Abstract
To evaluate the effect of elevated pCO2 exposure on the juvenile growth of the sea urchin Lytechinus variegatus, we reared individuals for three months in one of three target pCO2 levels: ambient seawater (380 µatm) and two scenarios that are projected to occur by the middle (560 µatm) and end (800 µatm) of this century. At the end of 89 days, urchins reared at ambient pCO2 weighed 12% more than those reared at 560 µatm and 28% more than those reared at 800 µatm. Skeletons were analyzed using scanning electron miscroscopy, revealing degradation of spines in urchins reared at elevated pCO2 (800 µatm). Our results indicate that elevated pCO2 levels projected to occur this century may adversely affect the development of juvenile sea urchins. Acidification-induced changes to juvenile urchin development would likely impair performance and functioning of juvenile stages with implications for adult populations.
Keywords: echinoderm, carbon dioxide, invertebrate, development, CO2, pH, pCO2
1. Introduction
As a result of oceanic uptake of atmospheric carbon dioxide, the world’s oceans are slowly becoming more acidic (ocean acidification, OA). Approximately 30% (148 Pg C) of anthropogenic CO2 emissions have been absorbed by the oceans (Sabine et al., 2011). Consequently, seawater carbonate concentrations have been depleted by ~30 µmol kg−1, simultaneously reducing the pH of the ocean’s surface waters by 0.1 units relative to the pre-industrial era (a 30% increase in acidity) (IPCC, 2007). Further reductions of 0.3–0.5 pH units are projected to occur by the end of this century as the oceans continue to absorb anthropogenic CO2 (IPCC, 2007).
Documenting and predicting the response(s) of various marine organisms to changing ocean chemistry has been of recent concern in the scientific community. Mounting experimental evidence suggests that ocean acidification may hold negative consequences for a variety of marine organisms (Gattuso and Hansson, 2011), primarily calcifiers that depend on the delicate balance of inorganic carbon species to form their CaCO3 shells or skeletons (Doney et al., 2009; Fabry et al., 2008; Kroecker et al., 2010).
Sea urchins are one of the most heavily studied groups of organisms with respect to ocean acidification, and early life history stages are thought to be particularly vulnerable to changing water chemistry (Kurihara, 2008; Dupont and Thorndyke, 2009). To date, the majority of work evaluating the effects of ocean acidification on early life history stages of urchins has focused on pre-metamorphic processes including pre-larval (e.g., fertilization, embryonic development) and larval stages (Table 1). While increasing information is available on the effects of ocean acidification on pre-metamorphic life history stages of sea urchins, there is a clear lack of information regarding the sensitivity of post-metamorphic (i.e., juvenile) stages. Only two studies to date have evaluated the effect(s) of elevated pCO2 exposure on juvenile urchin growth. Shirayama and Thornton (2005) reported decreased juvenile growth of two species of urchin, Echinometra mathaei and Hemicentrotus pulcherrimus, during six-months exposure to elevated pCO2 (560 µatm). Increased mortality was also reported in one of two trials. Byrne et al. (2011) reported an increased number of abnormal Heliocidaris erythrogramma juveniles exposed to depressed pH (−0.2 and −0.4 pH units) and elevated temperature (+2°C and +4°C). Metamorphosis marks the transition from a planktonic larva to a benthic juvenile and as such, larvae and juveniles differ substantially with respect to their morphology, physiology, and ecology and may differ in susceptibility to environmental stressors such as ocean acidification. It is, therefore, important to better understand post-metamorphic susceptibility to OA to couple with our growing knowledge of pre-metamorphic effects.
Table 1.
Compilation of recent studies investigating the effects of ocean acidification (pH change) on early life history stages of sea urchins. This listing is restricted to comprehensive studies for which methods, data, and statistical analyses are available and is focused on studies conducted over the last decade, under the context of near-future ocean acidification scenarios. Life stage abbreviations are as follows: PL = Pre-larval (fertilization and/or embryogenesis); L = Larval; J = Juvenile.
| Taxon | Life Stage |
CO2 or HCl (ppm) |
pH | Temp °C |
Exposure period |
Effect | Source |
|---|---|---|---|---|---|---|---|
| Echinometra mathaei | PL, L | HCl & CO2 (365–10,360) |
6.8–8.1 | 3 d | pH < 7.8, skeletal malformation, reduced larval size & fertilization | Kurihara & Shirayama 2004 | |
| Echinometra mathaei | J | CO2 (ambt., 560) |
7.90, 7.94 | <30 | 6 mo | Decreased growth at 12 wk; mortality in 1 of 2 expts. | Shirayama & Thornton 2005 |
| Hemicentrotus pulcherrimus | PL, L | HCl & CO2 (365–10,360) |
6.8–8.0 | 3 d | pH < 7.8, skeletal malformation, reduced larval size & fertilization | Kurihara & Shirayama 2004 | |
| Hemicentrotus pulcherrimus | J | CO2 (ambt., 560) |
7.90, 7.94 | <30 | 6 mo | Decreased growth at 14 wk; mortality in 1 of 2 expts. | Shirayama & Thornton 2005 |
| Heliocidaris erythrogramma | PL | CO2 (ambt.,1000) |
7.7, 8.1 | 20.5 | 24 h | Decreased sperm motility, swimming speed, and fertilization | Havenhand et al. 2008 |
| Heliocidaris erythrogramma | PL | CO2 (230–690) | 7.6–8.2 | 20–26 | 4 d | No effect of CO2 on fertilization or development; T effect on development, not fertilization; no T*CO2 interaction |
