Abstract
Pathology underlying behavioral variant frontotemporal dementia (bvFTD) is heterogeneous, with the most common pathologies being Pick’s disease (PiD), corticobasal degeneration (CBD), and FTLD-TDP type 1. Clinical features are unhelpful in differentiating these pathologies. We aimed to determine whether imaging atrophy patterns differ across these pathologies in bvFTD subjects. We identified 15 bvFTD subjects that had volumetric MRI during life and autopsy: five with PiD, five CBD and five FTLD-TDP type 1. Voxel-based morphometry was used to assess atrophy patterns in each bvFTD group compared to 20 age and gender-matched controls. All three pathological groups showed grey matter loss in frontal lobes, although specific patterns of atrophy differed across groups: PiD showed widespread loss in frontal lobes with additional involvement of anterior temporal lobes; CBD showed subtle patterns of loss involving posterior lateral and medial superior frontal lobe; FTLD-TDP type 1 showed widespread loss in frontal, temporal and parietal lobes. Greater parietal loss was observed in FTLD-TDP type 1 compared to both other groups, and greater anterior temporal and medial frontal loss was observed in PiD compared to CBD. Imaging patterns of atrophy in bvFTD vary according to pathological diagnosis and may therefore be helpful in predicting these pathologies in bvFTD.
Keywords: Frontotemporal dementia, behavioral variant, Pick’s disease, corticobasal degeneration, TDP-43, atrophy, voxel-based morphometry, MRI
INTRODUCTION
Behavioral variant frontotemporal dementia (bvFTD) is a progressive neurodegenerative syndrome characterized by change in personality and behavioral abnormalities (Neary D et al., 1998). The majority of bvFTD subjects have frontotemporal lobar degeneration (FTLD) pathology (Josephs KA, 2008; McKhann GM et al., 2001), with approximately half showing deposition of the microtubule associated protein tau (FTLD-tau) and half showing deposition of the TAR DNA binding protein of 43KD (FTLD-TDP) (Josephs KA et al., 2006; Snowden J et al., 2007). Subjects with bvFTD can also show deposition of the fused in sarcoma protein (FTLD-FUS) (Cairns NJ et al., 2004; Josephs KA et al., 2003; Urwin H et al., 2010), but it is rare. The FTLD-tau group can be sub-classified by pathological diagnosis into Pick’s disease (PiD) (Dickson DW, 2001), corticobasal degeneration (CBD) (Dickson DW et al., 2002), progressive supranuclear palsy (PSP) (Hauw JJ et al., 1994), and other rare entities (Bigio EH et al., 2001), while FTLD-TDP can be further sub-classified into FTLD-TDP types 1, 2, 3 and 4 depending on the morphology and distribution of the TDP-43 immunoreactive inclusions (Cairns NJ et al., 2007; Mackenzie IR et al., 2006; Sampathu DM et al., 2006). The most common specific pathologies that underlie sporadic bvFTD are PiD, CBD and FTLD-TDP type 1 (Josephs KA et al., 2006; Josephs KA et al., 2009; Snowden J et al., 2007). It is difficult to predict these pathologies during life based on clinical features alone, yet with the advent of treatments that will likely target these pathologies there is increasing need for biomarkers that can help predict pathology in bvFTD.
Subjects with bvFTD typically show progressive atrophy of the frontal and temporal lobes, although different combinations of frontal and temporal lobe atrophy results in a heterogeneous pattern of atrophy (Josephs KA, Jr. et al., 2010; Whitwell JL et al., 2009b). While patterns of atrophy do not help differentiate bvFTD subjects with FTLD-tau and FTLD-TDP as a group (Whitwell JL et al., 2009a), we have previously demonstrated that atrophy patterns differ across different FTLD-tau (Josephs KA et al., 2008) and across different FTLD-TDP pathologies (Whitwell JL et al., 2010b), suggesting that atrophy patterns of the specific pathologies may be useful biomarkers of molecular pathology. The aim of this study was therefore to assess whether patterns of atrophy differ in bvFTD subjects with the most common pathologies of PiD, CBD and FTLD-TDP type 1, and therefore whether imaging could be a useful biomarker of molecular pathology in bvFTD.
