Abstract
Animals can switch their behavioral priorities from ingestive to sex behaviors to optimize reproductive success in environments where energy fluctuates. We hypothesized that energy availability differentially affects the appetitive (motivation), consummatory (performance), and learned (rewarding) components of behavior. In Experiment 1, appetitive and consummatory aspects of sex behavior were dissociated in the majority of female Syrian hamsters restricted to 75% of their ad libitum food intake for 11 days. Food restriction significantly inhibited vaginal scent marking, decreased the preference for spending time with male hamsters vs. spending time with food, and increased food hoarding with no significant effect on consummatory behaviors such as the incidence of lordosis or food intake. In Experiments 2 and 3, we attempted to use a similar level of food restriction to dissociate sexual appetite from sexual reward. In hamsters, formation of a conditioned place preference (CPP) for copulatory reward is reflected in increased nucleus accumbens (NAc) neural activation, measured as immunocytochemical staining for c-Fos, the protein product of the immediate-early gene, c-fos. In Experiment 2, neural activation increased 1 h after copulation in the NAc core and shell, and did not differ significantly between 10-day food-restricted and ad libitum-fed females in any brain area examined. In Experiment 3, females were either food-restricted or fed ad libitum over 8-30 days of conditioning with copulatory stimuli. Food-restricted females showed significantly fewer appetitive behaviors, but no difference in formation of a CPP compared to females fed ad libitum. Together these data are consistent with the idea that mild levels of food restriction that inhibit appetitive behaviors fail to attenuate consummatory behaviors and the rewarding consequences of copulation. Thus, appetitive sex behaviors are, at least partially, neuroanatomically and behaviorally distinct from both consummatory behaviors and copulatory reward.
Keywords: food restriction, mating reward, hoarding, vaginal marking, lordosis, conditioned place preference
INTRODUCTION
Hormonal control of ingestive behavior is relevant to clinical problems such as obesity, metabolic syndrome, infertility, and addiction. An understanding of the neuroendocrine mechanisms underlying ingestion requires attention to the functional significance of hormone action for both survival and reproductive success [1-3]. For example, many investigators assume that the neuroendocrine mechanisms underlying ingestive behavior function to maintain body weight within narrow limits. Mounting evidence, however, suggests that many of these mechanisms function to prioritize conflicting behaviors in order to optimize reproductive success in environments where energy fluctuates [1-3]. Our experimental approach has been to examine the effects of food availability on the choice between food and sexual stimuli made by subjects housed and tested in semi-natural environments. The advantage of this approach lies in the fact that many neural mechanisms exist, not only because they maintain a particular body size, but also (or primarily) because they conferred reproductive success during evolutionary history.
The present experiments are focused on the neural mechanisms that mediate female motivation and reward underlying the choice between food and a mating partner in Syrian hamsters (Mesocricetus auratus). Hamsters exhibit regular, 4-day estrous cycles and easily-quantifiable sex and ingestive behaviors, and can be housed in an apparatus that mimics important aspects of the natural habitat. Syrian hamsters in the wild live in isolation in underground burrows, from which they emerge for 80 to 90 min per day, primarily for foraging and hoarding [4]. Mating has been observed in the wild, and was reported to occur just outside the female’s burrow [4]. Thus, in the present experiments, estrous-cycling females were housed in home cages, but were free to forage for 90 min per day via vertical tunnels leading in one direction to food and in another direction to an adult male.
Motivated behaviors such as eating and copulating have at least three components: motivation, performance and reward. Consummatory behaviors might, in some cases, reflect both motivation and performance. Some appetitive sex behaviors, however, are at least partially distinct from consummatory sex behaviors. Appetitive sex behaviors occur separated in time from copulation, reflect sexual motivation but not necessarily the ability to perform the sex act, serve to bring animals in close contact with opposite-sex conspecifics, and are assumed to induce arousal in the potential mating partner [5-11]. For example, Syrian hamsters respond to male odors by making vaginal scent marks and by spending more time in closer proximity to males on day 3 of the estrous cycle, one full day before ovulation and the consummatory act of mating [10, 12]. Syrian hamster appetitive ingestive behaviors include food hoarding, the latency to eat food, and the preference for food over sex. In contrast to appetitive behaviors, consummatory behaviors include food intake (the amount of food eaten within a particular time period) and the percentage of females that show the lordosis reflex on day 4 of the estrous cycle in an enclosed area in response to an adult, sexually-experienced male. Sex and other behaviors are said to have rewarding consequences when performance of these behaviors increases their future occurrence in the contexts in which they first occurred. Sexual reward in hamsters has been measured using the conditioned place preference (CPP) method, in which an estrous female develops a preference for a chamber previously associated with mating [13].
The extent to which appetitive, consummatory, and reward mechanisms are distinct is unknown. Experiment 1 was designed to determine whether there was a level of food restriction that could inhibit appetitive sex and ingestive behaviors without effects on consummatory behaviors. Experiments 2 and 3 were designed to determine whether the level of food restriction that inhibited appetitive sex behaviors also inhibited the rewarding consequences of copulation both in terms of formation of a CPP and neural activation of brain areas thought to mediate reward.
MATERIALS AND METHODS
Animals and housing
The subjects were adult (at least 3 months of age) female Syrian hamsters obtained from the breeding colony at Lehigh University (original stock from Charles River Laboratories) or directly from Charles River Laboratories (Stoneridge, NY). Hamsters were used at 140.0 ± 10 g body weight in Experiment 1, 148.0 ± 10 g in Experiment 2, and 148.0 ± 16 g in Experiment 3. Hamsters were housed individually in plastic laboratory cages (either 50 × 40 × 10 cm or 31 × 19 × 18 cm) with wire lids. The colony environment was maintained at 22°C with a 14:10 light-dark cycle (lights on at 2200 h) and food and water were provided ad libitum until the point at which half of the hamsters were food-restricted to 75% of the 4-day average of their ad libitum daily food intake. All procedures followed the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals, the United States Department of Agriculture, and a protocol approved by the University of Minnesota and Lehigh University Institutional Animal Care and Use Committees.
Estrous Cyclicity
Ovulation and estrous behavior occur on the last day of the 4-day estrous cycle in Syrian hamsters. Increased circulating concentrations of estradiol on days 3 and 4 culminates in the luteinizing hormone surge and ovulation. Estradiol and progesterone are critical for estrous behavior (the stationary, arched-back lordosis posture necessary for the male to gain intromission). Estrous cycle day 3 is characterized by an increase in circulating estradiol, a decrease in aggression, an increased tolerance for males, and increased vaginal scent marking, but no lordosis.
Only females that showed two consecutive 4-day estrous cycles were chosen for each experiment. Estrous cyclicity was determined by brief introduction of an adult male stimulus hamster into the female’s cage. Daily tests for estrous behavior (lordosis) were carried out each day within 30 min of the onset of the dark phase of the light-dark cycle until 4-day estrous cycles were established. Females were considered to be in heat (day 4 of the estrous cycle) if they showed lordosis within 5 min. Those that did not display lordosis within this time period were typically aggressive toward the male and the test was terminated; testing was resumed at the same time the next day. Tests for other behaviors (vaginal scent marking, male preference, food hoarding, etc.) occurred throughout the 20 days of food-restriction or ad libitum-feeding, even if the females failed to show lordosis on the expected day of estrous.
Food Restriction
Individually-housed females were given a pre-weighed amount of food (approximately 20 g) that was measured to the nearest 0.01 g each day at 1200 hours for 4 days. The baseline daily ad libitum food intake was calculated as the difference in the weight of food found in the cage each subsequent day. Mean daily ad libitum food intake (the sum of 4 daily food intakes divided by 4) was determined for each female. Food-restricted females received 75% of their ad libitum food intake divided into two rations given 12 h apart. Females were tested 12 h after their last food ration had been given, and no food was found in the cage at the time of testing.
PROCEDURES
Experiment 1. Time course of effects of food restriction on appetitive and consummatory behaviors
To test the idea that deficits in energy availability set behavioral priorities at the level of motivation rather than performance, we examined the time course of changes in sex and ingestive behaviors in estrous-cycling female Syrian hamsters every 4 days for 20 days of mild (25%) food restriction and after return to ad libitum food intake. This allowed us to pinpoint the duration of food restriction that significantly affected motivation but not performance, and to look for correlated changes in the mesolimbic dopamine system and other brain areas in Experiment 2, and changes in the formation of a CPP to copulation in Experiment 3.
The preference apparatus
In order to examine the motivation to engage in sex or ingestive behaviors, 16 hamsters (weights and ages described in Animals and Housing) were introduced to a preference apparatus described previously [1]. Each apparatus contained a home cage connected via plastic tubes to a food source box and another box that contained an adult male. The home cage was made from an opaque, Nalgene cage (31 × 19 × 18 cm) lined with fine wood shavings with a door that was kept closed when the animal was not being trained or tested. When the subject females were being trained or tested, the door to the home cage was opened, which allowed the females to ascend a vertical tube (134 cm in length) leading to 2 horizontal tubes (40-50 cm in length), connected in a T-configuration. One horizontal tube was connected to a clear, plastic box (the food box), containing a weighed amount (150 ± 5 g) of hoardable pellets. Hoardable pellets were made from standard laboratory chow (Harlan Rodent Chow 2016) that was broken into 2 cm pieces, a size that hamsters can easily fit into their cheek pouches and carry through the plastic tubes. A second horizontal tube was connected to a clear, Plexiglas cage (27 × 20 × 15 cm), containing an adult, sexually-experienced male (the male box). During assessment of appetitive behaviors, the male was separated from the female by a wire barrier that allowed olfactory, visual and tactile stimuli from the male, but prevented the male from mating with the female. The male box did not contain food or water.
Subject females were acclimated to the home cage that contained fine wood chip bedding, food and water for at least 1 week prior to testing. This reduced any tendencies to sleep or move bedding to other chambers.
