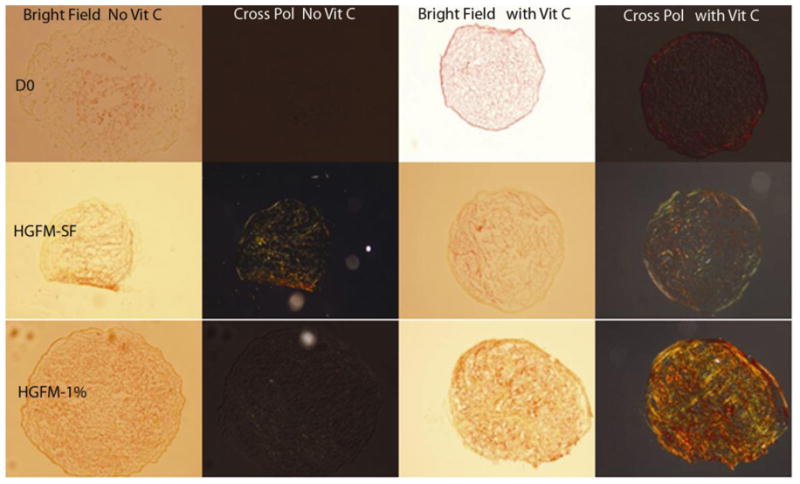
Under serum free and spartan media conditions, (D0), Vitamin C does not increase collagen content. However, in spheroids grown in HGFM-SF and especially HGFM-1%, Vitamin C induces increased collagen production within the extracellular matrix. The top, middle, bottom rows of the image are 5um picrosirius stained cryosection of 100k spheroids cultured in D0, HGFM-SF, and HGFM-1% respectively. Left column pair of pictures are bright field and cross-polarized results of control groups (without P-ascorbic acid) whereas the right column pair of pictures show the behavior of the experimental groups (with P-ascorbic acid).
With appropriate maintenance, ASC spheroids can survive for at least 6 months in suspension culture in ULA culture-ware (the longest time point tested), based on microscopic appearance, H&E histology, and their ability to spawn new, replication-competent cells when placed into adherent culture. Even ASC-aggregates grown in DMEM/F12 without any additives (D-0) remain viable for as long as one month - maintaining a compact architecture, exhibiting persistent DiI fluorescence and demonstrating the ability to readily attach to tissue culture plastic and spawn new cells that grow to monolayer confluence (data not shown). ASC spheroids routinely grow up to 400um diameter. When grown in suspension using HGFM spheroids exhibit extensive enlargement (elongation) and ‘polarization’ of fluorescent DiI signal. BrdU pulsing studies demonstrate actively replicating cells in regions that correspond to diluted fluorescent signal (figure 4, supplemental figure 1).
Figure 4. Proliferation of ASCs within spheroids in serum free suspension culture.
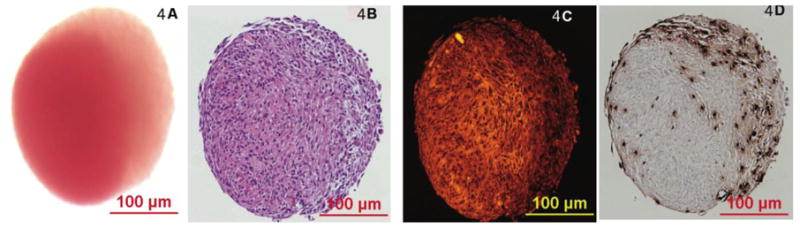
(A) Light micrograph of an ASC spheroid grown in suspension culture in serum-free medium (HGFM-SF), with early evidence of polarization and directional growth. (B) H&E histology of the same spheroid demonstrates defined nuclei throughout a background of abundant ASC-derived extracellular matrix. (C) Fluorescent micrograph of the same section in (B) demonstrates a more fluorescent intense cell and matrix-dense ‘core’ relative to a growing ‘apical’ edge (toward the right of the spheroid). (D) Reveals a light micrograph of a section from the same spheroid that has been immunohistochemically stained for BrdU (brown nuclei), demonstrating viable, replicating ASCs within the center and edge of the structure. The spheroid was grown in serum-free medium for 8 days, and pulsed with BrdU for 24 hours prior to fixation and staining.