Byrne et al. 2009 |
| Heliocidaris erythrogramma | PL | CO2 (370, 1100, 1850) |
7.6–8.2 | 20–26 | 2 h | No effect of T or CO2 on fertilization | Byrne et al. 2010a |
|
Strongylocentrotus franciscanus |
PL | CO2 (400, 800, 1800) |
7.6, 7.8, 8.0 | 10 | 3 h | Decreased fertilization efficiency; increased susceptibility to polyspermy at 1580 ppm; CO2 effect dependent on sperm concentration |
Reuter et al. 2010 |
|
Strongylocentrotus franciscanus |
PL, L | CO2 (380, 540, 970) |
7.87, 7.98, 8.04 |
15, 19, 21, 23, 25, 27, 29, 31 |
~96 h | Gametes (larvae) fertilized (reared) at elevated CO2 showed reduced expression of heat shock proteins in response to acute T stress (1h); T of max. induction was shifted |
O’Donnell et al. 2009 |
| Strongylocentrotus purpuratus | L | CO2 (380, 540, 1020) |
7.88, 7.96, 8.01 |
15 | < 72 h | Decreased gene expression in 4 major cellular processes: biomineralization, cellular stress response, metabolism, apoptosis |
Todgham & Hofmann 2009 |
| Tripneustes gratilla | L | CO2 (450, 1200, 1900) |
8.15, 7.8, 7.6 | 24, 27, 30 | 5 d |
pCO2 decreased larval growth; T < 30°C increased growth; interaction of CO2 *T − 3° warming (24°–27°) diminished CO2 effect |
Sheppard Brennand et al. 2010 |
|
Tripneustes gratilla (tropical spp.) |
L | CO2 (395– 1119) |
6.0–8.1 | 26 | 4 d | Decreased calcification and size; no effect on fine skeletal morphology; decreased survival at pH < 7.0 |
Clark et al. 2009 |
|
Pseudechinus huttoni (temp. spp.) |
L | CO2 (429– 1,282) |
6.0–8.1 | 10–15 | 9 d | Decreased calcification and size; degradation of fine skeletal morphology; decreased survival at pH < 7.0 |
Clark et al. 2009 |
|
Evechinus chloroticus (temp. spp.) |
L | CO2 (438– 1,320) |
6.0–8.1 | 10–15 | 13 d | Decreased calcification and size; degradation of fine skeletal morphology; decreased survival at pH < 7.0 |
Clark et al. 2009 |
|
Sterechinus neumayeri (polar spp.) |
L | CO2 (521– 1,380) |
6.0–8.1 | −1 | 7 d | No effect on calcification; no effect on fine skeletal morphology; decreased survival at pH < 7.0 |
Clark et al. 2009 |
| Lytechinus pictus | L | CO2 (380, 540, 970) |
7.78, 7.87, 7.93 |
18.5 | 6 d | Altered larval size, shape; down-regulation of genes central to energy metabolism, biomineralization; up-regulation of genes involved in ion regulation and acid-base balance pathways |
O’Donnell et al. 2010 |
| Paracentrotus lividus | PL, L | CO2 (400, 700, 1100, 1900, 3600, 6600) |
7.0, 7.25, 7.5, 7.7, 7.9, 8.1 |
20 | 3 d | No effect on fertilization or larval survival; slowed larval growth at low pH; upregulation of development and biomineralization candidate genes |
Martin et al. 2011 |
| Heliocidaris erythrogramma | PL | CO2 (330–1828) |
7.6–8.25 | 18–26 | 15 min. | No effect of T or CO2 on fertilization at the sperm concentration used | Byrne et al. 2010b |
| Heliocidaris tuberculata | PL | CO2 (330–1828) |
7.6–8.25 | 18–26 | 15 min. | No effect of T or CO2 on fertilization at the sperm concentration used | Byrne et al. 2010b |
| Tripneustes gratilla | PL | CO2 (330–1828) |
7.6–8.25 | 18–26 | 15 min. | No effect of T or CO2 on fertilization at the sperm concentration used | Byrne et al. 2010b |
| Centrostephanus rodgersii | PL | CO2 (330–1828) |
7.6–8.25 | 18–26 | 15 min. | No effect of T or CO2 on fertilization at the sperm concentration used | Byrne et al. 2010b |
| Strongylocentrotus purpuratus | L | CO2 (375, 1264) |
7.7, 8.1 | 14 | 21 d. | Developmental delay, increased respiration, no effect on feeding rate | Stumpp et al. 2011a |
| Strongylocentrotus purpuratus | L | CO2 (399, 1318) |
7.7, 8.17 | 16 | 7 d. | Developmental delay, altered gene expression - upregulation of metabolic genes that induce developmental delay, down regulation of calcification genes, altered ion regulation |
Stumpp et al. 2011b |
| Strongylocentrotus purpuratus | L | CO2 (ambt., 1000, 1450) |
7.5, 7.7, 8.07 | 15.6 | 6 d. | Smaller larvae at 1450 ppm after 3 d.; no effect on developmental timing | Yu et al. 2011 |
| Heliocidaris erythrogramma | PL, L, J |
CO2 | 7.6, 7.8, 8.2 | 20, 22, 24 | 5 d. | T & CO2 caused abnormal development; 2°C warming diminished negative effects of low pH; decreased number of spines with pCO2 |
Byrne et al. 2011 |
The objective of the present study was to evaluate the effect of elevated pCO2 exposure on post-metamorphic urchin growth using the tropical Atlantic variegated sea urchin, Lytechinus variegatus, as a model organism. L. variegatus is an ecologically important species with a broad geographical range. It is found in both intertidal and shallow sub-tidal habitats, ranging from North Carolina and Bermuda southward to Brazil, and throughout the Caribbean and the Gulf of Mexico (Mortensen, 1943; Watts et al., 2001). This species serves as a primary grazer in many seagrass habitats where it plays an important role in energy transfer between trophic levels and can influence the structure of animal communities (Greenway, 1995; Watts et al., 2001). L. variegatus has an echinopluteus larva that remains in the plankton for a minimum of 2 weeks but can stay for several months if the appropriate metamorphic cues are not encountered. Upon encountering suitable settlement cues, larvae metamorphose into benthic juveniles. Individuals typically start feeding within two days of metamorphosis and mature at a diameter of approximately 40 mm, or around the age of 1 year (Moore et al., 1963). The life-span of near-shore animals is estimated to be approximately 3–4 years (Allain, 1975; Beddingfield and McClinktock, 2000), but deep-water animals may live longer.