MATERIALS & METHODS
Subjects
The Mayo Clinic clinicopathological database of neurodegenerative diseases was queried to identify all subjects with a pathological diagnosis of FTLD (n=224). Of these 224 subjects we selected only those that had an antemortem clinical diagnosis of bvFTD according to research criteria (Neary D et al., 1998), a pathological diagnosis of PiD, CBD or FTLD-TDP type 1, and had completed an antemortem volumetric head MRI scan (n=24). From these 24 subjects we excluded one CBD subject that had coexisting Alzheimer’s disease since the presence of Alzheimer’s disease could influence the pattern of atrophy. In addition, we excluded all FTLD-TDP type 1 subjects with a progranulin gene mutation (n=6) or unknown progranulin status (n=2) because progranulin mutations alter patterns of atrophy in FTLD-TDP type 1 (Whitwell JL et al., 2010b). Furthermore, predicting pathology in subjects with a progranulin gene mutation is not a problem since the presence of a progranulin gene mutation guarantees FTLD-TDP type 1 pathology. All remaining subjects (n=15) were included in the study. This included five subjects with PiD, five subjects with CBD and five subjects with FTLD-TDP type 1 that had all screened negative for progranulin gene mutations. Clinical features for all 15 subjects were abstracted blinded to pathological diagnosis at initial presentation by a behavioral neurologist (KAJ) as previously described (Hu WT et al., 2007).
The 15 bvFTD subjects were frequency matched by age and gender to 20 healthy control subjects that had not yet come to post-mortem. Controls were recruited into the Alzheimer’s Disease Research Center (ADRC), performed within normal limits on standardized neurological and neuropsychological testing, including the Mini Mental State Examination (Folstein MF et al., 1975), and were selected from the ADRC database based purely on age and gender. Demographic features are shown in Table 1.
Table 1.
Demographics of bvFTD subjects with PiD, CBD and FTLD-TDP type 1 pathology, and controls
| Controls | PiD | CBD | FTLD-TDP type 1 | P value | |
|---|---|---|---|---|---|
| N | 20 | 5 | 5 | 5 | NA |
| % female | 50% | 40% | 40% | 80% | 0.51 |
| Education, years | 15 (10–20) | 17 (13–20) | 16 (12–20) | 16 (8–18) | 0.54 |
| Age at onset, years | NA | 58 (56–64) | 61 (40–68) | 64 (49–71) | 0.48 |
| Age at scan, years | 63 (50–70) | 64 (56–75) | 65 (42–69) | 68 (52–76) | 0.49 |
| Age at death, years | NA | 69 (62–83) | 67 (47–73) | 72 (52–83) | 0.28 |
| Time onset-scan, years | NA | 5.8 (0–7) | 3.4 (1–4) | 3.5 (1–5) | 0.58 |
| Disease duration, years | NA | 9.0 (6–14) | 5.0 (4–7) | 7.8 (3–12) | 0.05 |
| MMSE (/30) | 30 (27–30) | 24 (18–27) | 24 (21–28) | 21 (20–29) | 0.0001* |
Data shown as median (range); disease duration = time from onset to death; MMSE = Mini Mental State Examination; NA = Not available
No difference was observed across the PiD, CBD, and FTLD-TDP type 1 groups (p=0.79)
Pathological Diagnoses
All 15 subjects had undergone standardized neuropathological evaluations, by a experienced neuropathologist (DWD or JEP), as recommended in the diagnostic protocol for Alzheimer’s disease (Mirra SS et al., 1991). PiD was diagnosed based on the presence of argyrophilic and tau-positive rounded Pick bodies (Dickson DW, 2001); CBD by the presence of tau-positive cortical and striatal neuronal and glial lesions, in particular astrocytic plaques and thread-like lesions in grey and white matter, as well as focal cortical and substantia nigra neuronal loss (Dickson DW et al., 2002); and FTLD-TDP type 1 on the presence of TDP-43 immunoreactive neuronal cytoplasmic inclusions and dystrophic neuritis, as well as the presence of intranuclear inclusions, in frontotemporal neocortex (Mackenzie IR et al., 2006).