In an effort to characterize the relationship between starting cell numbers and spheroid size and biology, spheroids were formed using incremental cell numbers ranging from 10,000 to 50,000 and grown in suspension. Spheroids did not grow beyond a certain diameter regardless of starting cell number or culture medium. As might be expected, spheroids decreased in size when grown in unfortified, minimalist medium (D0), but increased in size when grown in growth factor-containing medium (figure 5). When normalized to starting cell number, larger spheroids secrete higher levels of HGF and VEGF over time, except when grown in D0 medium (figure 6). Spheriods, when grown in close proximity, are also observed to fuse to each other to form more complex second order structures such as chains (figure 7).
Figure 5. Change in spheroid size when placed in different media conditions.
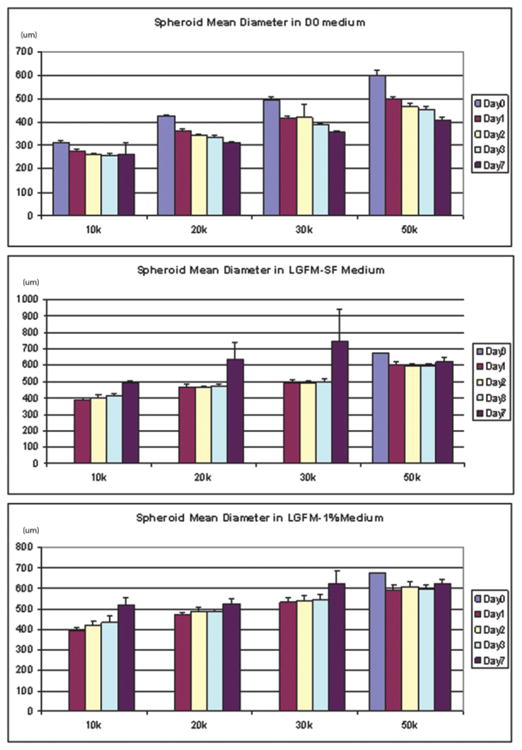
(A) Spheroids show a gradual decrease in mean diameter over a period of 7 days irrespective of starting size when maintained in spartan (D0) media conditions. (B) Spheroids show an increase in mean diameter over a period of 7 days when maintained in growth factor enriched (LGFM-SF) media conditions. (C) Spheroids show an increase in mean diameter over a period of 7 days when maintained in serum and growth factor enriched (LGFM-1%) media conditions. Diameter in micrometers.
Figure 6. Variation in growth factor production (HGF and VEGF) by spheroids when maintained in different media conditions.
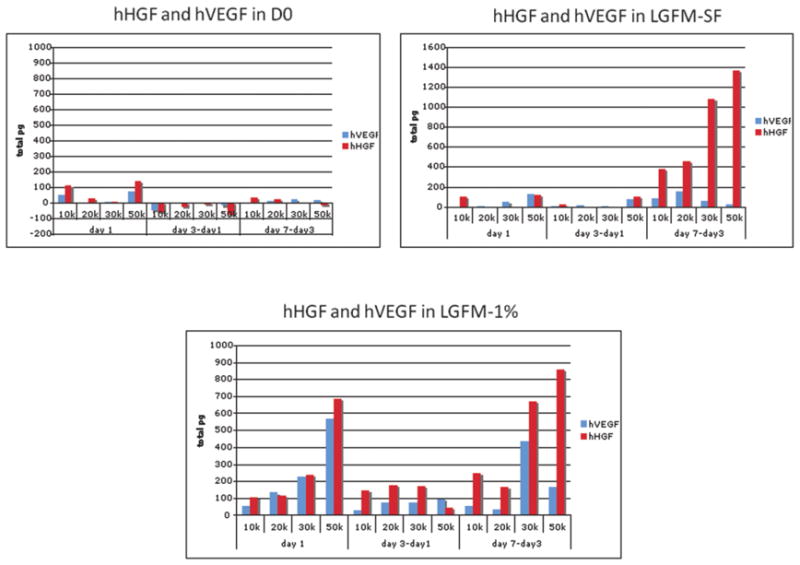
Spheroids show an increase in growth factor production (HGF and VEGF) when grown in growth factor only (LGFM-SF) (B) and growth factor + serum enriched (LGFM-1%) (C) media conditions. On the contrary, they show a decreased in growth factor production (HGF and VEGF) when grown in spartan serum free media conditions (D0) (A).
Figure 7. A, B, C.: ASC spheroids fuse together for form second order structures.