To evaluate the effect of near-future ocean acidification scenarios on juvenile urchin growth, we reared L. variegatus individuals (< 6 months old) under controlled pCO2 conditions over the course of three months (89 days) and evaluated the effect on post-metamorphic growth. Three pCO2 levels were targeted: ambient seawater (~380 µatm) and two scenarios that are projected to occur by the middle (560 µatm) and end (800 µatm) of this century (IPCC, 2007).
2. Materials and Methods
Juvenile urchins (< 6 months old) were sourced from the University of Miami’s Experimental Hatchery (UMEH). UMEH regularly collects adult specimens of Lytechinus variegatus from wild populations (< 5 m depth) offshore of Key Biscayne, Florida (ocean-side; 25°41’20”N, 80°9’21”W) to breed in-house. Details regarding the collection and breeding protocols are provided in the supplemental information. Urchins were approximately 5 months old at the time of retrieval. In the hatchery, urchins are maintained at 23°C. We, therefore, acclimated them to the experimental temperature (28°C) over the course of 2 weeks at a rate of approximately 0.4°C day−1. No mortality or signs of stress were observed during the acclimation period. On 18 January 2010, 175 d old juveniles were introduced to experimental conditions.
2.1 Seawater chemistry
Seawater was supplied from a source inlet in the nearby Bear Cut (Virginia Key, FL) and pumped into a 63,000-gallon settling tank and subsequent sand filter to remove particulate matter. Seawater was then supplied to indoor tanks wherein the carbonate system was manipulated prior to introduction to the experimental aquaria. One ambient pCO2 concentration (380 µatm) and two elevated pCO2 concentrations (560 and 800 µatm) were chosen for the study, based on near-future projections determined by the Intergovernmental Panel on Climate Change (IPCC) (IPCC, 2007). Seawater chemistry was manipulated via direct bubbling with carbon dioxide-enriched air. The control was bubbled with outside air. All treatments experienced natural fluctuations in pCO2; consistent bubbling with CO2-enriched air superimposed an acidification effect on top of diurnal variability.
Discrete water samples from treatment aquaria were analyzed for total alkalinity (TA) and pH on a weekly basis to verify distinct treatments. Because highest and lowest pCO2 levels are typically observed near dawn and dusk respectively, water samples were taken between 1200–1300h and represent average daily pCO2 levels. TA was determined in duplicate using an automated, open-cell Gran titration (Dickson et al., 2007, SOP3b), and accuracy was checked against certified seawater reference material (A. Dickson, Scripps Institute of Oceanography). pH (total scale) was determined using an Orion Ross combination pH electrode (Thermo Scientific) calibrated at 25°C against a seawater TRIS buffer (Dickson et al., 2007, SOP6a). Concentrations of HCO3−, CO32−, CO2, and Ωarag were computed from TA, pH, temperature, and salinity using the program CO2SYS (E.Lewis, Brookhaven National Laboratory), with dissociation constants for carbonate determined by Mehrbach et al. (1973), as refit by Dickson and Millero (1987) and dissociation constant for boric acid determined by Dickson (1990). Chemical and physical conditions that persisted during the experiment are provided in the Table 2.
Table 2.
Physical and chemical conditions of seawater during growth experiment (mean ± SD, N = 8 weekly samples). All measurements are based upon duplicate or triplicate analyses of pH (total scale) and total alkalinity (TA) for each sampling period. pHT, pCO2, HCO3−, CO32−, CO2, TCO2, and Ωcalcite were calculated using CO2SYS. Calcium concentration was calculated based on 10.28 mmol kg−1 of Ca2+ at a salinity of 35 ppt.
| Salinity | T (°C) |
TA (µmol kg−1) |
pHT |
pCO2 (µatm) |
HCO3− (µmol kg−1) |
CO32− (µmol kg−1) |
CO2 (µmol kg−1) |
TCO2 (µmol kg−1) |
bCa2+ (mmol kg−1) |
Ωcalcite | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ambient | 35.2 ± 0.6 | 28.1 ± 0.4 | 2467 ± 30 | 8.10 ± 0.06 | 367 ± 64 | 1799 ± 78 | 275 ± 22 | 10 ± 2 | 2084 ± 59 | 10.3 ± 0.2 | 6.6 ± 0.5 |
| Mid CO2 | 35.2 ± 0.7 | 28.2 ± 0.5 | 2446 ± 35 | 7.96 ± 0.04 | 542 ± 66 | 1928 ± 53 | 213 ± 15 | 14 ± 2 | 2155 ± 45 | 10.3 ± 0.2 | 5.2 ± 0.4 |
| High CO2 | 35.4 ± 0.8 | 28.1 ± 0.1 | 2447 ± 42 | 7.83 ± 0.03 | 765 ± 58 | 2039 ± 41 | 168 ± 11 | 20 ± 2 | 2227 ± 41 | 10.4 ± 0.2 | 4.1 ± 0.3 |
2.2 Experimental Design
Three individuals (initial weight 30.4 ± 3.5 mg; mean ± SD) were randomly assigned to each of twelve exposure tanks (four tanks per treatment x three pCO2 treatments) containing seawater as described in Table 2. Tanks were maintained at a constant temperature of 28 ± 0.2 °C. Urchins were fed algae (Ulva spp.) ad libitum and weighed approximately every two weeks using an analytical balance. Measurements were taken a total of 7 times on days 0, 12, 25, 40, 56, 68, and 89 of the experiment. To obtain accurate weights, excess water was gently removed with laboratory wipes, and organisms were immediately placed on the scale. Care was taken to minimize the time out of water to reduce stress on the animals. Mean urchin weight per tank was compared using a repeated measures two-way ANOVA with time and pCO2 as main effects. Bonferroni post-hoc comparisons were used to determine which treatments and time intervals differed from each other. Statistical analyses were conducted using GraphPad Prism® 5.0 statistical software.