Training to the preference apparatus
Once subjects were acclimated to the home cage, they were trained to expect food in the food box and a male in the male box for four consecutive days. Subjects were trained to each box separately, by blocking access to opposing tubes for 90 min. On days 1 and 2 of the estrous cycle, females were trained to the food box and allowed to keep the food they hoarded in their home cages. On days 3 and 4, females received additional training with the male box. On the eve of day 3, females were allowed to enter into the male box that contained an unrestrained, gonadally-intact male (females cannot become impregnated on this day). Females were allowed to interact with the males for 5 min or until a fight broke out. On the eve of day 4, the day of estrous, females were allowed to enter the male box, to interact with the male, and receive anogenital licks and ectopic mounts without intromissions or ejaculations for 5 min. During training, the experimenter prevented male hamsters’ intromission so that no females would become pregnant.
Testing in the preference apparatus
Testing began at the onset of the dark phase of the light-dark cycle (1200 h) and was conducted under dim, red illumination on days 3 and 4 of the estrous cycle. To start the test, the door to the home cage was opened and females were allowed access to both the food and male boxes for a total of 90 min. The first 15 min of the 90 min test were conducted while the experimenter recorded all behaviors every 5 sec. In the remaining 75 min of the test, the females were allowed to move freely about the apparatus, interact with males, hoard or eat food, or remain in the home cage. During this period the experiment did not record the subjects’ behaviors. Behaviors that were recorded during the 15 min on day 3 included vaginal marking, flank marking, hoarding, and eating. At the end of the full 90 min, all food was weighed in the food box and the home cage. No food was found in the male box.
On day 4 of the estrous cycle, females received a 10-min test with a gonadally-intact, male hamster restrained behind a wire barrier in the male box. After the female hamster entered the male box, stimulation was provided to her flanks by an experimenter to induce the lordosis reflex (while the male continued to be restrained behind the wire barrier). The latency and duration of the females’ lordosis was recorded.
Previous research showed that food-restricted females that do not show lordosis when the male is restrained will often show lordosis when the male is allowed to mount [1]. Thus, in the present experiment, if the females did not show lordosis during the 10-min period, the wire barrier was removed and the male hamster was allowed to mount the female, and the occurrence or absence of lordosis was recorded. The male was quickly removed if a fight broke out between the two hamsters.
Following the lordosis test with the free male, recording was stopped and the male was placed back behind the wire barrier and the test continued for an additional 80 min while the female still had access to both the food box and the male box. Throughout the total 90 min, the females were free to hoard food, eat food, investigate the male, travel through the tubes, or remain in the home cage. At the end of the full 90 min, all food was weighed in the food box and the home cage.
Food hoarding was measured as the difference between the weight of food in the food box at the start of the 90 min and the weight of the food left in the food box at the end of the 90 min. Food intake was measured as the sum of the food in all compartments (home + food box) at the start of 90 min minus the sum of the food in all compartments at the end of 90 min.
Baseline behaviors were measured on days 3 and 4 of the estrous cycle prior to the start of food restriction. Females were randomly placed into one of two groups that did not differ significantly in body weight. During 20 days of food restriction or ad libitum feeding, all hamsters were tested in the preference apparatus on days 3 and 4 of each subsequent estrous cycle. Thus, they were tested on days 3 and 4, 7 and 8, 11 and 12, 15 and 16, and 19 and 20 of restriction and days 3 and 4, and 7 and 8 after return to ad libitum feeding.
After 16 days of restriction when the majority of food-restricted hamsters stopped showing lordosis, those that failed to show lordosis were returned to ad libitum feeding, whereas the rest were food-restricted for 1 more cycle. After 20 days of restriction, the remaining hamsters were returned to ad libitum feeding whether they showed lordosis or not. After the return to ad libitum feeding, hamsters were re-tested in the preference apparatus on days 3 and 4 for two additional estrous cycles.
Statistical analysis
Data were analyzed using repeated measures analysis of variance (ANOVA) to determine whether there were changes over time, and whether these effects differed according to food-restriction or ad libitum feeding. Repeated measures ANOVA were performed on the amount of food hoarded and eaten during the 90-min period, male preference ((the time with the male minus the time with the food)/total time), and the number of vaginal and flank marks across all time points of restriction followed by planned contrasts when the main effects were significant. The percentages of hamsters displaying lordosis were compared using Fisher’s exact probability test. Differences were considered significant at P < 0.05.
Experiment 2. Mating-induced neural activation in the nucleus accumbens (NAc) in food-restricted and ad libitum-fed females
This experiment was designed to determine whether food restriction could dissociate appetitive behaviors from the neural activation known to occur as a consequence of copulatory experience. In Experiment 1, appetitive sex behaviors were not inhibited by 7 days of food restriction, but were significantly inhibited by 11 days of food restriction at 75% of ad libitum intake (Fig. 1A), with no significant effect on consummatory behavior in the majority (4/6) of females (Fig. 1B). Because Experiment 2 required females in which appetitive, but not consummatory behaviors were inhibited, we used 10 days of food restriction in Experiment 2. We knew 7 days would be unlikely to inhibit appetitive behavior, and that 8 days was sufficient to inhibit appetitive sex behavior in a previous experiment [1], but 12 days might be to long, since there were 2 females in Experiment 1 that failed to show lordosis after 12 days of restriction.
Figure 1.
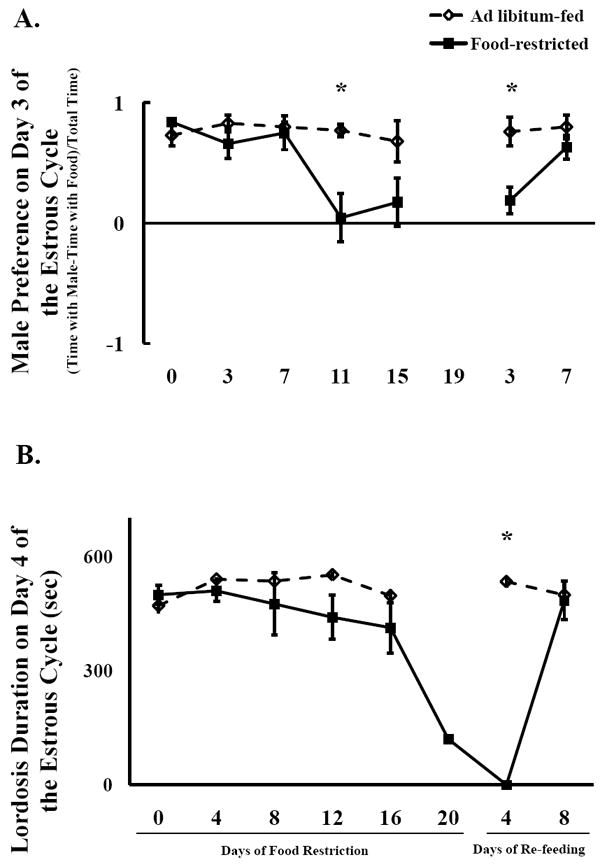
Mean and standard error of the mean for A) Male preference (Time with male – time with food)/Total time on Day 3 of the estrous cycle (top), and B) Lordosis duration on day 4 of the estrous cycle (bottom). Only females that showed lordosis on day 4 of the same estrous cycle are included. Females were fed ad libitum or food-restricted to 75% of their ad libitum intake until they stopped showing lordosis, at which point they were re-fed ad libitum. * = significant difference between food-restricted and ad libitum-fed hamsters at P < 0.05.
The mesolimbic dopamine system, including the nucleus accumbens (NAc), is of interest because this brain area is thought to play a role in appetitive behavior, [14-18], effortful approach to reinforcing stimuli [19], and is activated by experience with addictive drugs [20, 21] and naturally rewarding stimuli including copulatory experience [22-26]. Whether there is increased release of dopamine and neural activation after vaginal scent marking or other appetitive behaviors in hamsters is unknown, and since mild food restriction decreases these behaviors, it would be difficult to determine whether food restriction also inhibits the rewarding aspects of these behaviors. However, there are instances where the rewarding aspects of copulation are inhibited even when the performance of copulation is not inhibited. In Syrian hamsters, for example, copulatory experience increases neural activation and dopamine release in the NAc [26, 27], and antagonists to dopamine receptors block the rewarding aspects of sexual experience without decreasing copulatory behavior (lordosis) [13]. Perhaps food restriction, like a dopamine receptor antagonist, also decreases the rewarding consequences of copulation. In Experiment 2, we compared food-restricted to ad libitum-fed females’ mating-induced neural activation in the NAc, as measured by immunoreactivity to c-Fos, the protein product of the immediate-early gene, c-fos. If brain mechanisms involved in reward, specifically the NAc, share neuroanatomical and functional overlap with mechanisms that control appetitive behavior, it would be expected that food restriction would attenuate mating-induced neural activation in the NAc. However, no attenuation of neural activation would be expected in brain areas more strictly involved in lordosis, such as the ventromedial hypothalamus (VMH) or medial nucleus of the amygdala (MeA). Alternatively, mating-induced neural activation in the NAc might be dissociated from appetitive behaviors by mild food restriction.
c-Fos immunoreactive cells were also counted in brain areas containing various peptides implicated in energetic control of food intake and reproduction, such as the arcuate nucleus (Arc) and paraventricular nucleus of the hypothalamus (PVN), which contain leptin receptors, neuropeptide Y/agouti-related protein, proopiomelanocortin and corticotropin releasing hormone [28-36].