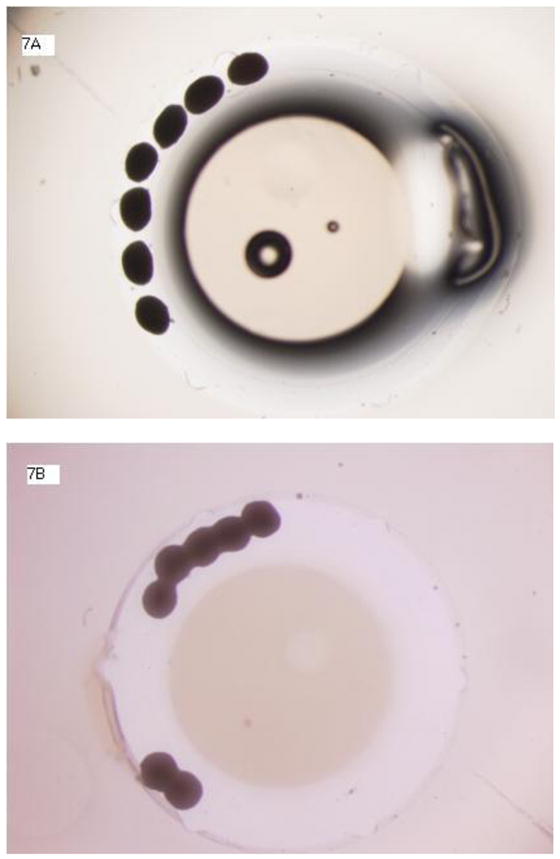

When ASC Spheroids are grown in close proximity they can fuse together to form secondary structures such as chains. The following figures show fusion of 7 spheroids over a period of 2 days. Figure 7A shows spheroids at day 0. Figure 7B shows spheroids at day 1 and figure 7C shows spheroids at day 2. Magnification 20x.
3.2. Developmental Plasticity of ASC Spheroids in Suspension Culture
ASC spheroids were tested for multilineage developmental plasticity while maintained in suspension culture by exposing them to lineage induction medias. Real-time PCR demonstrates up-regulation of lineage specific markers in spheroids cultured in inductive media compared to control spheroids maintained in non-inductive media (figure 8). These findings are corroborated on a histological level by lineage-associated stains (figure 9).
Figure 8. A,B, C. PCR Data showing adipogenic, chondrogenic and osteogenic differentiation of ASC spheroids in suspension culture.
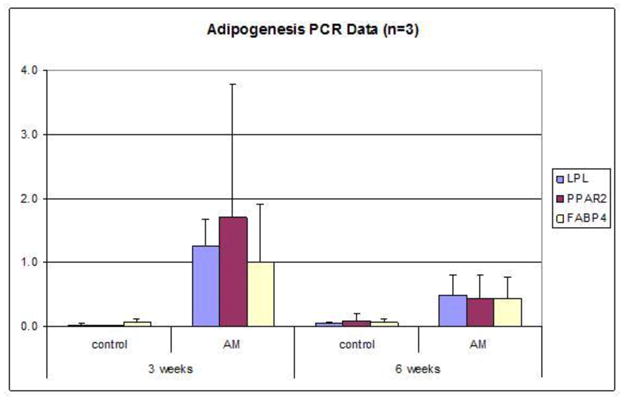
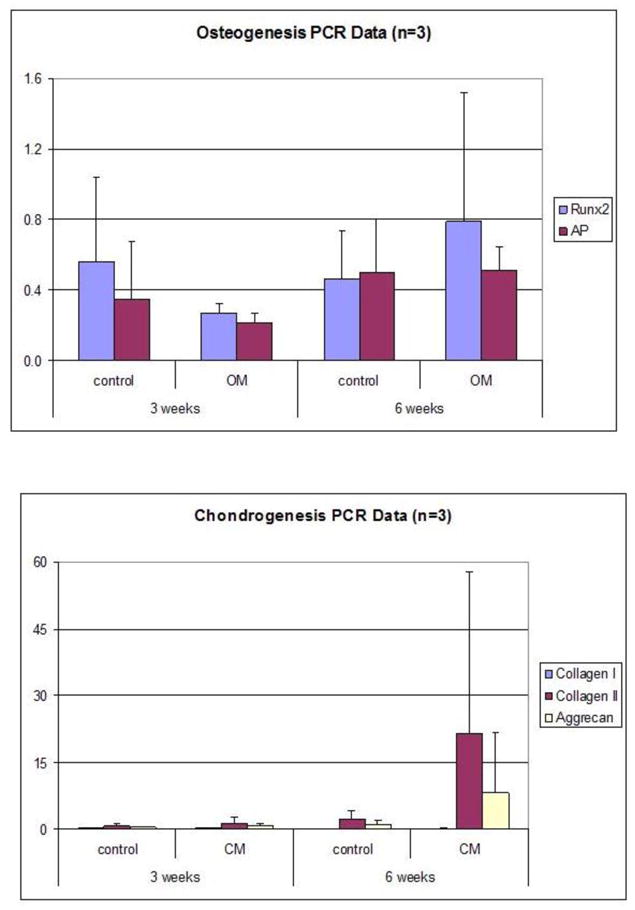
PCR analysis of RNA taken from 50k spheroids grown in inductive media for 3 weeks and 6 weeks. Bar graphs compare expression of differentiation specific markers in spheroids grown in inductive media conditions to spheroids grown in control medium. N=3 for all experiments. The means and standard deviation of this data is shown in the Supplementary Tables (Supplementary Tables 1–16).