2.3 Scanning Electron Microscopy
Upon termination of the 89-day growth experiment, urchins were sampled for use in scanning electron microscopy (SEM) to examine for potential effects of seawater pH/pCO2 on fine-scale skeletal morphology. Three urchins from each treatment (N=3) were randomly chosen and bleached in 10% sodium hypochlorite overnight to remove organic material. Skeletons were rinsed three times with tap water and once with distilled water to remove bleach residue. Skeletal subsamples were mounted on aluminum stubs using carbon adhesive tabs and coated with palladium for three minutes using a Cressington 108 Auto sputter coater. Samples were imaged in an FEI XL-30 environmental scanning electron microscope (ESEM) to assess the presence of skeletal abnormalities at low pH (e.g. malformation or dissolution of the skeleton).
3. Results
3.1 Post-metamorphic growth and survivorship
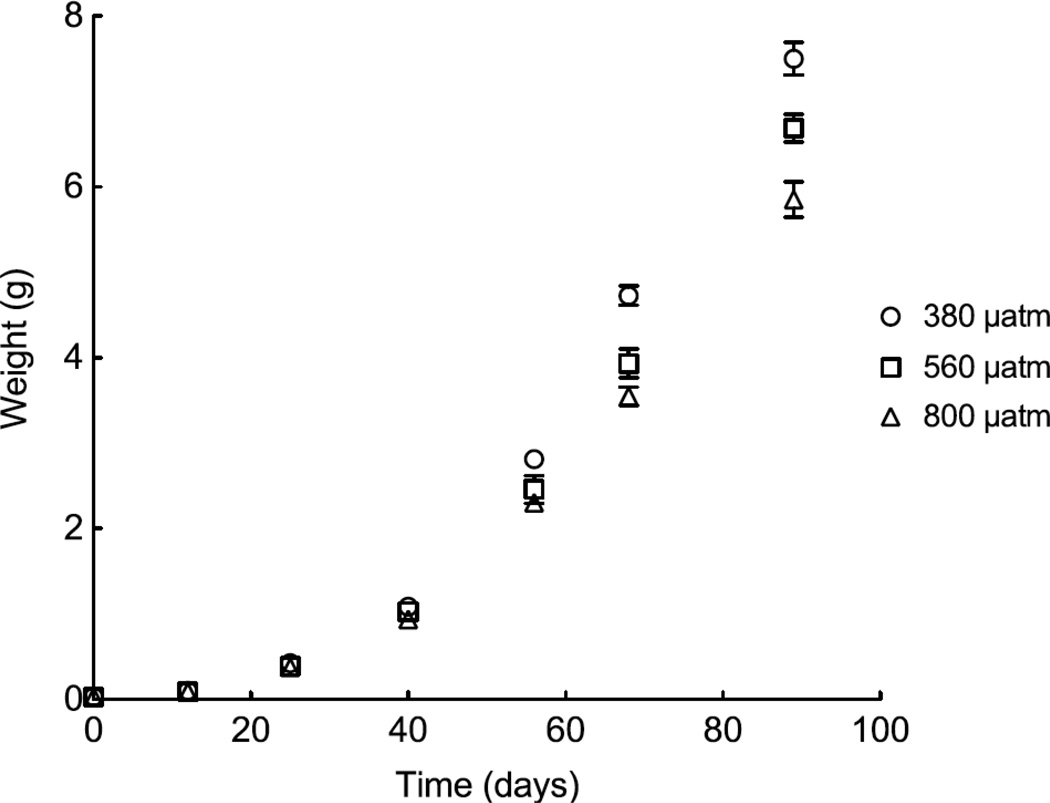
Mean initial urchin weight did not differ significantly between treatments (F2,9=0.761, P>0.05). Initial weights (mg) by treatment were as follows (mean ± SD): 31.17 ± 4.15 (380 µatm); 28.62 ± 3.67 (560 µatm); 31.48 ± 2.89 (800 µatm). We observed 100% survival of all sea urchins over the course of the 89-day experiment. Upon termination of the experiment, urchins reared at 380 µatm weighed, on average, 12% more than those reared at 560 µatm and 28% more than those reared at 800 µatm. Final weights (mg) by treatment were as follows (mean ± SD): 7500 ± 380 (380 µatm); 6690 ± 324 (560 µatm); 5851 ± 414 (800 µatm). A repeated measures two-way ANOVA indicated significant effects of both time (F6,54=1957; P<0.001) and pCO2 (F2,54=30.46; P<0.001) on urchin weight. A significant interaction between time and pCO2 was also detected (F12,54=11.55; P<0.001). Post-hoc tests using the Bonferroni correction revealed that no significant differences were detected between treatments during the first 40 days of the experiment. Mean weights began to separate by day 56 (380 µatm > 800 µatm; P<0.05); further separation of treatments was observed by day 68 (380 µatm > 560 µatm, P<0.001 and 380 µatm > 800 µatm, P<0.001), and full separation (i.e., all treatment means significantly different from one another) was observed by day 89 (P<0.001).