Animal testing
Twenty-four female hamsters were either fed ad libitum or food-restricted for 10 days as described under Food Restriction (weights and ages reported above in Animals and Housing). On the tenth day, half of the females from each group in Experiment 2 (6 food-restricted hamsters; 6 ad libitum-fed hamsters) received a 10 min mating test in which a sexually naïve male stimulus hamster was introduced to each female’s cage and mating behavior was video recorded. All tests were conducted within the first 2 h of the dark portion of the light-dark cycle. The mating behavior recorded included lordosis latency, total lordosis duration, and total numbers of mounts, intromissions, and ejaculations. Hit rate (proportion of mounts that included intromission) was derived, and for this purpose, ejaculations were scored as intromissions.
c-Fos immunocytochemistry
One h following sex testing, females were deeply anesthetized with Sleepaway (Nembutal, Fort Dodge Laboratories, Fort Dodge, IA 0.2 ml/animal, I.P.) and intracardially perfused with 25 mM phosphate-buffered saline (PBS, pH 7.6, 4°C) for 2 min followed by 4% paraformaldehyde in 25 mM PBS (4°C) for 20 min. Brains were post-fixed in 4% paraformaldehyde for 2 h and cryoprotected in 10% sucrose in PBS at 4°C overnight. Coronal 40 μm sections were cut on a freezing microtome at the level of the NAc, and at the level of the MeA/VMH and Arc. Sections were rinsed in wash buffer (25 mM PBS + 0.1% BSA) and incubated in c-Fos primary antibody (Santa Cruz Biotechnologies, Inc, Santa Cruz, CA; cat. # sc 52; 1:3000 in wash buffer + 0.3% Triton X-100) for 24 h at room temperature followed by 24 h at 4°C. Following appropriate buffer washes, the sections were incubated for 45 min each in biotinylated anti-rabbit IgG (1:200 in wash buffer, Elite Vectastain ABC Kit, Vector Laboratories, Burlingame, CA, USA) and avidin–biotin horseradish peroxidase complex (1:50 in wash buffer, Elite Vectastain ABC Kit, Vector Laboratories), separated by appropriate rinses in wash buffer. Sections were then further rinsed in 0.05 M Tris buffer (pH 7.6), and then incubated for 5 min in 0.07% diaminobenzidine (DAB) (Sigma-Aldrich Chemicals, St. Louis, MO) in Tris activated by 0.003% hydrogen peroxide. To intensify staining 0.015% nickel ammonium sulfate was added to the DAB reactions. Finally, the sections were rinsed with Tris buffer and deionized water and mounted on slides to dry overnight. The sections were then cleared and cover-slipped with DPX (Sigma-Aldrich Chemicals).
Slides were analyzed using a Leica DM4000B light microscope coupled to a Leica DFC500 digital camera. Brain regions analyzed included the dorsal and ventral subdivisions of the posterior dorsal MeA, the lateral and medial divisions of the ventromedial hypothalamus (VMH shell and VMH core), the NAc core and shell, the PVN, and the arcuate nucleus of the hypothalamus.
The sampling procedure insured that the counting regions were consistent among animals and that the individual who counted stained cells was blind to the experimental condition. Counting boxes were sized to encompass most of the identified region by positioning boxes on appropriate archived digital cresyl violet stained hamster brain images previously generated in the laboratory. These counting boxes were then digitally layered onto images of immunocytochemically-processed tissue with Adobe Photoshop. Boxed images were then analyzed for number of c-Fos-IR labeled cells by using ImageJ, and were only included in the cell count if completely enclosed within the counting box. Differences among the groups were analyzed using a 2 × 2 (food restriction × test) ANOVA, followed by Student–Newman–Keuls post hoc test if the main effects were significant. Differences were considered statistically significant if P was less than 0.05.
Experiment 3. Effects of energy restriction on formation of a conditioned place preference to mating
This experiment was designed to determine whether food restriction can dissociate appetitive sex behaviors from the formation of a conditioned place preference (CPP), a change in behavior that indicates that a stimulus has been rewarding or reinforcing.
24 ovariectomized (OVX) Syrian hamsters (weights and ages reported above in Animals and Housing) were randomly placed into treatment groups, ad libitum or food-restricted, that did not differ in body weights. Hamsters in both groups were fed 1 daily ration 4 h after the onset of the dark phase of the light-dark cycle. Females in the food-restricted group received 75% of their ad libitum food intake for 8 days until the start of conditioning in the CPP apparatus, and then their body weights were maintained for the rest of the experiment to approximately 10 g below the body weights of the ad libitum-fed controls. After the first conditioning trial, we maintained the food-restricted females’ body weights and level of restriction by alternating days of ad libitum feeding with days of restriction. Ad libitum feeding days occurred after each CPP conditioning trial, whereas restriction days occurred on the 3 days prior to the CPP conditioning trial.
Ovariectomy
Hamsters were deeply anesthetized using 80 mg/kg of sodium pentobarbital (Ovation Pharmaceuticals, Inc., Deerfield, IL) and OVX through bilateral flank incisions closed with suture (muscle incision) and wound clips (skin incision). An analgesic (Metacam, Boehringer Ingelheim Vetmedica, Inc., St. Joseph, MO; 0.06 ml) was given at the time of anesthesia to minimize pain and discomfort after surgery. After at least one week of recovery, hamsters were randomly placed into treatment groups that did not differ in body weight.
Females were primed with an estrous-inducing regimen of estradiol and progesterone prior to each conditioning or testing session. Forty-eight hours before the conditioning or testing in the CPP apparatus, OVX females were given a subcutaneous (S.C.) injection of 10 μg estradiol, and six hours before testing or conditioning a S.C. injection of 500 μg of progesterone. Both steroids were dissolved in canola oil.
The CPP apparatus
The CPP apparatus consisted of a gray chamber and white chamber (each 60 × 45 × 38 cm) that were separated by a clear, neutral chamber (37 × 22 × 38 cm) described previously [24]. Compartments were further differentiated by corn cob bedding (Harlan 7092) in the white chamber and fine wood shavings (same as females’ home cages) in the gray chamber. Sliding partitions made of the same color as the compartments were used to isolate the females during conditioning.
Pre-testing in the preference apparatus
Pre-tests were used to familiarize the animals to the apparatus and to determine whether the females displayed any preference for either of the two compartments. Beginning at the onset of the dark phase of the light-dark cycle, 48 h after estradiol injection, and 6 h after progesterone injection, females were placed in the clear, neutral compartment of the preference apparatus, partitions were removed, and animals were allowed to roam freely in all chambers for 10 min under supervision by an observer. The number of seconds in each compartment were recorded.
All testing was performed under fluorescent illumination within 3 h of the onset of the dark portion of the light-dark cycle. The procedure was repeated 3 times prior to conditioning, and an average of the last 2 pre-tests was used to calculate the initial preference. The apparatus was cleaned with 70% ethanol and fresh bedding was replaced after each animal was tested.
Twenty-four h food intake was measured for 4 days during pre-testing by placing approximately 20 g of food inside the home cage and weighing the food remaining 24 h later.
Conditioning in the preference apparatus
In conditioning sessions, subject females were paired with a stimulus in the compartment that had been less preferred in the pre-test. The mated group was placed in the previously less preferred compartment with an adult, sexually-experienced male hamster. The unmated group was never placed in a compartment with a male, but was placed alone in the previously less preferred compartment. Half the mated and half the unmated females were either fed ad libitum or food restricted as described above. All females received the same hormonal priming prior to the conditioning test and received their conditioning sessions every 4 days.
Each conditioning session had two 10-min phases, a conditioning phase and an isolation phase. In one phase, the females were paired with the stimulus common to all members of the groups (the mated group was placed with the male stimulus, the unmated group was placed in an empty compartment). In the other phase, the females (regardless of group) were placed alone in the compartment not used in the first phase. The two phases were repeated every 4 days alternating the order of conditioning and isolation.
Post-conditioning testing in the preference apparatus
Two post-conditioning tests were performed to determine whether the females had formed a CPP. One occurred after the first conditioning session and the other occurred after the fourth conditioning session.
Four days after the first conditioning session, females were hormonally-primed as described above and given a post-conditioning preference test 48 h after the estradiol injection and 6 h after the P injection. Briefly, females were placed in the clear, neutral chamber and given free access to the apparatus in the absence of a male for 10 min. The amount of time spent in each compartment was recorded by an observer who was blind to prior treatments. After the initial post-test, conditioning continued every 4 days. After a total of 4 conditioning sessions, females were again given a post-test in the absence of a male and the time spent in each compartment of the apparatus recorded.
Appetitive sexual behavior
Four days after the last post-test, hamsters were injected with estradiol 48 h and 24 h prior to being tested for appetitive sexual behavior and aggressive behavior using a sexually-experienced male stimulus hamster. Test subjects were placed into a clear, Plexiglas cage (43 × 20 × 20 cm) containing a male and a 100 ml of a standard olfactory stimulus made from the soiled bedding collected and pooled from multiple individual, sexually-active male hamsters. Subjects were observed for 15 min with behaviors recorded every 5 sec including number of vaginal scent marks, an appetitive sex behavior known to be inhibited by food restriction [1].
Statistical Analysis
Time spent in the conditioned chamber was analyzed by a paired t-test to compare pre-test to post-test times. Change in body weight, food intake, and appetitive behaviors were analyzed using 2-way ANOVA with food availability and mating experience as the main effects. Post hoc tests for significant differences between groups were conducted by Duncan’s Multiple Range method when the main effects were significant. Results were considered significant when P < 0.05.
RESULTS
Experiment 1. Time course of effects of food restriction on appetitive and consummatory behaviors
Sex Behaviors
Male preference on day 3 of the estrous cycle was calculated as (the amount of time females spent with a male – the amount of time spent with food) / the total time in the preference apparatus, and was inhibited by food restriction some time between day 7 and day 11 (P < 0.05) (Figs. 1A and 2A).
Figure 2.
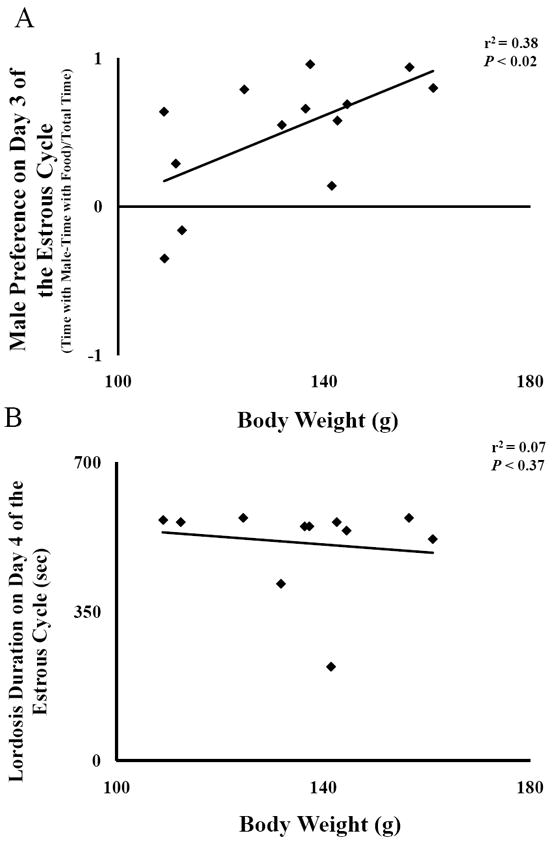
Regression of A) male preference (top), and B) lordosis (bottom) on body weight at the time of behavioral testing. Male preference was calculated as (Time with a male – time with food)/Total time on Day 3 of the estrous cycle.