Figure 9 . 9B, 9C. Adipogenic, and osteogenic differentiation of ASC spheroids in suspension culture.
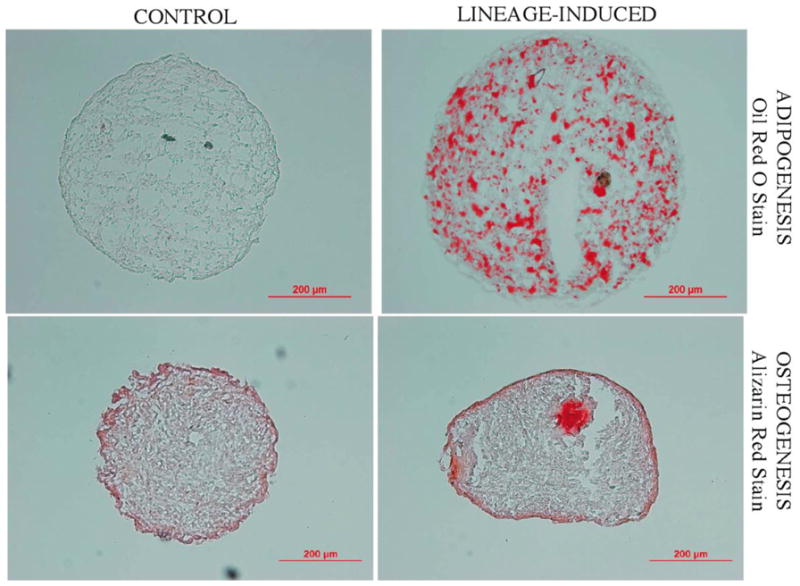
Human ASC spheroids were cultured for 2 weeks in serum-free suspension (LGFM-SF) and then exposed to either adipogenic or osteogenic medium for 3 weeks. Control spheroids remained in LGFM-SF medium for a total of 5 weeks. Oil Red O staining of spheroid sections grown in control (left upper) or adipogenic (right upper) medium demonstrates notable adipogenesis in the latter. Alizarin Red staining of spheroid sections grown in osteogenic medium (right lower) demonstrates the presence of calcified matrix. Figure 9B shows control spheroids in LGFM-SF medium and Figure 9C demonstrates Alcian Blue staining of spheroid section grown in osteogenic medium.
3.3. Self-renewal, and Developmental Plasticity of Monolayer Progeny
When plated onto regular culture plates, spheroids readily attach and ‘generate’ fibroblast-like progeny at their perimeter, which then grow to monolayer confluence in supportive media (figure 10(A,B)). If spheroids are manually lifted off of the culture surface within 3–5 days of plating and placed into a new dish/well, they will give rise to new/additional replicative-competent adherent cells (figure 10(C)). This process can be repeated with retained viability and generative capability of spheroids for at least 20 passages (moves) (data not shown). Adherent progeny cells derived from serially passaged spheroids maintain their ability to undergo adipogenesis, chrondrogenesis and osteogenesis when grown in appropriate induction conditions based on histological and morphological analysis (supplemental figure 2, supplemental figure 3).
Figure 10. Extensive growth and self-renewal of ASCs from spheroids in adherent culture. (A-C).
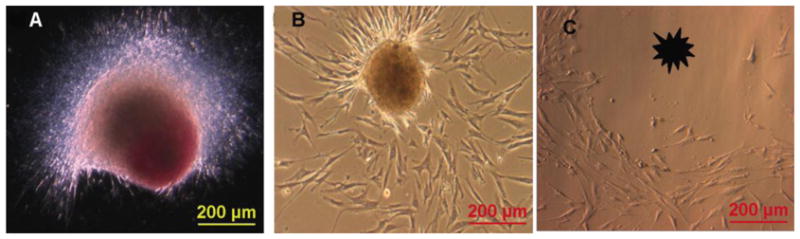
ASC spheroids demonstrate excellent growth and self-renewal in ‘traditional’ adherent culture. Image A shows a DiI labeled ASC spheroid that has been placed onto standard culture plastic. ASC spheroids readily attach to standard culture surfaces. Following attachment, the 3D structure generates cells that will eventually repopulate the culture surface as a confluent monolayer (B,C). The spheroids can also be lifted off the plate (asterisk in C) and repeatedly re-plated for the generation of new monolayer cells.