3.2 Electron Microscopy
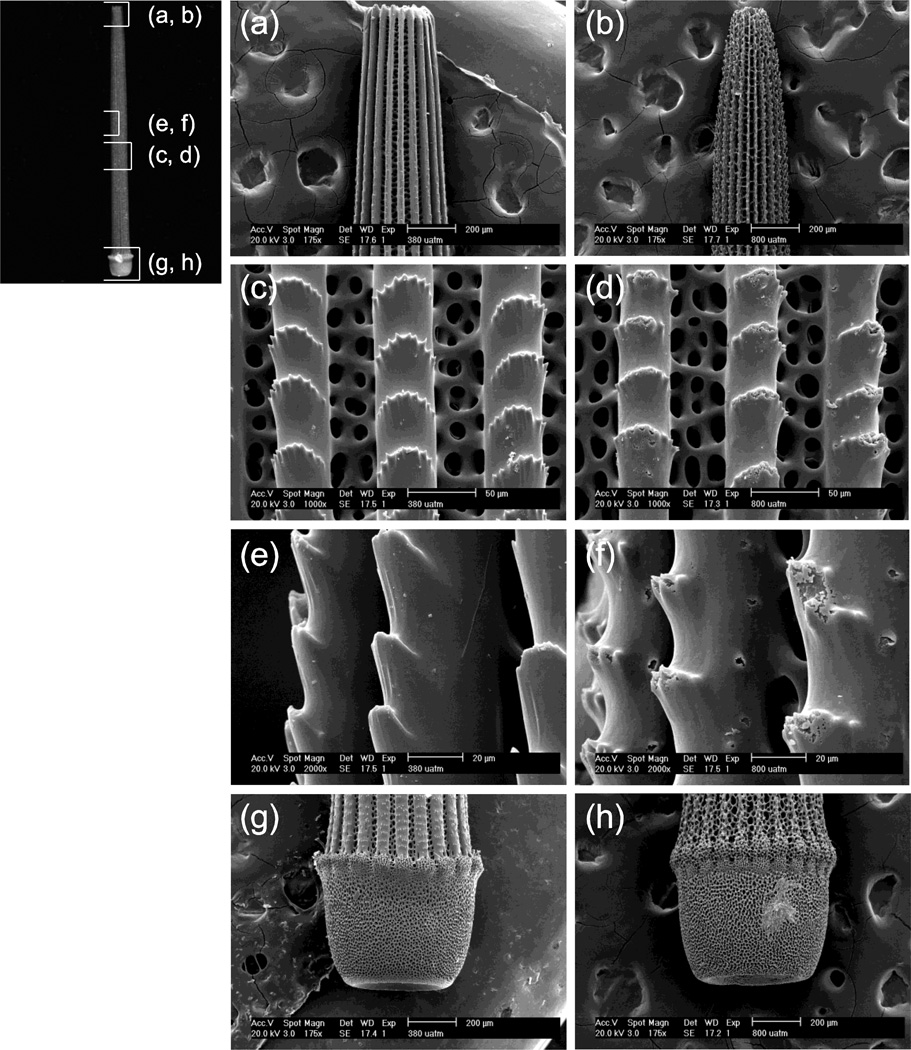
Quantitative reductions in weight with increasing pCO2 are supported by the SEM photographs, showing a loss of structural integrity (malformation or dissolution) in urchin spines grown under 800 µatm pCO2 (Fig. 2). Qualitative differences in skeletal morphology were primarily evident in the spines, in contrast to the tests. Evidence of skeletal malformation and/or dissolution was apparent along the length of the spines of individuals reared at 800 µatm. Individuals grown in this treatment had thinner longitudinal ribs (Fig. 2b). No consistent differences were observed between controls and individuals reared at 560 µatm.
Figure 2.
Scanning electron micrographs of Lytechinus variegatus spines from individuals reared at ambient (380 µatm) and elevated (800 µatm) pCO2 over the course of 89 days. The regions of the spines shown are indicated on the representative spine at left. Scale bars for each view are indicated on SEM micrographs. (a,b) lateral view of spine tip, showing the control (a) in contrast to thinning of longitudinal striations at 800 µatm (b); (c,d) middle of spine (front view) comparing the fenestrated structure and longitudinal striations of the control (c) with evidence of malformation and/or dissolution at 800 µatm (d); (e,f) middle of spine (high magnification, side view), showing normal skeleton (e) and evidence of malformation and/or dissolution at 800 µatm (f); (g,h) base of spine and calcareous annular ridge in the control (g) compared to thinning of longitudinal striations at 800 µatm (h).
4. Discussion
This study demonstrates that juvenile growth, as determined by increase in wet weight, and skeletal integrity of L. variegatus are negatively affected by exposure to elevated pCO2. Previous studies indicate that numerous physiological and biological processes of several sea urchin species are vulnerable to rising CO2 levels, including: fertilization (Havenhand et al., 2008; Kurihara et al., 2004; Kurihara and Shirayama, 2004; Reuter et al., 2010; but see Byrne et al., 2010a, 2010b; Martin et al., 2011); larval development (Kurihara et al., 2004; Kurihara and Shirayama, 2004; Kurihara, 2008; Clark et al., 2009; Sheppard Brennand et al., 2010; O’Donnell et al., 2010; Martin et al. 2011); gene expression in larvae (Martin et al., 2011; O’Donnell et al., 2010; Stumpp et al. 2011a, 2011b; Todgham and Hofmann, 2009); physiology (Miles et al., 2007); and juvenile/adult growth (Ries et al., 2009; Shirayama and Thornton, 2005). Given the range of life history stages and physiological processes that may be negatively impacted by ocean acidification (OA), carryover effects, and/or compounding effects on successive life history stages are likely.
In our study, we observed 100% survival and positive net growth (albeit it slower at elevated pCO2) of all individuals over the course of the 89-day experiment. Significant differences in weight between treatments were not observed during the first 40 days of the experiment. Growth rates between treatments began to separate at 56 days (week 8), and full separation (i.e., all treatment means significantly different from one another) was observed at 89 days (week 13). The lack of significant differences during the first 7 weeks of the experiment is most likely due to the slower growth rates observed early on in the experiment, rendering it difficult to detect significant differences between treatments. Our results are consistent with Shirayama and Thornton (2005), who documented decreased juvenile wet weight of two urchin species, Echinometra mathaei and Hemicentrotus pulcherrimus, following a six-month exposure to 560 µatm pCO2. Similar to our study, treatment weights did not diverge until part-way through the experiment at weeks 12–16, depending on the species.