As food restriction progressed beyond 8 days, some females became anestrous, i.e., they failed to show lordosis on day 4 of the estrous cycle, even in response to a male that was freed from his wire restraint. As shown in Fig. 3B, 6/6, 6/6, 5/6, 4/6, 2/6, 1/6, 0/6, and 4/6 showed lordosis in response to an adult male on days 0, 4, 8, 12, 16, and 20 days after the start of food restriction, and 4 and 8 days after re-feeding. It is known from previous experiments that food-deprived or restricted female Syrian hamsters that fail to show lordosis also show an absence of appetitive behaviors and low circulating estradiol and progesterone [3, 37, 38]. We wanted to know whether appetitive behaviors could be inhibited in females in which the consummatory behavior (lordosis to a free male) was not yet inhibited. It is important to use hamsters in which lordosis was not inhibited by food restriction to determine whether it is possible for food restriction to inhibit appetitive behaviors independent of lordosis and the hypothalamic-pituitary-gonadal system. Thus, in Fig. 1, mean male preference scores on day 3 of the estrous cycle were calculated using only females that showed lordosis to a free male on the subsequent day. Male preference on day 3 of the estrous cycle (anestrous females excluded) was significantly decreased by food restriction at 11 days after restriction and at 3 days after re-feeding (P < 0.05) (Fig. 1A). In contrast, the same females that showed decreased male preference showed no significant decrease in lordosis duration until day 20 after the start of food restriction (Fig. 1B).
Figure 3.
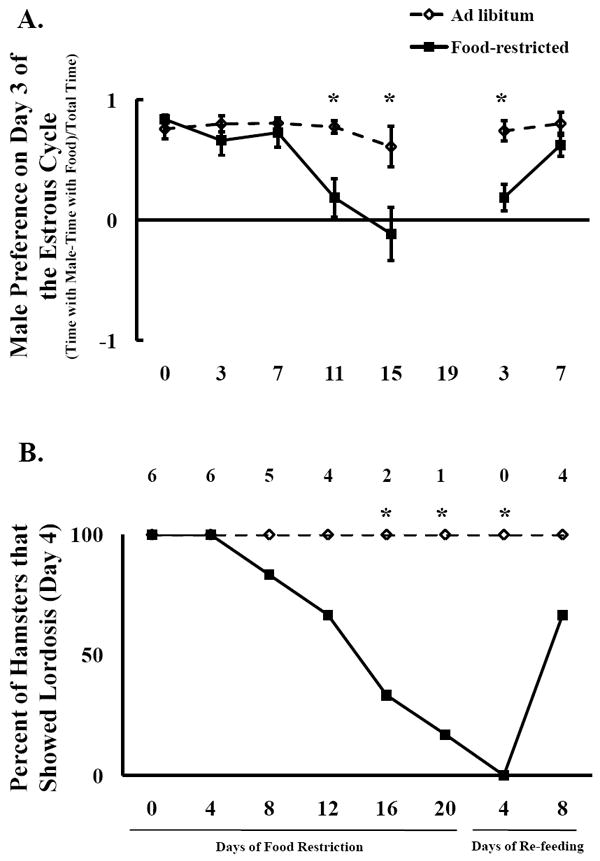
Mean and standard error of the mean for A) male preference (Time with male – time with food)/Total time for all females on Day 3 of the estrous cycle including those that did not show lordosis (those that did not show lordosis received a score of 0), and B) the frequency of females that showed lordosis. Females were fed ad libitum or food-restricted to 75% of their ad libitum intake until they stopped showing lordosis, at which point they were re-fed ad libitum. * = significant difference between food-restricted and ad libitum-fed hamsters by P < 0.05.
Linear regression showed a significant portion of the variance in male preference was accounted for by variation in body weight (r2 = .38, P < 0.02), whereas there was no significant association between lordosis duration and body weight (Fig 2A and 2B).
A similar pattern in male preference on day 3 of the cycle was apparent when all females were included in the male preference calculations (Fig. 3A). Food restriction decreased lordosis duration (P <).05) and this effect was magnified as restriction continued, and was reversed by 8 days of re-feeding. This was reflected in the repeated measures ANOVA as a significant main effect of food availability (F(1,40) = 18.85, P < 0.002), time (F(4,40) = 9.27, P < 0.002), and food availability × time interaction (F(4,40) = 6.90, P < 0.0003). Furthermore, male preference was significantly lower in food-restricted females after 11 days (P < 0.003) and 15 days (P < 0.02) of food restriction and 3 days of re-feeding (P < 0.007) compared to ad libitum-fed hamsters (Fig. 3A). A similar pattern was seen when male preference was measured on day 4 of the estrous cycle, in which there was a main effect of food availability (F(1,40) = 10.89, P < 0.008), but no effect of time and no interaction (Table 2).
Table 2.
Day 4 of the Estrous Cycle
| Length of Restriction (d) | Length of Re-feeding (d) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 0 | 4 | 8 | 12 | 16 | 4 | 8 | |
| 90-min Food Hoarding (g)
| |||||||
| Ad libitum | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Food-restricted | 2.7 ± 2.7 | 0 ± 0 | 40.1 ± 25.2 | 50.6 ± 31.0 | 42.7 ± 28.6 | 65.3 ± 29.0 | 36.9 ± 23.5 |
|
| |||||||
| 90-min Food Intake (g)
| |||||||
| Ad libitum | 0.6 ± 0.4 | 0.2 ± 0.1 | 0.5 ± 0.2 | 0.8 ± 0.5 | 1.0 ± 0.3 | 2.8 ± 2.2 | 0.4 ± 0.1 |
| Food-restricted | 0.8 ± 0.5 | 2.4 ± 0.4 | 0.9 ± 0.3 | 2.2 ± 0.9 | 2.5 ± 1.1 | 0.9 ± 0.5 | 0.8 ± 0.3 |
|
| |||||||
| Male Preference for All Females (Time with Male -Time with Food) / Total Time
| |||||||
| Ad libitum | 0.92 ± 0.01 | 0.95 ± 0.01 | 0.97 ± 0 | 0.96 ± 0.01 | 0.94 ± 0.02 | 0.95 ± 0.01 | 0.96 ± 0.01 |
| Food-restricted | 0.94 ± 0.01 | 0.94 ± 0.03 | 0.72 ± 0.16 | 0.51 ± 0.21* | 0.61 ± 0.22 | 0.67 ± 0.10* | 0.82 ± 0.09 |
|
| |||||||
| Male Preference Without Anestrous Females (Time with Male -Time with Food) / Total Time
| |||||||
| Ad libitum | 0.92 ± 0.01 | 0.95 ± 0.01 | 0.97 ± 0 | 0.96 ± 0.01 | 0.94 ± 0.02 | 0.95 ± 0.01 | 0.96 ± 0.01 |
| Food-restricted | 0.94 ± 0.01 | 0.94 ± 0.03 | 0.80 ± 0.17 | 0.69 ± 0.27 | 0.95 ± 0.01 | 0 ± 0* | 0.86 ± 0.11 |
Food-restricted hamsters different from hamsters fed ad libitum by P < 0.05.
The frequency of females that showed lordosis was not significantly affected by food restriction until day 16 (P < 0.05), at least 5 days after male preference was affected. At 20 days after the start of restriction, all hamsters were re-fed, whether they showed lordosis or not, because we were concerned about low body weights of those hamsters that continue to show lordosis at day 20. At 4 days after re-feeding, none of the food-restricted females showed lordosis. This has been observed previously when very lean animals were restricted on day 4 of the cycle, but did not recover estrous cyclicity by 4 days of re-feeding (Schneider, unpublished data). By 8 days of re-feeding, the majority of females showed lordosis, and the frequency of lordosis did not differ between food-restricted and ad libitum-fed females (Fig. 3B).
The majority of vaginal scent marks were produced on day 3 of the estrous cycle, the day before behavioral estrous (Table 1). Ad libitum-fed females tended to show higher levels of vaginal marking compared to restricted, but this difference was only significant on day 11 of restriction (P < 0.03). Repeated measures ANOVA showed no main effect of food availability or time, but there was a significant interaction between time and food availability (F(4,40) = 49.61, P < 0.03) on the number of vaginal marks per 15 min. In the present experiment, the rate of vaginal scent marking did not significantly increase after re-feeding.
Table 1.
Day 3 of the Estrous Cycle
| Length of Restriction (d) | Length of Re-feeding (d) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 0 | 3 | 7 | 11 | 15 | 3 | 7 | |
| Number of Vaginal Marks
| |||||||
| Ad libitum | 2.4 ± 1.1 | 3.8 ± 1.7 | 4.1 ± 1.3 | 9.3 ± 2.9 | 3.5 ± 1.4 | 2.0 ± 2.0 | 9.8 ± 2.4 |
| Food-restricted | 3.2 ± 1.7 | 5.0 ± 1.6 | 1.7 ± 0.8 | 1.0 ± 0.8* | 1.8 ± 1.1 | 1.7 ± 1.3 | 5.0 ± 2.2 |
|
| |||||||
| Number of Flank Marks
| |||||||
| Ad libitum | 3.6 ± 1.5 | 7.1 ± 2.4 | 8.8 ± 2.6 | 7.0 ± 2.6 | 3.9 ± 1.4 | 6.5 ± 4.0 | 12.5 ± 2.1 |
| Food-restricted | 5.0 ± 1.8 | 3.0 ± 1.7 | 4.3 ± 3.2 | 0.3 ± 0.3* | 1.0 ± 0.6 | 3.5 ± 2.6 | 12.8 ± 3.8 |
Food-restricted hamsters different from hamsters fed ad libitum by P < 0.05.