Voxel based morphometry
Patterns of cerebral atrophy were assessed using the automated and unbiased technique of VBM implemented using SPM5 (http://www.fil.ion.ucl.ac.uk/spm) (Ashburner J and KJ Friston, 2000). All subjects in this study had their images normalized to a customized template and segmented into grey matter, white matter and CSF using customized tissue probability maps and the unified segmentation routine (Ashburner J and KJ Friston, 2005) followed by the HMRF clean-up step. The customized probability maps were created from 116 subjects (35 controls and 81 subjects with FTLD-tau or FTLD-TDP). Grey matter images were modulated and smoothed at 8 mm full width at half maximum. A full factorial statistical model was used including the controls, PiD, CBD and FTLD-TDP type 1 subjects. Each bvFTD pathological group was compared to the control group, and compared to each other. Results were assessed after correction for multiple comparisons using family wise error (FWE) at p<0.05, and uncorrected for multiple comparisons at p<0.001.
Statistical Analyses
Statistical analyses were performed using JMP computer software (JMP Software version 8.0.0; SAS Institute Inc, Cary, NC) with statistical significance set at p< 0.05. Non-parametric Kruskal-Wallis testing was used to assess differences in continuous variables across groups. χ2 test was used to compare categorical variables across groups.
RESULTS
No significant differences were observed across the PiD, CBD and FTLD-TDP type 1 groups in any demographic feature, although there was a trend for a difference in disease duration (Table 1). All three groups did however perform worse on the MMSE than controls. All subjects showed behavioral changes, with the majority also showing personality changes (Table 2). Neglect of personal hygiene (grooming, bathing and regularly changing ones clothing, e.t.c.) was only observed in the PiD subjects, whereas vertical supranuclear palsy was only observed in the CBD subjects. Parkinsonism was also most common in CBD.
Table 2.
Clinical features observed at initial presentation in bvFTD subjects with PiD, CBD and FTLD-TDP type 1 pathology
| PiD | CBD | FTLD-TDP type 1 | |
|---|---|---|---|
| Personality change | 100 | 80 | 80 |
| Behavioral change | 100 | 100 | 100 |
| Disinhibition | 20 | 40 | 20 |
| Apathy | 40 | 40 | 60 |
| Aggression | 20 | 20 | 0 |
| Obsessive compulsive | 60 | 20 | 60 |
| Eating changes | 20 | 40 | 0 |
| Stereotypy | 0 | 0 | 0 |
| Neglect of personal hygiene | 80 | 0 | 0 |
| Executive dysfunction | 80 | 60 | 40 |
| Aphasia | 0 | 20 | 40 |
| Parkinsonism | 20 | 40 | 0 |
| Vertical supranuclear palsy | 0 | 40 | 0 |
Data shown as percentage.
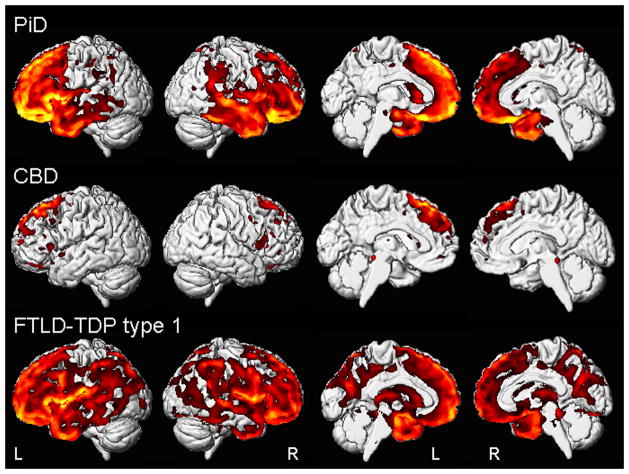
The PiD group showed a bilateral pattern of frontotemporal grey matter loss, with greater involvement of the left hemisphere (Figure 1). Loss of the frontal lobes was widespread, with temporal loss observed in the temporal pole and anterior medial and lateral temporal regions. Grey matter loss was also observed in the anterior insula and caudate nucleus, with a small amount of loss observed in the parietal lobes. After correction for multiple comparisons (FWE p<0.05), grey matter loss was observed predominantly in medial, orbital and superior lateral frontal regions, as well as insula (Table 3).
Figure 1.
Patterns of grey matter loss observed in bvFTD subjects with PiD, CBD or FTLD-TDP type 1 pathology compared to controls. Results are shown uncorrected for multiple comparisons at p<0.001 on medial and lateral three dimensional renderings of the brain.
Table 3.