4. Discussion
Gravity-mediated assembly of cell aggregates has been described using several different cell types including cell lines, tumor cells, and certain primary human cells (hepatocytes, chondrocytes, cardiomyocytes) [1,23,24]. Our studies describe definitive, reproducible methods for the formation and culture of human adipose-derived cells as self-assembling spheroids. The use of gravity mediated 3-D cell assembly has many potential advantages for tissue engineering and biofabrication objectives. For example, spheroids of hepatocytes have been shown to exhibit liver specific functions over longer periods of times than hepatocyte monolayers. Spheroids have also served as biological models for microtumors and metastases, as drug screening assays, and recently they have garnered interest for their potential to serve as building blocks of engineered tissue [25–32]. The studies herein differ from those of Dromard et al. in that they describe the use of culture-expanded cells, as well as methodology to prepare spheroids of uniform, reproducible and specific size. The culture expanded ASCs used to engineer these spheroids are obtained from patients that vary significantly in age and BMI. Studies have shown the preadipocytes isolated from different donors vary significantly in their response to an adipogenic stimulus [33]. Yet we are able to successfully characterize ASC-spheroids because the ASC spheroids have a more consistent transcriptome compared to ASC monolayers cells and the spheroid culture system seems to diminish donor-donor variability when compared to other monolayer culture systems [16]. Recent data also shows that age and BMI do not significantly affect the proliferative capacity of ASCs [34].
The maximal size obtained by ASC spheroids is ultimately determined by the nature of the growth medium, starting cell number and diffusion distance to core cells As seen in figure 5, when ASCs are grown in growth factor enriched media, higher starting cell numbers give rise to larger spheroids such that ASC spheroids can grow to lengths measuring 1mm and diameters measuring 400–600μm. Ultimate spheroids size does not directly correlate with higher starting numbers once spheroids reach a diameter of about 600um. We also see that when placed in a minimalist medium, spheroids consistently show a decrease in size over a period of time irrespective of starting number. These data suggest that an equilibrium is reached between the ultimate size of the spheroid and the ability of the medium to sustain it. Others have shown that at a diameter of 400um and above, a central region of necrosis develops within cell spheroids. Research using hepatocytes has shown that oxygen diffusion is limited to 150–200um and that spheroids with diameters greater than 500um exhibit a layered structure consisting of a necrotic core surrounded by viable cells [35]. Although our studies demonstrate similarities to these earlier reports with respect to spheroid sizes, H&E and BrdU staining of ASC spheroids of 500um diameter or more strongly suggest viable cells even in the core. The emerging body of evidence that shows that ASCs are not only tolerant of hypoxia, but are also induced toward an angiogenic phenotype by such may explain this. This finding might also explain elevated levels of certain hypoxia-related growth factors such as VEGF by ASCs cultured as spheroids as compared to monolayer culture (figure 6) [16,36–37].
In high growth factor conditions, ASC spheroids demonstrate a distinct topographical polarity. Interestingly, others have previously shown that during growth of tumor spheroids an internal population of non-cycling viable cells develops [38]. Indeed, our BrdU staining trials with ASC spheroids support this notion, showing a clear demarcation, or polarization, within the spheroid of non-proliferating and proliferating cells. From a stem cell niche perspective, current theory suggests that the majority of stem cells within a given niche are quiescent in the G0 phase of the cell cycle when a tissue is in equilibrium [39]. Perturbation of the niche, however, can stimulate the stem cell reservoir to re-establish equilibrium (i.e. regenerate) by self-renewal, apoptosis, and/or differentiation - all of which is believed to be governed by the surrounding micromilieu [40,41]. In the case of dramatic polarization observed in a few of our studies, the micromilieu consists of several growth factors in high concentrations. It is possible that the non-dividing, fluorescence-intense core (figure 4, supplemental figure 1) represents a ‘quiescent reservoir’ within the spheroid, but this cannot be definitively concluded base on these findings alone. Alternatively, the polarized nature of the spheroids may reflect asymmetric cell division and/or active replication of a subpopulation of cells within the spheroid. The appearance of the spheroid in Figure 4 is the result of self-directed cell activity. No sorting was performed. We cannot definitively explain this dramatic polarization based on the phenotypic differences of the cells concerned at this time. A better understanding of this phenomenon is a subject of ongoing research in our lab.