Food-restricted females showed significantly fewer flank marks compared to ad libitum-fed females after 11 days of food restriction (P < 0.04) (Table 1).
Ingestive Behavior
Food hoarding (day 3 of the estrous cycle) increased in food-restricted females as reflected by the repeated measures ANOVA, which showed a significant main effect of food availability (F(1,40) = 7.35, P < 0.02), time (F(4, 40) = 4.71, P < 0.003), and an interaction between food availability and time (F(4,40) = 6.40, P < 0.0004). The amount of food hoarded on day 3 was significantly greater in food-restricted hamsters compared to hamsters fed ad libitum after 11 days (P < 0.04) and 15 days (P < 0.01) of food restriction and remained increased through 3 days of re-feeding (P < 0.03) (Fig. 4A). Similarly, for food hoarding on day 4 of the estrous cycle, there was a significant main effect of time (F(4,40) = 3.24, P < 0.02) and an interaction between food availability and time (F(4,40) = 3.61, P < 0.01) (Table 2).
Figure 4.
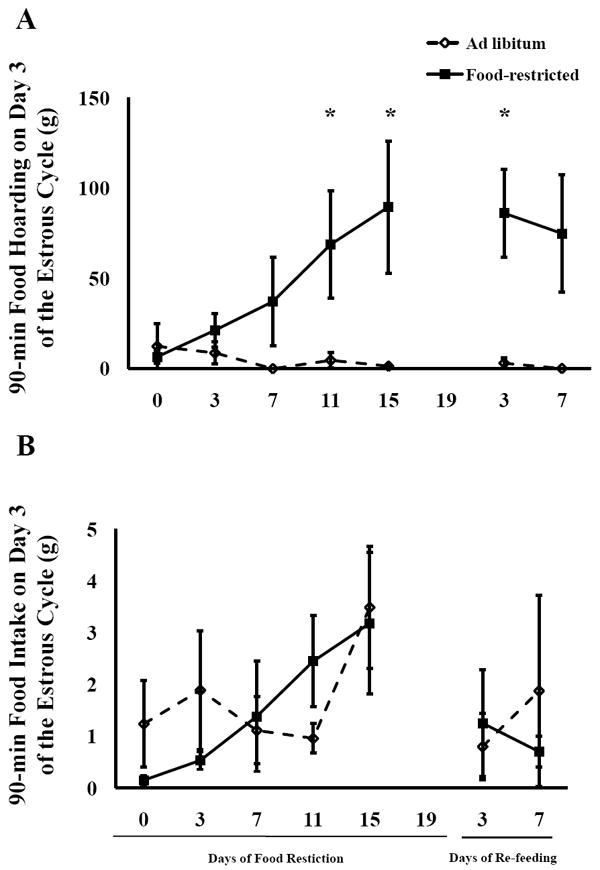
Mean and standard error of the mean for A) 90-min food hoarding, and B) 90-min food intake on Day 3 of the estrous cycle. Females were fed ad libitum or food-restricted to 75% of their ad libitum intake until they stopped showing lordosis, at which point they were re-fed ad libitum. Hamsters were tested for food hoarding and intake at the same time they had access to an adult sexually-experienced male restrained behind a wire barrier * = significant difference between food-restricted and ad libitum-fed hamsters by P < 0.05.
There was no effect of food restriction on food intake on day 3 of the cycle (Fig. 4B). On day 3 of the cycle, there was a main effect of time on the amount of food eaten (F(4,40) = 2.74, P < 0.04), but there was no interaction between food availability and time (Table 1). Food-restricted females never differed significantly in their food intake from ad libitum-fed females on any day of day 3 testing.
On day 4 of the estrous cycle, ad libitum-fed most females went directly to the male and spent all of their time with males, whereas late in food restriction, some females made brief visits to the food box, particularly those who were anestrous. Food intake did not differ significantly between food-restricted and ad libitum-fed females on any day (Table 2). However, on day 4 of the estrous cycle, there were main effects of food availability (F(1,28) = 27.90, P < 0.001) and time (F(4,28) = 3.83, P < 0.01) and an interaction between food availability and time (F(4,28) = 2.76, P < 0.05) on food intake (Table 2).
Body Weight
Female hamsters food-restricted for 16 days lost weight (an average of -31.5 ± 5.58 g S.E.M.) whereas females fed ad libitum gained weight (+10.5 ± 3.28 g S.E.M.) and the change in body weight was significant (F(1,12) = 47.20, P < 0.0001) (Fig. 5). After eight days of re-feeding the previously food-restricted hamsters increased body weight, but remained significantly lower than ad libitum-fed hamsters (P < 0.018) (Fig. 5).
Figure 5.
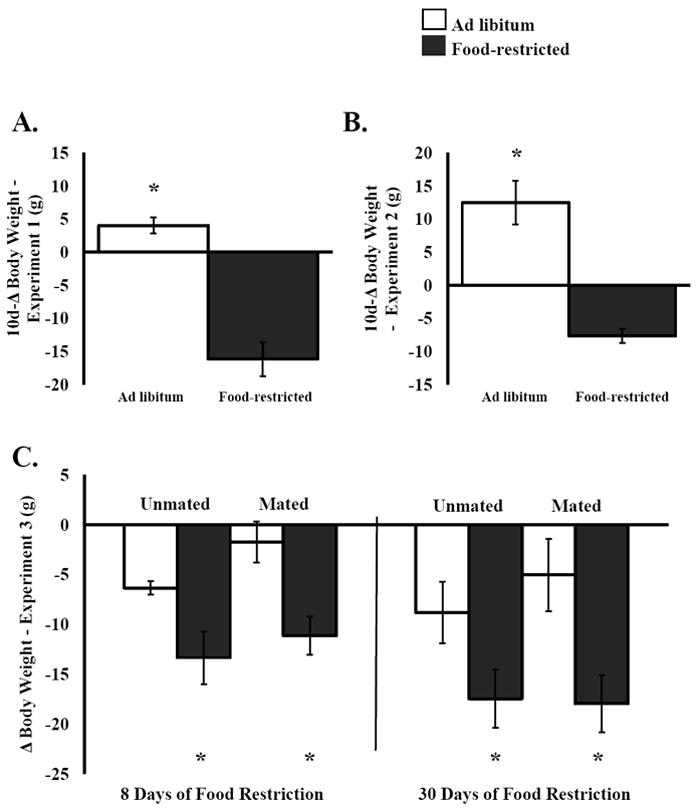
Mean and standard error of the mean for the change in body weight from the start of restriction to day 10 in females from A) Experiment 1 and B) Experiment 2. Mean and standard error of the mean for the change in body weight from the start of restriction to the first conditioning session (8 days of restriction) and from the start of the experiment to the last post-conditioning test (30 days of restriction) from Experiment 3. Females received a conditioning session or a post-conditioning test every 4 days. * = significantly different at P < 0.05.
Experiment 2. Mating-induced neural activation in food-restricted and ad libitum-fed females Lordosis frequency and duration
Ten days of food restriction at 75% of ad libitum intake resulted in significant body weight loss (F(1,22) = 37.08, P < 0.0001) (Fig. 5), and did not result in significant differences in the frequency of or duration of lordosis (Fig. 6) compared to ad libitum-fed hamsters. Male hit rate was determined by dividing the number of intromissions/ejaculations by the number of mounts. Males that mated with food-restricted females did not show a significantly different hit rate or number of sexual attempts than those that mated with ad libitum-fed female hamsters (Fig. 6).
Figure 6.
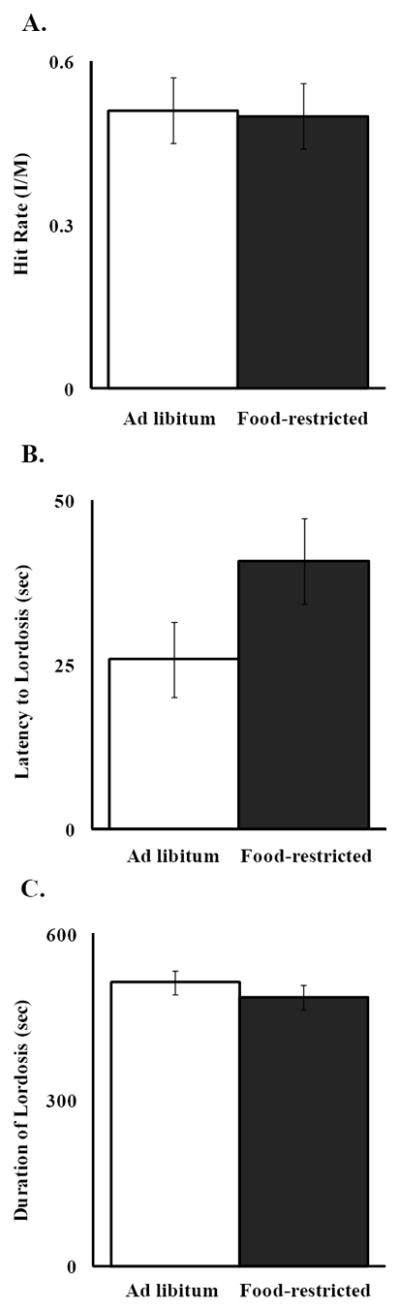
Mean and standard error of the mean for A) hit rate (calculated as the number of intromissions/the number of mounts), B) latency to lordosis, and C) lordosis duration, * = significantly different at P < 0.05.
c-Fos immunoreactivity
For c-Fos-IR in the NAc core, there was a significant main effect of mating (F(1,19) = 9.45, P < 0.006), but no significant effect of food treatment and no interaction between food treatment and mating (Fig. 7). Analysis of the NAc shell yielded similar results with a significant main effect of mating (F(1,19) = 10.42, P < 0.004) and no significant effect of food treatment and no significant interaction between food treatment and mating (Fig. 7).
Figure 7.