Peak voxel coordinates from the VBM analyses comparing the PiD, CBD and FTLD-TDP type 1 groups to controls. Results are shown after correction for multiple comparisons using the family wise error at p<0.05.
| Cluster size | Z score | x | y | z | Hem | Region |
|---|---|---|---|---|---|---|
| PiD | ||||||
| 4803 | 6.31 | −33 | 18 | 3 | L | Anterior insula |
| 10703 | 6.10 | −27 | 51 | 19 | L | Middle frontal gyrus |
| −23 | 60 | −4 | L | Orbitofrontal gyrus | ||
| −3 | 39 | 42 | L | Superior medial frontal lobe | ||
| 1214 | 5.79 | 33 | 19 | −14 | R | Anterior insula |
| 223 | 5.77 | 59 | 12 | 6 | R | Inferior frontal gyrus |
| 503 | 5.75 | 4 | 40 | 36 | R | Superior medial frontal lobe |
| 240 | 5.64 | 9 | 64 | 15 | R | Temporal pole |
| 743 | 5.60 | 6 | 33 | −28 | R | Medial orbitofrontal cortex |
| 543 | 5.49 | −27 | 38 | 37 | L | Superior frontal gyrus |
| 1093 | 5.37 | −45 | 29 | −15 | L | Lateral orbitofrontal cortex |
| CBD | ||||||
| 128 | 5.19 | −4 | 19 | 59 | L | Supplemental Motor Area |
| 56 | 5.12 | −3 | 38 | 43 | L | Superior medial frontal lobe |
| FTLD-TDP type 1 | ||||||
| 4121 | 7.12 | 58 | 11 | 6 | R | Inferior frontal gyrus |
| 11424 | 6.44 | −53 | 5 | 1 | L | Inferior frontal gyrus |
| −35 | 21 | −9 | L | Insula | ||
| 726 | 6.34 | −27 | 51 | 19 | L | Superior frontal gyrus |
| 3336 | 6.31 | −10 | 61 | −18 | L | Frontal pole |
| 2693 | 6.20 | −2 | 31 | 52 | L | Superior medial frontal lobe |
| 963 | 5.88 | −47 | 17 | −21 | L | Temporal pole |
| 354 | 5.80 | −11 | 12 | 9 | L | Caudate nucleus |
| 964 | 5.78 | −28 | 12 | 57 | L | Superior frontal gyrus |
| 314 | 5.78 | 9 | 64 | −16 | R | Frontal pole |
| 321 | 5.68 | 51 | −36 | 50 | R | Superior lateral parietal lobe |
| 983 | 5.67 | 6 | 57 | 28 | R | Superior frontal gyrus |
| 246 | 5.44 | −62 | −47 | 26 | L | Lateral parietal lobe |
In contrast, grey matter loss in the CBD patients was relatively restricted to posterior superior lateral and medial frontal lobe, including supplemental motor area (Figure 1). Only minor loss was observed in inferior frontal lobe and orbitofrontal cortex. Only two regions of loss were observed after correction for multiple comparisons, with both located in superior medial frontal lobe (Table 3).
The FTLD-TDP type 1 patients showed the most widespread pattern of loss involving frontal, temporal and parietal lobes (Figure 1). Temporal lobe loss was observed more posteriorly than in PiD, with involvement of the anterior and posterior medial and lateral temporal lobes. Parietal loss was observed both medially, involving predominantly the precuneus, and laterally. Loss was also observed throughout the insula and in the caudate nucleus, thalamus and occipital lobe. After correction for multiple comparisons (FWE p<0.05), grey matter loss was observed predominantly in the inferior frontal gyri and insula, with additional loss in the temporal and parietal lobes (Table 3).
Direct comparisons were also performed between the three disease groups (uncorrected for multiple comparisons at p<0.001). The PiD group showed greater loss in the anterior temporal lobes and anterior medial frontal lobes compared to the CBD group (Figure 2), but did not show greater loss in any regions compared to FTLD-TDP type1. The FTLD-TDP type 1 group showed greater loss in the inferior frontal gyri, medial frontal lobe, insula, temporal lobe and parietal lobe compared to CBD, and greater loss in the medial and lateral parietal lobes and thalamus than PiD (Figure 2). The CBD group did not show greater loss in any regions compared to PiD and FTLD-TDP type 1.