ASC spheroids can be cultured and maintained in defined media free of serum (D0, HGFM, LGFM). This has both basic science and translational implications since FBS is associated with lot to lot variability, contains various unknown and/or undefined components, and cell therapies formulated and manufactured in FBS have been associated with allergic complications when administered to humans [42–44]. In addition, given their robust and durable nature, handling and transfer of these 3-D structures is quite easy. This durability is due, at least in part, to a self-generated matrix that contains variable amounts of collagen, depending on the specific environmental cues (figure 3). For example, the addition of Vitamin C was associated with increased levels of collagen production, a finding consistent with similar previously published reports [45,46].
ASC spheroids can be cultured two different ways: floating in suspension, or as adherent monolayers. We explored and characterized the differences of these two systems in the context of growth and differentiation. Until now, it was unknown whether ASCs could undergo multilineage differentation while maintained in suspension as 3-D spheroids. Our data clearly demonstrates that ASC spheroids grown in suspension in inductive media conditions show progression down defined lineage pathways. The up-regulation of appropriate lineage specific markers is seen in PCR analysis (figure 8) and appropriate lineage specific staining is revealed in histology studies (figure 9). The calcifications seen in spheroids undergoing osteogenic differentiation are most likely related to the formation of calcified matrix as opposed to a sign of necrosis. This calcification, if due to necrosis, would be seen in the control spheroids as well as in spheroids undergoing adipogenic differentiation. We do not see such calcification and therefore assert that the presence of calcification in spheroids placed in osteoinductive media to be a sign of osteogenic differentiation. When placed into adherent culture, suspension-grown spheroids readily attach, and can repeatedly generate replication-competent ASCs that grow to confluence on adherent tissue culture plastic in supportive medium. Even more, our work shows that monolayer cells derived from suspension cultured spheroids continue to display multilineage developmental plasticity capabilities when challenged with traditional inductive assays, and do so even after serial, long-term culture expansion.
5. Conclusion
In summary, these studies demonstrate that human ASCs can reproducibly form and subsequently be maintained as dynamic, 3-dimensional cell spheroids in suspension culture. Whether cultured in suspension or as adherent entities, our studies demonstrate that ASC-spheroids display a capacity for extensive renewal, developmental plasticity, and internally directed homeostasis and (re)organization. Even more, their capacity for renewal and differentiation is manifest after maintenance in a variety of different culture media – including serum free conditions. These attributes make this ‘system’ useful for the mechanistic study of ASC biology in the context of 3 dimensions under defined and reproducible conditions. Even more, this work unequivocally demonstrates that ASC spheroids may be used to provide a flexible and practical modular foundation with which to build tissues and organs using emerging bio-printing, layered fabrication and similar techniques.
Supplementary Material
Acknowledgments
The author’s would like to mention special thanks to Roy Ogle, PhD and Shayn Peirce-Cottler, PhD for their advice, expertise and use of pertinent lab equipment.
Funding for this work was provided in part by NIH/NHLBI Grant HL72141, NIH/NIBIB Grant EB009140 and The Department of the Army, Armed Forces Institute of Regenerative Medicine (AFIRM) Award Number W81XWH-08-2-0034.
Footnotes
Disclosure Statement
AJK is a named inventor on issued and pending patents related to the isolation and use of Adipose-derived Cells and Matrix and received royalty payments related to this IP.
The information does not necessarily reflect the position or the policy of the Government, and no official endorsement should be inferred.