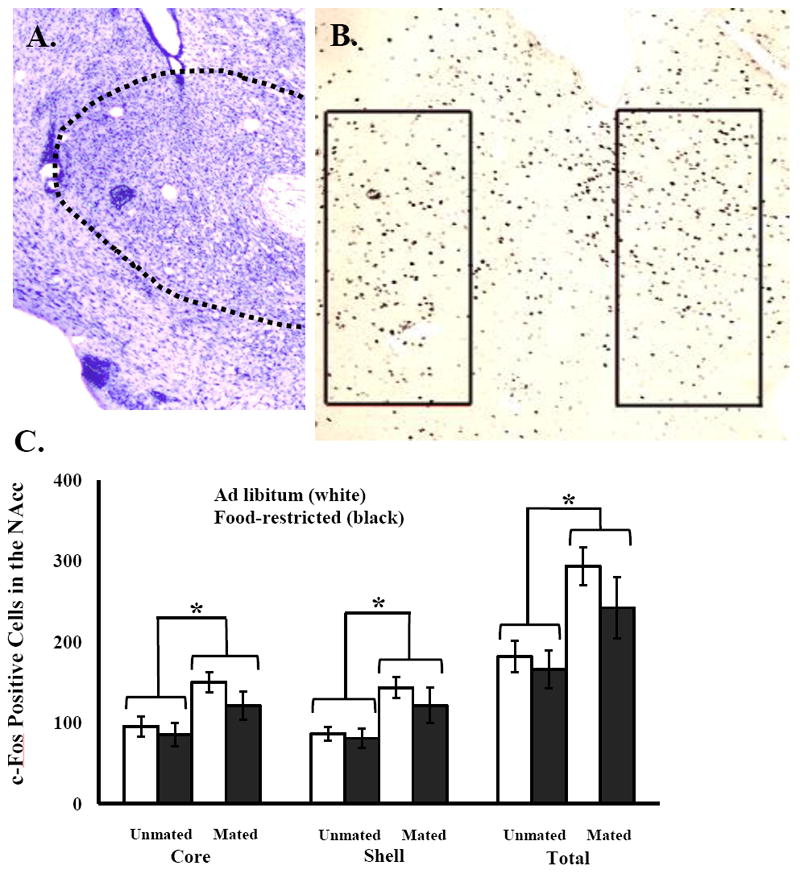
Representative cresyl violet (Top left) and DAB stained (Top right) images of the NAc. Food-restricted hamsters were fed 75% of their ad libitum intake for 10 days. Mated hamsters were given a 10 min mating test with a sexually-naïve male hamster and unmated hamsters had no contact with a male prior to sacrifice 1 h later. Mean and standard error of the mean for c-Fos immunoreactivity in the NAc (Bottom). * = Mated hamsters significantly different from unmated hamsters by P < 0.05.
For c-Fos-IR in the lateral VMH there was a significant main effect of mating (mating experience increased c-Fos-IR) (F(1,19) = 22.0, P < 0.0002) but no significant effect of food treatment and no significant interaction between food treatment and mating (Figure 8).
Figure 8.
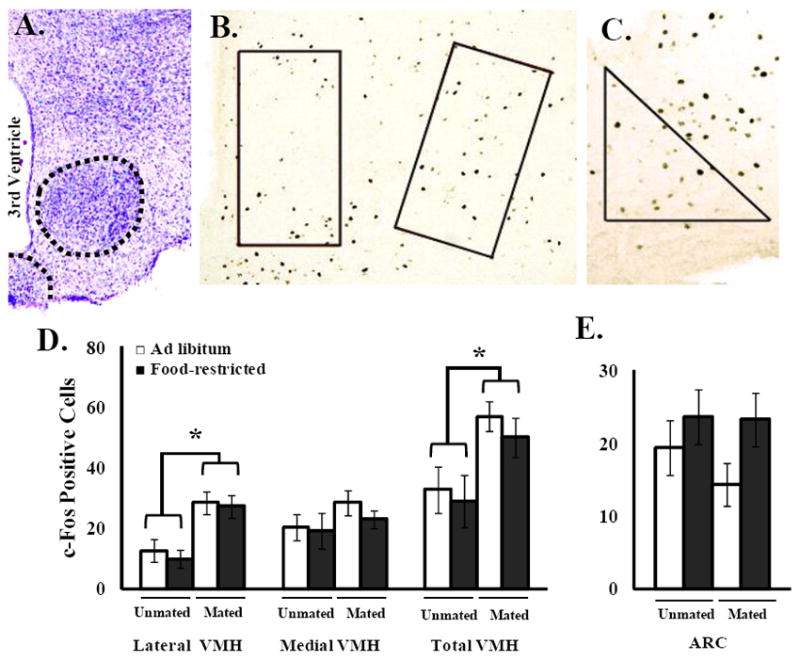
Representative cresyl violet (Top left) and DAB stained (Top middle and right) images of the VMH and Arc. Food-restricted hamsters were fed 75% of their ad libitum intake for 10 d. Mated hamsters were given a 10-min mating test with a sexually-naïve male hamster and unmated hamsters had no contact with a male prior to sacrifice 1 h later. Mean and standard error of the mean for c-Fos immunoreactivity in the lateral VMH and arcuate nucleus (Bottom). * = Mated hamsters significantly different from unmated hamsters by P < 0.05.
Within the medial VMH, there was no significant main effect of mating, food treatment or interaction between food treatment and mating F(1,19) = 0.23, P < 0.60). c-Fos-IR in the VMH did not differ between food-restricted and ad libitum-fed hamsters after mating at 10 days of restriction in either the lateral or medial VMH (Fig. 8).
For c-Fos-IR in the Arc, the general pattern was different than the VMH, with food-restricted females always showing somewhat higher levels than ad libitum-fed, and mated lower than non-mated females, but there was no significant main effect of mating or food treatment and no significant interaction between food treatment and mating (Fig. 8).
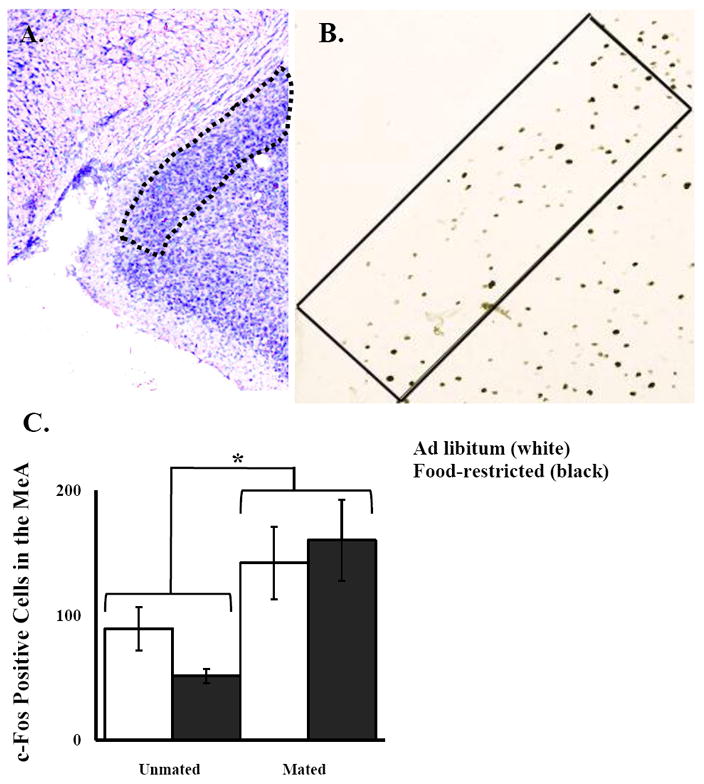
For c-Fos-IR in the MeA, there was a significant main effect of mating (F(1,19) = 11.07, P < 0.004) and no significant effect of food treatment and no significant food treatment by mating interaction, although it appeared that the mating response in food-restricted females might be exaggerated compared to that of ad libitum-fed females (Fig. 9).
Figure 9.
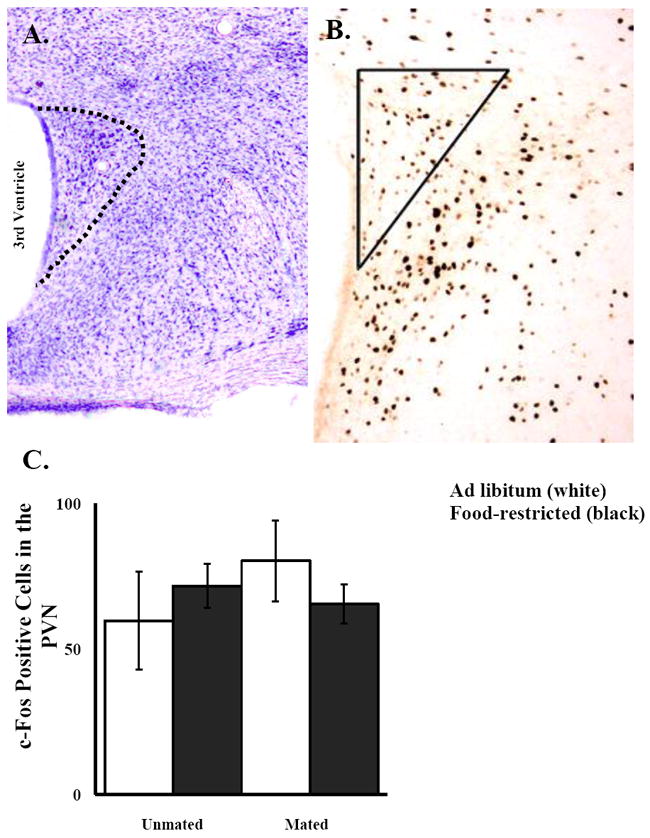
Representative cresyl violet (Top left) and DAB stained (Top right) images of the PVN. Food-restricted hamsters were fed 75% of their ad libitum intake for 10 d. Mated hamsters were given a 10-min mating test with a sexually-naïve male hamster and unmated hamsters had no contact with a male prior to sacrifice 1 h later. Mean and standard error of the mean for c-Fos immunoreactivity in the PVN (Bottom). No significant differences were detected among mated or unmated and food-restricted or ad libitum-fed hamsters.
For c-Fos-IR in the PVN there was no significant main effect of food treatment, mating, and no significant interaction between food treatment and mating (Fig. 10).