Figure 2.
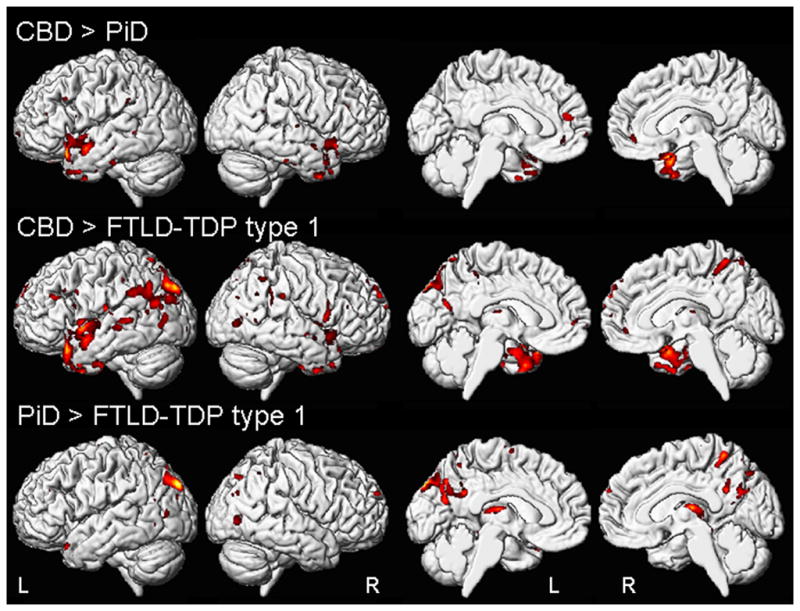
Regions of grey matter loss that were different between the bvFTD subjects with PiD, CBD or FTLD-TDP type 1 pathology. The top panel shows regions of greater loss in PiD compared to CBD; the middle panel shows regions of greater loss in FTLD-TDP type 1 compared to CBD; and the bottom panel shows regions of greater loss in FTLD-TDP type 1 compared to PiD. Results are shown uncorrected for multiple comparisons at p<0.001 on medial and lateral three dimensional renderings of the brain.
DISCUSSION
Our results show different patterns of atrophy in patients meeting criteria for bvFTD (Neary D et al., 1998), but having different molecular pathology. Subjects with PiD, CBD, and FTLD-TDP type 1 all showed involvement of the frontal lobes, consistent with their clinical diagnosis, but showed different overall patterns of atrophy consistent with the view that molecular pathology contributes to pattern of atrophy and that imaging could help predict pathology in bvFTD.
Patterns of grey matter loss observed in subjects with CBD pathology were strikingly different from those observed in subjects with either PiD or FTLD-TDP type 1. Subjects with CBD showed less severe patterns of atrophy compared to the other groups, with loss mainly focused in an area of the posterior medial and lateral superior frontal lobe, including the supplemental motor area. While this region of the frontal lobes was also atrophic in both PiD and FTLD-TDP type 1, both of these pathologies showed more widespread and severe frontal lobe atrophy, particularly involving the prefrontal cortex, as well as the temporal lobes. Atrophy of the prefrontal cortex and temporal lobes could therefore be a useful anatomical signature to suggest the presence of either PiD or FTLD-TDP type 1 pathology in bvFTD subjects. Our results also highlighted differences across PiD and FTLD-TDP type 1 that could help differentiate these two entities further. A more widespread pattern of atrophy with more involvement of the posterior temporal and parietal lobes would suggest FTLD-TDP type 1, whereas loss more restricted to anterior regions of the brain, with frontal and anterior temporal grey matter loss would be more indicative of PiD. In addition, atrophy of the orbitofrontal and medial frontal cortex appeared to be particularly associated with PiD, whereas atrophy of the dorsolateral inferior frontal lobes was associated with FTLD-TDP type 1.