While the precise physiological mechanism behind reductions in sea urchin growth at elevated pCO2 is not yet known, it has been demonstrated that urchins are poor regulators of internal pH (Johansen and Vadas, 1967; Miles et al., 2007; Spicer, 1995). It has been suggested that some marine invertebrates, such as mussels (Lindinger et al., 1984, Michaelidis et al., 2005) and sea urchins (Miles et al., 2007), use passive shell dissolution to support acid-based regulation at high internal pCO2 levels. Structural dissolution of the magnesian calcite test of the purple-tipped sea urchin, Psammechinus miliaris, results in spikes in the concentrations of HCO3− and Mg2+; such ‘compensation events’ occur episodically during exposure to acidified conditions and may serve to temporarily, yet often insufficiently, buffer internal change in pH (Miles et al., 2007).
Whether the skeletal degradation observed in this experiment was evidence of dissolution used by the organism to partially abate changing external seawater chemistry is unknown. Qualitative differences in skeletal morphology were primarily evident in the spines, in contrast to the test, with dissolution/malformation evident along the longitudinal ribs (sensu D’yakonov, 1969, Fig. 2). The degradation of skeletal components (primarily spines/rods) in urchins at elevated pCO2 is consistent with previous studies conducted on both larval (Kurihara and Shirayama, 2004; Kurihara et al., 2004; Clark et al. 2009) and adult (Ries, 2010) life history stages. Urchin spines form via a transient amorphous calcium carbonate (ACC) precursor (Beniash et al., 1997; Politi et al., 2004) that is 30 times more soluble (200 mg/liter) than the crystalline calcite of mature spines (6.7 mg/liter) (Brecevic and Nielsen, 1989, Politi et al., 2004). The presence of this transient ACC phase may render growing and/or regenerating urchin spines particularly vulnerable to acidified conditions.
Compromised structural integrity of urchin spines, in conjunction with reductions in overall weight, are likely to weaken the supportive and protective skeleton of urchins, rendering them more susceptible to predation and/or mechanical damage (implications for urchin survival). Urchins use their spines for protection and motility, and spines play a critical role in preventing structural damage to tests by absorbing energy and spreading impact over a broader area (Strathmann, 1981). Following injury, spine reparation can indirectly affect other vital biological functions, as energy channeled into spine repair is unavailable for test growth, grazing, reproduction, motility, etc. (Ebert, 1968). Additionally, strength under impact increases with size, such that smaller urchins are less resistant to predation pressure as well as other biological and mechanical damage (Ebert, 1968).
As a present-day analog for near-future ocean acidification scenarios, a recent study investigated community composition along pH gradients near a natural CO2 vent [normal pH (8.1–8.2) to low pH (mean 7.8–7.9, minimum 7.4–7.5)] and found significant reductions in the abundance of sea urchins and other calcifying organisms (e.g. scleractinian corals and coralline algae) approaching the vent (Hall-Spencer et al., 2008). Sea urchins are often the dominant grazer in many shallow, marine communities and often determine community structure (Lawrence, 1975; Lawrence and Sammarco, 1982) by acting as bioturbators and keystone species (Brown, 1997; Karlson, 1999). It is, therefore, essential to better understand how they will be affected by changes occurring in the chemistry of the global oceans. Future experimental work should take care to continue the use of ecologically, biologically, and physiologically relevant pCO2 scenarios and experimental timescales. Additional efforts should be made to evaluate synergistic effects with other stressors (e.g., warming) to determine if, and how, these stressors alter the acidification response. Whenever possible, studies should employ a combined mechanistic approach (e.g. molecular and experimental) to allow the investigator(s) to determine if an organism is physiologically compensating for changing external conditions (e.g., metabolically or through the regulation of gene expression).
Supplementary Material
Figure 1.
Mean weight (±1 SEM) of Lytechinus variegatus individuals over time (89 days) by pCO2 level. Day 0 represents initial weights taken at the start of the experiment.
Highlights.
We evaluate the effect of near-future ocean acidification scenarios on juvenile urchin growth
We show that elevated pCO2 depresses juvenile urchin growth
Scanning electron microscopy demonstrates that elevated pCO2 alters skeletal integrity
Acknowledgments
We are grateful to P. Blackwelder and H. Al-Sayegh of the University of Miami’s Center for Advanced Microscopy (UMCAM) for their assistance with SEM analyses and Dustin Stommes for laboratory assistance. Support for this project was provided by NIH-NCRR (RR010294, awarded to TC), and National Science Foundation Grant (NSF OCE 0547169) and the Korein Foundation (awarded to CL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rebecca Albright, Email: r.albright@aims.gov.au.
Charnelle Bland, Email: charnelleb@aol.com.
Phillip Gillette, Email: pgillette@rsmas.miami.edu.
Joseph E. Serafy, Email: joe.serafy@noaa.gov.
Chris Langdon, Email: clangdon@rsmas.miami.edu.
Thomas R. Capo, Email: tcapo@rsmas.miami.edu.