Figure 10.
Representative cresyl violet (Top left) and DAB stained (Top right) images of the MeA. Food-restricted hamsters were fed 75% of their ad libitum intake for 10 d. Mated hamsters were given a 10-min mating test with a sexually-naïve male hamster and unmated hamsters had no contact with a male prior to sacrifice 1 h later. Mean and standard error of the mean for c-Fos immunoreactivity in the amygdala (Bottom). * = mated hamsters significantly different from unmated hamsters by P < 0.05.
Experiment 3. Effects of energy restriction on formation of a conditioned place preference to mating
Conditioned place preference
Both food-restricted and ad libitum-fed females developed a CPP to mating after 4 conditioning sessions (Fig. 11). Food-restricted and ad libitum-fed hamsters did not form a preference to mating after 1 conditioning session (P < 0.26 and P < 0.15 respectively). However, after 4 conditioning sessions, both food-restricted and ad libitum-fed hamsters formed a preference to mating (P < 0.004 and P < 0.03). There were also significant differences between 1 and 4 mating sessions for hamsters food-restricted and fed ad libitum (P < 0.03 and P < 0.05 respectively). Food-restricted and ad libitum-fed females conditioned to an empty box failed to form a preference after 1 (P < 0.61 and P < 0.56 respectively) or 4 conditioning sessions (P < 0.84 and P < 0.26 respectively).
Figure 11.
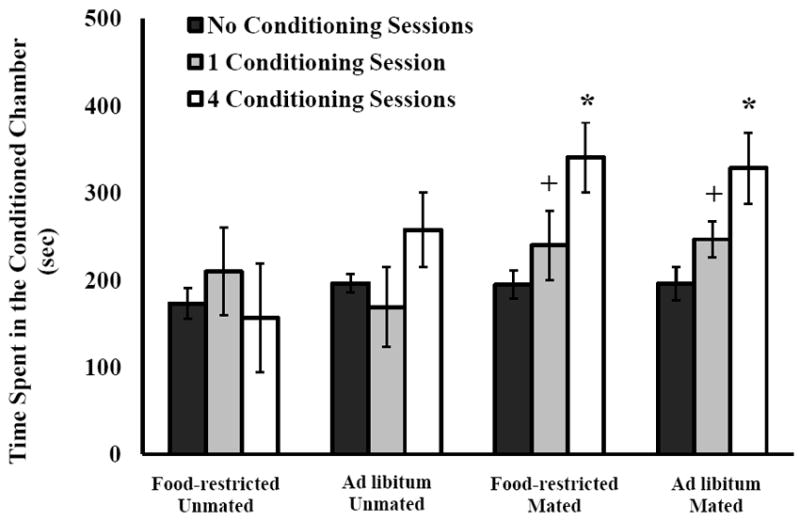
Mean and standard error of the mean of time spent in the conditioned chamber of the CPP apparatus in hamsters tested after 0, 1 or 4 post-conditioning tests. Females were all ovariectomized and treated with estradiol and progesterone and half were either fed ad libitum or food-restricted to about 75% of their ad libitum intake beginning 8 days prior to conditioning through the last post-test. Half of each food group was either conditioned with a male (mated) or conditioned with the empty compartment (unmated). During post-conditioning tests, female subjects had a choice between a chamber where 10 min of copulatory experience had occurred (conditioned compartment) or where no male had been present (unconditioned compartment). Females were tested for their preference for the either the conditioned or unconditioned compartment after 0, 1 or 4 conditioning tests. + = 1 conditioning session significantly different from 4 conditioning sessions by P < 0.05. * = 0 conditioning sessions (pre-test) significantly different from 4 conditioning sessions by P < 0.05.
Food intake
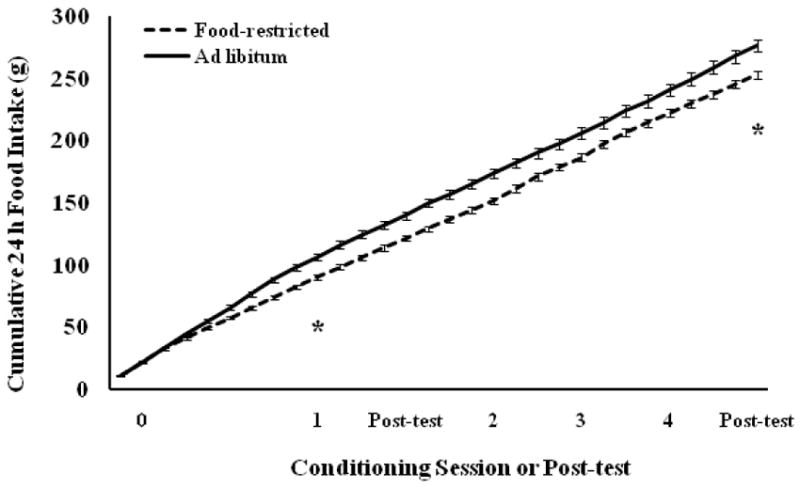
Cumulative food intake (Fig. 12) differed significantly between food-restricted and ad libitum-fed hamsters by the first conditioning session (F(1,20) = 15.43, P < 0.0008) and this difference continued through the last post-test (F(1,20) = 10.85, P < 0.0036) and appetitive sex test (data not shown).
Figure 12.
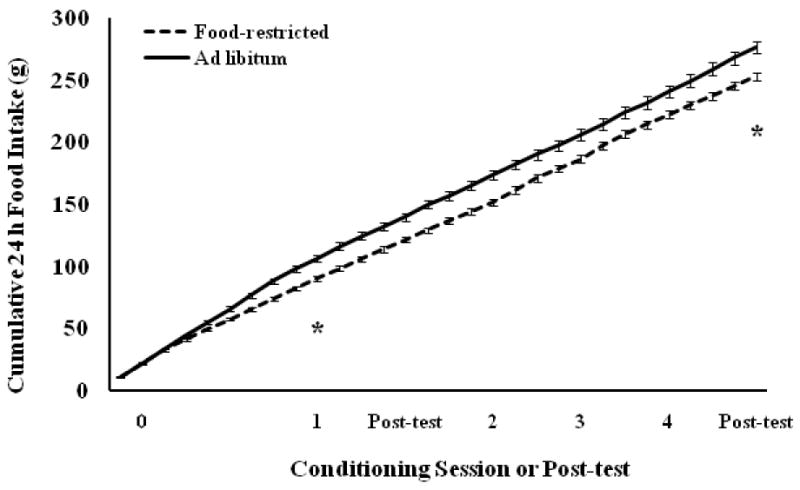
Mean and standard error of the mean of cumulative food intake over the course of Experiment 3. Females were food-restricted to 75% of their ad libitum food intake or fed ad libitum for 8 days prior to the first CPP conditioning trial and throughout conditioning. * = cumulative food intake of food-restricted hamsters was significantly less than that of ad libitum-fed hamsters between 8 and 30 days of food restriction.
Body weight
Both food-restricted and ad libitum-fed hamsters lost weight throughout Experiment 3 (Fig. 5), however, hamsters that were food-restricted lost significantly more weight than hamsters fed ad libitum. By the first conditioning session (8 days of restriction) food-restricted hamsters lost significantly more weight (weights subtracted from pre-test) than hamsters fed ad libitum (F(1,20) = 13.31, P < 0.002) and this difference remained throughout the last post-test (30 days of food restriction) and appetitive sex test (F(1,20) = 8.79, P < 0.008).
Appetitive sex test
Similar to Experiment 1, hamsters that were food-restricted were less sexually-motivated than hamsters fed ad libitum. For example, food-restricted hamsters produced significantly less vaginal marks (Fig. 13) than hamsters fed ad libitum (F(1,20) = 4.42, P < 0.05) and there was no effect of prior mating experience obtained during conditioning (F(1,20) = 0.67, P < 0.42) or an interaction between food and mating (F(1,20) = 1.10, P < 0.31). Although not significant, there was a tendency for food-restricted hamsters to have a longer latency to vaginal mark (F(1,20) = 3.61, P < 0.07) and shorter latency to aggression (F(1,20) = 3.31, P < 0.08), compared to hamsters fed ad libitum (data not shown). Also, twice as many females fed ad libitum (8 hamsters) allowed mounting attempts by a male compared to food-restricted hamsters (4 hamsters).
Figure 13.
Mean and standard error of the mean of the number of vaginal marks produced during a test for appetitive sex behavior in ovariectomized females treated with estradiol in Experiment 3. Females were food-restricted or fed ad libitum for 30 days prior to the test. Half of each food group, the “Mated” females were conditioned with males whereas the other half, the “Unmated” females were conditioned with an empty compartment. * = 0 conditioning sessions (pre-test) significantly different from 4 conditioning sessions by P < 0.05.
DISCUSSION
The main findings from these experiments were threefold. First, 25% food restriction for greater than 7 but less than 12 days significantly decreased the motivation for sex without a significant decrease in sexual performance in the majority of females (Figs. 1 and 2). These results are consistent with an earlier study in which 8 days of 25% food restriction significantly decreased sexual motivation but not performance [1]. Second, ten days of 25% food restriction failed to prevent or significantly attenuate lordosis duration, copulatory interactions with males, or mating-induced neural activation in the NAc, VMH, PVN, MeA, or Arc in Experiment 2 (Figs. 7-10). Third, between 8 and 30 days of 25% food restriction significantly decreased vaginal marking, but failed to effect formation of a CPP to mating in Experiment 3.