No previous studies have assessed patterns of atrophy across bvFTD subjects with these three pathologies. Our atrophy-molecular pathology correlations observed in this study do however concord with a few studies that have assessed atrophy in PiD, CBD and FTLD-TDP type 1 subjects, although these previous studies included subjects with a wide range of different clinical diagnoses. For example, frontotemporal atrophy has been observed in a group of PiD subjects with a mixture of different language and behavioral syndromes (Whitwell JL et al., 2005); posterior frontal atrophy has been observed in CBD subjects with a mixture of different dementia and extrapyramidal syndromes (Josephs KA et al., 2008); and parietal atrophy has been observed in FTLD-TDP type 1 subjects with a mixture of behavioral, language, pyramidal and extrapyramidal syndromes (Rohrer JD et al., 2010; Whitwell JL et al., 2010b). In addition to studies that include a wide range of different clinical diagnoses, one study assessing subjects with corticobasal syndrome similarly found patterns of frontotemporal and parietal atrophy in subjects with FTLD-TDP type 1, and posterior superior frontal atrophy in subjects with CBD (Whitwell JL et al., 2010a). The fact that similar patterns of atrophy were observed across subjects with bvFTD, a mixture of different clinical diagnoses, as well as corticobasal syndrome, suggests that molecular pathologies may have characteristic patterns of atrophy. Variations of these characteristic patterns of atrophy, for example, asymmetry, may account for the differing clinical syndromes (Whitwell JL et al., 2010a). It is well known that atrophy of the frontal lobes can result in behavioral abnormalities, and studies of bvFTD subjects that do not have pathology have also associated this syndrome with atrophy of the frontal lobes (Boccardi M et al., 2005; Seeley WW et al., 2008). Since the specific frontal regions affected in each pathological group differed, it is likely that the bvFTD syndrome observed in each of these groups resulted from atrophy of differing regions of the frontal lobe which explains why behavioral abnormalities observed in bvFTD are typically heterogeneous (Le Ber I et al., 2006; Rosen HJ et al., 2005; Snowden JS et al., 2001). Indeed, while our clinical data was only retrospective and we observed little difference across the groups, there was some suggestion that there may be differences in specific behaviors. Executive dysfunction has been reported to be associated with tau pathology (Hu WT et al., 2007), and indeed was more frequent in the PiD and CBD groups in our study. Decline in personal hygiene was only observed in patients with PiD at presentation but was found to be more associated with non-tau pathology when assessed over the entire disease course in another study (Hu WT et al., 2007). This suggests that the region of atrophy associated with decline in personal hygiene may be affected early in the disease course in PiD and then later in FTLD-TDP. All subjects in this study were characterized by behavioral abnormalities and met criteria for bvFTD (Neary D et al., 1998), yet these findings suggest that refining the clinical features of bvFTD, by possibly creating bvFTD subtypes, may improve clinical prediction of molecular pathology. Indeed, recent evidence suggests that bvFTD dominated by stereotypic and obsessive compulsive features, i.e. “stereotypic bvFTD”, is associated with one specific type of FTLD-FUS pathology (Snowden JS et al., 2011). These results therefore support the suggestion that bvFTD is anatomically heterogeneous (Whitwell JL et al., 2009b), and further suggests that patterns of atrophy vary across different molecular pathologies. It also supports the notion that the current diagnosis of bvFTD is heterogeneous and could be further refined. The number of subjects in the current study was small and VBM is a group-level technique. Larger prospective studies will be needed to confirm these results, determine the value of these characteristic patterns of atrophy in predicting pathology in individual bvFTD cases, and further examine the utility of splitting bvFTD based on subtle clinical differences.