References
- 1.Kelm JM, Fussenegger M. Microscale tissue engineering using gravity-enforced cell assembly. Trends Biotechnol. 2004;22:195–202. doi: 10.1016/j.tibtech.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 3.Vacanti JP, Morse MA, Saltzman WM, Domb AJ, Perez-Atayde A, Langer R. Selective cell transplantation using bioabsorbable artificial polymers as matrices. J Pediatr Surg. 1988;23:3–9. doi: 10.1016/s0022-3468(88)80529-3. [DOI] [PubMed] [Google Scholar]
- 4.Hutmacher DW. Scaffold design and fabrication technologies for engineering tissues – state of the art and future perspectives. J Biomater Sci Polym. 2001;12:107–24. doi: 10.1163/156856201744489. [DOI] [PubMed] [Google Scholar]
- 5.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci (U S A) 2006;103:2480–7. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langer R. Tissue engineering: perspectives, challenges, and future directions. Tissue Eng. 2007;13:1–2. doi: 10.1089/ten.2006.0219. [DOI] [PubMed] [Google Scholar]
- 7.Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ Printing: Tissue spheroids as building blocks. Biomaterials. 2009;30:2164–74. doi: 10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lareu RR, Arsianti I, Subramhanya KH, Yanxian P, Raghunath M. In vitro enhancement of collagen matrix formation and crosslinking for applications in tissue engineering: a preliminary study. Tissue Eng. 2007;13:385–91. doi: 10.1089/ten.2006.0224. [DOI] [PubMed] [Google Scholar]
- 9.Lareu RR, Subramhanya KH, Peng Y, Benny P, Chen C, Wang Z, Rajagopalan R, Raghunath M. Collagen matrix deposition is dramatically enhanced in vitro when crowded with charged macromolecules: the biological relevance of the excluded volume effect. FEBS Lett. 2007;581:2709–14. doi: 10.1016/j.febslet.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Foty R, Steinberg M. The differential adhesion hypothesis: a direct evaluation. Developmental Biology. 2005;278:255–63. doi: 10.1016/j.ydbio.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Foty R, Pfleger C, Forgacs G, Steinberg M. Surface tension of embryonic tissues predict their mutual envelopment behavior. Development. 1996;122:1611–20. doi: 10.1242/dev.122.5.1611. [DOI] [PubMed] [Google Scholar]
- 12.Norotte C, Marga F, Niklason L, Forgacs G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30:5910–7. doi: 10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasza KE, Rowat AC, Liu J, Angelini TE, Brangwynne CP, Koenderink GH, Weitz DA. The cell as a material. Curr Opin Cell Biol. 2007;19:101–7. doi: 10.1016/j.ceb.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Layer PG, Robitzki A, Rothermel A, Willbold E. Of layers and spheres: the reaggregate approach in tissue engineering. Trends Neurosci. 2002;25:131–4. doi: 10.1016/s0166-2236(00)02036-1. [DOI] [PubMed] [Google Scholar]
- 15.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–60. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amos PJ, Kapur SK, Stapor PC, Shang H, Bekiranov S, Khurgel M, Rodeheaver GT, Pierce SM, Katz AJ. Human adipose-derived stromal cells accelerate diabetic wound healing: impact of cell formulation and delivery. Tissue Eng. 2010;16:1595–1606. doi: 10.1089/ten.tea.2009.0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dromard C, Bourin P, Andre’ M, De Barros S, Castiella L, Planat-Benard V. Human adipose derived stroma/stem cells grow in serum-free medium as floating spheres. Exp Cell Res. 2011;317:770–80. doi: 10.1016/j.yexcr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23:412–423. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- 19.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 20.Prichard HL, Riechert W, Klitzman B. IFATS collection: adipose-derived stromal cells improve the foreign body response. Stem Cells. 2008;26:2691–5. doi: 10.1634/stemcells.2008-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. PNAS. 2001;98:7841–5. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J. Differentiations of human embryonic stem cells on three-dimensional polymer scaffolds. PNAS. 2003;100:12741–6. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunz-Schughart LA. Multicellular tumor spheroids: intermediates between monolayer culture and in vivo tumor. Cell Biol Int. 1999;23:157–61. doi: 10.1006/cbir.1999.0384. [DOI] [PubMed] [Google Scholar]
- 24.Bjerkvig R, Lund-Johansen M, Edvardson K. Tumor cell invasion and angiogenesis in the central nervous system. Curr Opin Oncol. 1997;9:223–9. doi: 10.1097/00001622-199709030-00002. [DOI] [PubMed] [Google Scholar]
- 25.Fukuda J, Nakazawa K. Orderly arrangement of hepatocyte spheroids on a microfabricated chip. Tissue Eng. 2005;11:1254–62. doi: 10.1089/ten.2005.11.1254. [DOI] [PubMed] [Google Scholar]