References
- Allain JY. Contribution a la biologie de Lytechinus variegatus (Lamarck) de la baie de Carthagene (Colombie) (Echinodermata ; Echinoidea) Union Oceanographes France. 1975;7:57–62. [Google Scholar]
- Beddingfield SD, McClintock JB. Demographic characteristics of Lytechinus variegatus (Echinodermata: Echinoidea) from three habitats in a North Florida Bay, Gulf of Mexico. Mar. Ecol. 2000;21:17–40. [Google Scholar]
- Beniash E, Aizenberg J, Addadi L, Weiner S. Amorphous calcium carbonate transforms to calcite during sea urchin larval spicule growth. Proc. R. Soc. Lond. B. 1997;264:461–465. [Google Scholar]
- Brecevic L, Nielsen AE. Solubility of amorphous calcium carbonate. J. Cryst. Growth. 1989;98:504–510. [Google Scholar]
- Brown BE. Disturbances to reefs in recent times. In: Birkeland C, editor. Life and Death of Coral Reefs. New York: Chapman & Hall; 1997. [Google Scholar]
- Byrne M, Ho M, Selvakumaraswamy P, Nguyen H, Dworjanyn S, Davis A. Temperature, but not pH, compromises sea urchin fertilization and early development under near-future climate change scenarios. Proc. R. Soc. Lond. B Biol. Sci. 2009;276:1883–1888. doi: 10.1098/rspb.2008.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne M, Soars N, Selvakumaraswamy P, Dworjanyn SA, Davis AR. Sea urchin fertilization in a warm, acidified and high pCO2 ocean across a range of sperm densities. Mar. Environ. Res. 2010a;69:234–239. doi: 10.1016/j.marenvres.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Byrne M, Soars NA, Ho MA, Wong E, McElroy D, Selvakumaraswamy P, Symon AD, Davis AR. Fertilization in a suite of coastal marine invertebrates from SE Australia is robust to near-future ocean warming and acidification. Mar. Biol. 2010b;157:2061–2069. [Google Scholar]
- Byrne M, Ho M, Wong E, Soars NA, Selvakumaraswamy P, Shepard-Brennand H, Dworjanyn SA, Davis AR. Unshelled abalone and corrupted urchins: development of marine calcifiers in a changing ocean. Proc. R. Soc. Lond. B Biol. Sci. 2011;278:2376–2383. doi: 10.1098/rspb.2010.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D, Lamare M, Barker M. Response of sea urchin pluteus larvae (Enchinodermata: Echinoidea) to reduce seawater pH: a comparison among a tropical, temperate, and a polar species. Mar. Biol. 2009;156:1125–1137. [Google Scholar]
- Dickson AG. Thermodynamics of the dissociation of boric acid in synthetic seawater from 273.15 to 318.15°K. Deep Sea Res. 1990;37:755–766. [Google Scholar]
- Dickson AG, Millero FJ. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res. 1987;34:1733–1743. [Google Scholar]
- Dickson AG, Sabine CL, Christian JR, editors. Guide to best practices for ocean CO2 measurements. North Pacific Marine Science Organization; 2007. PICES Special Publication 3. [Google Scholar]
- Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean acidification: the other CO2 problem. Ann. Rev. Mar. Sci. 2009;1:169–192. doi: 10.1146/annurev.marine.010908.163834. [DOI] [PubMed] [Google Scholar]
- Dupont S, Thorndyke MC. Impact of CO2-driven ocean acidification on invertebrates early life history – what we know, what we need to know and what we can do. Biogeosciences Discussions. 2009;6:3109–3131. [Google Scholar]
- Dupont S, Ortega-Martinez O, Thorndyke M. Impact of near-future ocean acidification on enchinoderms. Ecotoxicology. 2010;19:449–462. doi: 10.1007/s10646-010-0463-6. [DOI] [PubMed] [Google Scholar]
- D’yakonov AM. Fauna of Russia and adjacent countries, Volume I: Echinoidea. Jerusalem: IPST Press; 1969. [Google Scholar]
- Ebert TA. Growth rates of the sea urchin Strongylocentrotus purpuratus related to food availability and spine abrasion. Ecology. 1968;49:1075–1091. [Google Scholar]
- Fabry VJ, Seibel BA, Feely RA, Orr JC. Impacts of ocean acidification on marine fauna and ecosystem processes. International Council for the Exploration of the Sea (ICES) J. Mar. Sci. 2008;65:414–432. [Google Scholar]
- Gattuso JP, Hansson L. Ocean acidification: background and history. In: Gattuso JP, Hansson L, editors. Ocean Acidification. New York: Oxford University Press; 2011. pp. 1–17. [Google Scholar]
- George SB, Lawrence JM, Lawrence AL. Complete larval development of the sea urchin Lytechinus variegatus fed an artificial feed. Aquaculture. 2004;242:217–228. [Google Scholar]
- Greenway M. Trophic relationships of macro fauna within a Jamaican seagrass meadow and the role of echinoid Lytechinus variegatus (Lamarck) Bull. Mar. Sci. 1995;56:719–736. [Google Scholar]
- Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, Turner SM, Rowley SJ, Tedesco D, Buia MC. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature. 2008;454:96–99. doi: 10.1038/nature07051. [DOI] [PubMed] [Google Scholar]
- Havenhand JN, Buttler FR, Thorndyke MC, Williamson JE. Near-future levels of ocean acidification reduce fertilization success in a sea urchin. Curr. Biol. 2008;18:R651–R652. doi: 10.1016/j.cub.2008.06.015. [DOI] [PubMed] [Google Scholar]
- IPCC. New York: Cambridge University Press; 2007. Climate Change 2007: The Physical Science Basis: Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. [Google Scholar]
- Johansen K, Vadas RL. Oxygen uptake and responses to respiratory stress in sea urchins. Biol. Bull. 1967;132:16–22. doi: 10.2307/1539873. [DOI] [PubMed] [Google Scholar]
- Karlson RH. Dynamics of coral communities. Dordrecht: Boston Kluwer Academic Publishers; 1999. [Google Scholar]
- Kroeker KJ, Kordas RL, Crim RN, Singh GG. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecology Letters. 2010;13:1419–1434. doi: 10.1111/j.1461-0248.2010.01518.x. [DOI] [PubMed] [Google Scholar]