Acknowledgments
The authors would like to acknowledge Dr. Rosa Rademaker and Matt Baker, Mayo Clinic Jacksonville FL, for performing the genetic analysis. This study was funded by grants NIH R01 DC10367, RO1 AG11378, and P50 AG16574
References
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bigio EH, Lipton AM, Yen SH, et al. Frontal lobe dementia with novel tauopathy: sporadic multiple system tauopathy with dementia. Journal of neuropathology and experimental neurology. 2001;60:328–341. doi: 10.1093/jnen/60.4.328. [DOI] [PubMed] [Google Scholar]
- Boccardi M, Sabattoli F, Laakso MP, et al. Frontotemporal dementia as a neural system disease. Neurobiology of aging. 2005;26:37–44. doi: 10.1016/j.neurobiolaging.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Bigio EH, Mackenzie IR, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns NJ, Grossman M, Arnold SE, et al. Clinical and neuropathologic variation in neuronal intermediate filament inclusion disease. Neurology. 2004;63:1376–1384. doi: 10.1212/01.wnl.0000139809.16817.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW. Neuropathology of Pick’s disease. Neurology. 2001;56:S16–20. doi: 10.1212/wnl.56.suppl_4.s16. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Bergeron C, Chin SS, et al. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61:935–946. doi: 10.1093/jnen/61.11.935. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hauw JJ, Daniel SE, Dickson D, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy) Neurology. 1994;44:2015–2019. doi: 10.1212/wnl.44.11.2015. [DOI] [PubMed] [Google Scholar]
- Hu WT, Mandrekar JN, Parisi JE, et al. Clinical features of pathologic subtypes of behavioral--variant frontotemporal dementia. Arch Neurol. 2007;64:1611–1616. doi: 10.1001/archneur.64.11.1611. [DOI] [PubMed] [Google Scholar]
- Josephs KA. Frontotemporal dementia and related disorders: deciphering the enigma. Annals of neurology. 2008;64:4–14. doi: 10.1002/ana.21426. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Holton JL, Rossor MN, et al. Neurofilament inclusion body disease: a new proteinopathy? Brain. 2003;126:2291–2303. doi: 10.1093/brain/awg231. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Jr, Whitwell JL, Weigand SD, et al. Predicting functional decline in behavioural variant frontotemporal dementia. Brain. 2010;134:432–448. doi: 10.1093/brain/awq348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Petersen RC, Knopman DS, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006;66:41–48. doi: 10.1212/01.wnl.0000191307.69661.c3. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Stroh A, Dugger B, Dickson DW. Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta Neuropathol. 2009;118:349–358. doi: 10.1007/s00401-009-0547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Dickson DW, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiology of aging. 2008;29:280–289. doi: 10.1016/j.neurobiolaging.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Ber I, Guedj E, Gabelle A, et al. Demographic, neurological and behavioural characteristics and brain perfusion SPECT in frontal variant of frontotemporal dementia. Brain. 2006;129:3051–3065. doi: 10.1093/brain/awl288. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Baborie A, Pickering-Brown S, et al. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol (Berl) 2006;112:539–549. doi: 10.1007/s00401-006-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Geser F, Zhou J, et al. TDP-43 subtypes are associated with distinct atrophy patterns in frontotemporal dementia. Neurology. 2010;75:2204–2211. doi: 10.1212/WNL.0b013e318202038c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612–2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampathu DM, Neumann M, Kwong LK, et al. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. The American journal of pathology. 2006;169:1343–1352. doi: 10.2353/ajpath.2006.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, et al. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol. 2008;65:249–255. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden J, Neary D, Mann D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathol. 2007;114:31–38. doi: 10.1007/s00401-007-0236-3. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. Journal of neurology, neurosurgery, and psychiatry. 2001;70:323–332. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Hu Q, Rollinson S, et al. The most common type of FTLD-FUS (aFTLD-U) is associated with a distinct clinical form of frontotemporal dementia but is not related to mutations in the FUS gene. Acta Neuropathol. 2011 doi: 10.1007/s00401-011-0816-0. [DOI] [PubMed] [Google Scholar]
- Urwin H, Josephs KA, Rohrer JD, et al. FUS pathology defines the majority of tau- and TDP-43-negative frontotemporal lobar degeneration. Acta Neuropathol. 2010;120:33–41. doi: 10.1007/s00401-010-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Jr, Boeve BF, et al. Imaging correlates of pathology in corticobasal syndrome. Neurology. 2010a;75:1879–1887. doi: 10.1212/WNL.0b013e3181feb2e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Jr, Parisi JE, et al. Does TDP-43 type confer a distinct pattern of atrophy in frontotemporal lobar degeneration? Neurology. 2010b;75:2212–2220. doi: 10.1212/WNL.0b013e31820203c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Jr, Senjem ML, et al. MRI correlates of protein deposition and disease severity in postmortem frontotemporal lobar degeneration. Neurodegener Dis. 2009a;6:106–117. doi: 10.1159/000209507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Josephs KA, Rossor MN, et al. Magnetic resonance imaging signatures of tissue pathology in frontotemporal dementia. Arch Neurol. 2005;62:1402–1408. doi: 10.1001/archneur.62.9.1402. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, et al. Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: a cluster analysis study. Brain. 2009b;132:2932–2946. doi: 10.1093/brain/awp232. [DOI] [PMC free article] [PubMed] [Google Scholar]