- 26.Tong JZ, Lagausie PD, Furlan V, Cresteil T, Bernard O, Alvarez F. Long term culture of adult rat hepatocyte spheroids. Exp Cell Res. 1992;200:326–32. doi: 10.1016/0014-4827(92)90179-c. [DOI] [PubMed] [Google Scholar]
- 27.Sakai Y, Nakazawa K. Technique for the control of spheroid diameter using microfabricated chips. Acta Biomater. 2007;3:1033–1040. doi: 10.1016/j.actbio.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Kale S, Biermann S, Edwards C, Tarnowski C, Morris M, Long MW. Three dimensional cellular development is essential for ex vivo formation of human bone. Nat Biotechnol. 2000;18:954–958. doi: 10.1038/79439. [DOI] [PubMed] [Google Scholar]
- 29.Santini MT, Rainaldi G. Three-dimensional spheroid model in tumor biology. Pathobiology. 1999;67:148–57. doi: 10.1159/000028065. [DOI] [PubMed] [Google Scholar]
- 30.Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA. Spheroid-based drug screen: considerations and practical approach. Nat Protoc. 2009;4:309–324. doi: 10.1038/nprot.2008.226. [DOI] [PubMed] [Google Scholar]
- 31.Kelm JM, Fussenegger M. Microscale tissure engineering using gravity enforced cell assembly. TRENDS in Biotechnology. 2004;22:195–202. doi: 10.1016/j.tibtech.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Kelm JM, Timmins NE, Brown CJ, Fussenegger M, Nielsen LK. Method for generation of homogenous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol Bioeng. 2003;83:173–80. doi: 10.1002/bit.10655. [DOI] [PubMed] [Google Scholar]
- 33.Sen A, Lea-Currie R, Sujkowska D, Franklin DM, Wilkison WO, Halvorsen YC, Gimble JM. Adipogenic Potential of Human Adipose Derived Stromal Cells From Multiple Donors is Heterogeneous. J Cell Biochem. 2001;81:312–19. doi: 10.1002/1097-4644(20010501)81:2<312::aid-jcb1046>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 34.Ali Mojallal, Lequeux C, Shipkov C, Duclos A, Braye F, Rohrich R, Brown S, Damour O. Influence of Age and Body Mass Index on the Yield and Proliferation Capacity of Adipose-Derived Stem Cells. Aesth Plast Surg. 2011;35:1097–105. doi: 10.1007/s00266-011-9743-7. [DOI] [PubMed] [Google Scholar]
- 35.Ota H, Yamamoto R, Deguchi K, Tanaka Y, Kazoe Y, Sato Y, Miki N. Three-dimensional spheroid-forming lab-on-a-chip using micro-rotational flow. Sens Actuators B Chem. 2010;147:359–65. [Google Scholar]
- 36.Kelm JM, Diaz Sanchez-Bustamante C, Ehler E, Hoerstrup SP, Djonov V, Itner L, Fussenegger M. VEGF profiling and angiogenesis in human microtissues. J Biotechnol. 2005;118:213–29. doi: 10.1016/j.jbiotec.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 37.Bhang SH, Cho S, La W, Lee T, Yang HS, Sun A, Baek S, Rhie J, Kim B. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials. 2011;32:2734–47. doi: 10.1016/j.biomaterials.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 38.Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–9. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- 39.Kindler V. Postnatal stem cell survival: does the niche, a rare harbor where to resist the ebb tide of differentiation, also provide lineage-specific instructions? J Leukoc Biol. 2005;78:836–44. doi: 10.1189/jlb.0505272. [DOI] [PubMed] [Google Scholar]
- 40.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–9. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 41.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–78. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 42.Mackensen A, Drager R, Schlesier M, Mertelsmann R, Lindemann A. Presence of IgE antibodies to bovine serum albumin in a patient developing anaphylaxis after vaccination with human peptide-pulsed dendritic cells. Cancer Immunol Immunother. 2000;49:152–156. doi: 10.1007/s002620050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spees JL, Gregory CA, Singh H, Tucker A, Peister A, Lynch PJ, Hsu S, Smith J, Prockop DJ. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther. 2004;9:747–756. doi: 10.1016/j.ymthe.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 44.Tuschong L, Soenen SL, Blaese RM, Candotti F, Muul LM. Immune response to fetal calf serum by two adenosine deaminase-deficient patients after T cell gene therapy. Hum Gene Ther. 2002;13:1605–1610. doi: 10.1089/10430340260201699. [DOI] [PubMed] [Google Scholar]
- 45.Trottier V, Marceau-Fortier A, Germain L, Vincent C, Fradette J. IFATS Collection: Using human adipose-derived stem/stromal cells for the production of new skin substitutes. Stem Cells. 2008;26:2713–23. doi: 10.1634/stemcells.2008-0031. [DOI] [PubMed] [Google Scholar]
- 46.Vermette M, Trottier V, Menard V, Saint-Pierre L. Production of a new tissue-engineered adipose substitue from human adipose-derived stromal cells. Biomaterials. 2007;28:2850–60. doi: 10.1016/j.biomaterials.2007.02.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