- Kurihara H. Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar. Ecol. Prog. Ser. 2008;373:275–284. [Google Scholar]
- Kurihara H, Shirayama Y. Effects of increased atmospheric CO2 on sea urchin early development. Mar. Ecol. Prog. Ser. 2004;274:161–169. [Google Scholar]
- Kurihara H, Shimode S, Shirayama Y. Sub-Lethal Effects of Elevated Concentration of CO2 on Planktonic Copepods and Sea Urchins. J. Oceanogr. 2004;60:743–750. [Google Scholar]
- Lawrence JM. The relationships between echinoids and marine plants. Oceanogr. Mar. Biol. Annu. Rev. 1975;13:213–286. [Google Scholar]
- Lawrence JM, Sammarco PW. Effects of feeding: Echinoidea. In: Jangoux M, Lawrence JM, editors. Echinoderm nutrition. Rotterdam: AA Balkema; 1982. [Google Scholar]
- Lindinger MI, Lauren DJ, McDonald DG. Acid-base balance in the sea mussel, Mytilus edulis. III. Effects of environmental hypercapnia on intra- and extracellular acid-base balance. Mar. Biol. Lett. 1984;5:371–381. [Google Scholar]
- Martin S, Richier S, Pedrotti ML, Dupont S, Castejon C, Gerakis Y, Kerros ME, Oberhänsli F, Teyssié JL, Jeffree R, Gattuso JP. Early development and molecular plasticity in the Mediterranean sea urchin Paracentrotus lividus exposed to CO2-driven acidification. J. Exp. Biol. 2011;214:1357–1368. doi: 10.1242/jeb.051169. [DOI] [PubMed] [Google Scholar]
- Mehrbach C, Culberson CH, Hawley JE, Pytkowicz RM. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 1973;18:897–907. [Google Scholar]
- Michaelidis B, Ouzounis C, Paleras A, Pörtner HO. Effects of long-term moderate hypercapnia on acid-base balance and growth rate in marine mussels Mytilus galloprovincialis. Mar. Ecol. Prog. Ser. 2005;293:109–118. [Google Scholar]
- Miles H, Widdicombe S, Spicer JI, Hall-Spencer J. Effects of anthropogenic seawater acidification on acid-base balance in the sea urchin Psammechinus miliaris. Mar. Pollut. Bull. 2007;54:89–96. doi: 10.1016/j.marpolbul.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Moore HB, Jutare T, Bauer JC, Jones JA. The biology of Lytechinus variegatus. Bull. Mar. Sci. 1963;13:25–53. [Google Scholar]
- Mortensen T. Orthopsidae, glyphocyphidae, temnopleuridae and toxopneustidae. Copenhagen: CA Reitzel Publisher; 1943. A monograph of the Echinoidea: III.2 Camarodonta. I. [Google Scholar]
- O'Donnell MJ, Todgham AE, Sewell MA, Hammond LM, Ruggiero K, Fangue NA, Zippay ML, Hofmann GE. Ocean acidification alters skeletogenisis and gene expression in larval sea urchins. Mar. Ecol. Prog. Ser. 2010;398:157–171. [Google Scholar]
- Politi Y, Arad T, Klein E, Weiner S, Addadi L. Sea urchin spine calcite forms via a transient amorphous calcium carbonate phase. Science. 2004;306:1161–1164. doi: 10.1126/science.1102289. [DOI] [PubMed] [Google Scholar]
- Reuter KE, Lotterhos KE, Crim RN, Thompson CA, Harley CDG. Elevated pCO2 increases sperm limitation and risk of polyspermy in the red sea urchin Strongylocentrotus franciscanus. Global Change Biol. 2010 [Google Scholar]
- Ries JB. Shell-shocked: marine creatures exhibit varied responses to ocean acidification. Earth. 2010:46–53. [Google Scholar]
- Ries JB, Cohen AL, McCorkle DC. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology. 2009;37:1131–1134. [Google Scholar]
- Sabine CL, Feely RA, Wanninkhof R, Takahashi T, Khatiwala S, Park G. Global oceans [The Global Ocean Carbon Cycle] in State of the Climate in 2010. Bull. Am. Meteorol. Soc. 2011;92(6):S100–S105. [Google Scholar]
- Sheppard Brennand HS, Soars N, Dworjanyn SA, Davis AR, Byrne M. Impact of ocean warming and ocean acidification on larval development and calcification in the sea urchin Tripneustes gratilla. PLoS ONE. 2010;5:e11372. doi: 10.1371/journal.pone.0011372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Thornton H. Effect of increased atmospheric CO2 on shallow water marine benthos. J. Geol. Res. 2005;110:108–115. [Google Scholar]
- Spicer JI. Oxygen and acid-base status of the sea-urchin Psammechinus miliaris during environmental. Mar. Biol. 1995;124:71–76. [Google Scholar]
- Strathmann RR. The role of spines in preventing structural damage to echinoid tests. Paleobiology. 1981;7:400–406. [Google Scholar]
- Stumpp M, Wren J, Melzner F, Thorndyke MC, Dupont ST. CO2 induced seawater acidification impacts sea urchin larval development I: Elevated metabolic rates decrease scope for growth and induce developmental delay. Comparative Biochem. and Physiology, Part A. 2011a;160:331–340. doi: 10.1016/j.cbpa.2011.06.022. [DOI] [PubMed] [Google Scholar]
- Stumpp M, Wren J, Melzner F, Thorndyke MC, Dupont ST. CO2 induced seawater acidification impacts sea urchin larval development II: Gene expression patterns in pluteus larvae. Comparative Biochem. and Physiology, Part A. 2011b;160:320–330. doi: 10.1016/j.cbpa.2011.06.023. [DOI] [PubMed] [Google Scholar]
- Todgham AE, Hofmann G. Transcriptomic response of sea urchin larvae Strongylocentrotus purpuratus to CO2-driven seawater acidification. J. Exp. Biol. 2009;212:2579–2594. doi: 10.1242/jeb.032540. [DOI] [PubMed] [Google Scholar]
- Watts SA, McClintock JB, Lawrence JM. The ecology of Lytechinus variegates. In: Lawrence JM, editor. Edible Sea Urchins: Biology and Ecology. Amsterdam: Elsevier Science Press; 2001. [Google Scholar]
- Yu PC, Matson PG, Martz TR, Hofmann GE. The ocean acidification seascape and its relationship to the performance of calcifying marine invertebrates: Laboratory experiments on the development of urchin larvae framed by environmentally-relevant pCO2/pH. J. Exp. Mar. Bio. Ecol. 2011;400:288–295. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.