The results of Experiment 1 are consistent with previous studies demonstrating that the mechanisms that control the motivation to engage in sex and/or ingestive behavior are more sensitive to the effects of energy availability than are the mechanisms that control copulatory performance [1, 3]. Specifically, after 11 days of food restriction, appetitive sex behaviors were significantly lower and appetitive ingestive behaviors were significantly higher in food-restricted compared to libitum fed females (Figs. 1A and 4A), even in females that showed lordosis on the next day of the same estrous cycle (Fig. 1B). The 12-day food-restricted females that showed lordosis did not differ significantly from ad libitum-fed females in lordosis duration (Fig. 1B). Also, on day 12 of food restriction, only two of six food-restricted females failed to show lordosis when the male was free (Fig. 3B). Thus, although copulatory performance can be affected by 12 days of restriction in some females, inhibition of lordosis and the underlying HPG system is not required in order for this level of food restriction to inhibit appetitive sex behaviors and increase appetitive ingestive behaviors on day 11. In addition, there was a significant positive correlation between male preference on day 3 of the estrous cycle and body weight, but the correlation between lordosis duration on day 4 of the estrous cycle and body weight was not significant (Fig. 5). Together, these results reveal and crystallize an important interaction between energy availability, male stimuli and ovarian steroids in setting behavioral priorities that is not apparent by limiting observation to consummatory behaviors such as lordosis and food intake.
The effects of this mild food restriction are not explained by decreases in circulating ovarian steroid concentrations because in Experiment 1 appetitive behavior was inhibited even in females that showed lordosis, a behavior dependent upon high circulating levels of estradiol and progesterone. In addition, in Experiment 3 vaginal scent marking was significantly decreased by food restriction despite treatment with estradiol (Fig. 14). In other experiments, two days of complete food deprivation significantly decreased vaginal scent marking by estrous-cycling Syrian hamsters, even though these hamsters did not show food deprivation-induced anestrus or a significant decrease in circulating estradiol concentrations [3]. In other experiments, neither estradiol nor progesterone concentrations were significantly lower after 4, 8 or 12 days of 75% food restriction (Klingerman, Williams, Kriegsfeld and Schneider, in unpublished data). Finally, significant effects on appetitive sex and ingestive behavior were seen when ovariectomized females were food restricted to 75% of their ad libitum intake for 8 days treated with estrous-inducing doses of exogenous estradiol and progesterone [1].
It is likely that the effects of food restriction on appetitive behaviors involves decreased sensitivity or responsiveness to estradiol because effects of energy availability occur prior to endogenous changes in circulating ovarian steroids and in spite of exogenous treatment with ovarian steroids. Energetic effects on neural estrogen receptors have been noted in the literature, but the energetic challenges were far more severe and inhibited both lordosis and the hypothalamic-pituitary-gonadal system. In earlier studies, food deprivation in lean female hamsters (less than 100 g in body weight) inhibited follicle development and ovarian steroid secretion, induced anestrous, and decreased the number of cells immunoreactive for estrogen receptor (ER-IR) in the VMH in hamsters [38-40], and induced hypogonadotropism in other species [41-43]. Although ER-IR is lower in the VMH and higher in the PVN and caudal preoptic area (POA) after 48 or more hours of total food deprivation in lean hamsters (less than 120 g) [40], the effects of the current food-restriction paradigm (25% food restriction in 140 g hamsters) on ER-IR remain to be investigated.
An additional new finding from the present experiment was that a similar level and duration of food restriction relative to that used in Experiment 1 did not have a significant effect on either the females’ lordosis duration, or on the naïve stimulus males’ hit rate (number of intromissions per number of mounts) in Experiment 2 (Fig. 6). Other experiments show that when sexually-naïve male hamsters mate with sexually-experienced females, they achieve a significantly greater hit rate than when they mate with sexually-naïve females. Furthermore, male hit rate is improved when their female mating partners receive treatments that increase the expression of ΔFosB in the NAc of females [44]. Given these results, it might be expected that the females’ energetic condition might also influence their ability to improve male hit rate. This idea was refuted because there was no significant difference in hit rate between those males that mated with females that were food-restricted and those that mated with females that were fed ad libitum (Fig. 6).
Having identified a duration during which 25% food restriction blocks appetitive but not consummatory behaviors in Experiment 1, Experiment 2 was designed to test the idea that food-restriction-induced changes in appetitive behaviors might be dissociated from changes that result from the rewarding consequences of copulation. The nucleus accumbens (NAc) was of particular interest because a 10-min period of sexual experience increases neural activation and dopamine release in the NAc, and the effect is exaggerated in females with prior sexual experience [26, 27]. Furthermore, just as the effects of 25% food restriction decreased appetitive behaviors prior to consummatory behaviors, treatment with a dopamine receptor antagonist blocked the formation of a conditioned place preference after sexual experience without significant effects on lordosis [13]. Would food restriction also inhibit copulation-induced increases in NAc neural activation in the same way that it inhibited appetitive behavior, or would appetitive behavior be dissociated from the rewarding aspects of copulation? A third possible outcome would be that food restriction would actually enhance NAc neural activation, similar to the food restriction-induced sensitization to the effects of drugs of abuse [45, 46]. In Experiments 2 and 3, inhibition of appetitive behavior was dissociated from mechanisms that mediate the rewarding consequences of copulation because both ad libitum-fed and food-restricted females showed mating-induced increases in neural activation in the NAc, similar to the pattern of neural activation found in the VMH and MeA (Figs. 7-9). Furthermore, food-restricted and ad libitum-fed females both showed evidence that they experienced a reward during copulation, because these groups did not differ significantly in the formation of a CPP (Fig. 11), despite the fact that food-restricted females decreased appetitive sex behavior (Fig. 12).
Whereas mating experience increased neural activation in the NAc, VMH and MeA (Figs. 8 and 9), a different pattern of neural activation was seen in the Arc and PVN (Fig.8 and 10). In the Arc, mating experience did not stimulate c-Fos expression. In the Arc, neural activation in the food-restricted group was always slightly higher than in females fed ad libitum, and these effects were not significant (Fig. 8). Similarly, in the PVN, effects of mating and food regimen were not significant (Fig. 10). Together these results suggest that the effects of energy availability on sexual motivation might be mediated by mechanisms that are not reflected by mating-induced neural activation in the NAc. Changes in these latter areas would be consistent with their high concentration of corticotropin releasing hormone, urocortin, leptin receptors, neuropeptide Y/agouti-related protein, and proopiomelanocortin neurons and their putative roles in response to energy deficits [28-36] but 25% food restriction might not be enough to significantly increase activation of these cells.
The present experiment addressed only the idea that energy deficits decrease the rewarding aspects of a mating experience that involves mounting, intromission and ejaculation. A separate question is whether precopulatory, appetitive behaviors are reinforcing in ad libitum-fed females (whether they can serve as a reinforcing stimulus in a CPP paradigm or whether these experiences can sensitize the dopamine transmission or neural activation in the NAc) and if so, whether the reinforcing aspects of behavior are susceptible to energy deficits. The answer to these questions with regard to ad libitum-fed females are unknown and thus, there is currently no basis for comparisons to food-restricted females.
In summary, the present experiments confirm that 25% food restriction can be used to reliably decrease appetitive aspects of sex and ingestive behavior without affecting consummatory behavior in the majority of female hamsters and mating reward. The behavioral results suggest that hamster precopulatory behaviors, including the choice between courtship and hoarding, represent an important locus of effect of energy availability on reproduction. This is a useful paradigm for elucidating the functional significance of neuropeptides and neurohormones previously thought to be important for body weight regulation, but which might have evolved to orchestrate the appetites for food and sex. Furthermore, the choice between engaging in sex or ingestive behaviors might be the point at which the reproductive outcome (and Darwinian fitness) is decided, and thus, the mechanisms that control this choice might be the mechanisms that have been under selection. If so, it will be important to reexamine the assertions that have come from many years of studying food intake in animals in an enclosed space with no opposite-sex conspecifics available. For example, if the adaptive function of so-called ‘satiety peptides,’ such as leptin and estradiol, has been to temporarily decrease ingestive behaviors and to stimulate reproductive behaviors, it might be imprudent to expect that these hormones limit anticipatory shopping, hoarding and body weight gain.
Research Highlights.
-
▪
Appetitive behaviors (sexual motivation) were dissociated from consummatory behaviors. A period of 7 to 11 days of mild food restriction (75% of ad libitum intake) significantly decreased the motivation for sex without a significant decrease in sexual performance in the majority of hamsters. This confirms previously published research showing that 8 days of food restriction inhibited appetitive but not consummatory sex behaviors. There was a significant positive correlation between body weight and the preference for food vs. males, but there was no significant correlation between lordosis duration and body weight.
-
▪
Appetitive behaviors were also dissociated from the rewarding effects of copulation. Both food-restricted and ad libitum-fed females formed a conditioned place preference to copulatory reward, and food restriction failed to prevent or significantly attenuate lordosis duration, hit rate, or mating-induced neural activation in the NAc VMH, PVN, or MeA in Experiment 2.
-
▪
The behavioral results suggest that hamster precopulatory behaviors, including the choice between courtship and hoarding, represent an important locus of effect of energy availability on reproduction. Together, these results demonstrate an interaction between energy availability, male stimuli and ovarian steroids, which sets behavioral priorities. This interaction, however, is not apparent when observations are limited to consummatory behaviors such as lordosis and food intake.
Acknowledgments
These experiments were supported by IBN0645882 from the National Science Foundation, R01DA13680 (RLM) and F31DA026255 (VLH) and R01DK069981 from the National Institute of Health, and Grants-in-aid of Research from Sigma Xi. The authors would like to thank Balaji Sridhar for his histological expertise, Timothy Garelick for statistical advice, Jenifer Golley for technical assistance, Jess Kohlert for use of the CPP apparatus, and Murray Itzkowitz, Kimberly Little, Joseph Leese, Jennifer Dautrich, Jeremy Brozek, and Noah Benton for their helpful discussion and comments on the manuscript.
Abbreviations
- NAc
nucleus accumbens
- VMH
ventromedial hypothalamus
- PVN
paraventricular nucleus of the hypothalamus
- MeA
medial amygdala
- Arc
arcuate nucleus of the hypothalamus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klingerman CM, Krishnamoorthy K, Patel K, Spiro AB, Struby C, Patel A, Schneider JE. Energetic challenges unmask the role of ovarian hormones in orchestrating ingestive and sex behaviors. Hormones and Behavior. 2010;58:563–74. doi: 10.1016/j.yhbeh.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 2.Schneider JE. Metabolic and hormonal control of the desire for food and sex: implications for obesity and eating disorders. Horm Behav. 2006;50:562–71. doi: 10.1016/j.yhbeh.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 3.Schneider JE, Casper JF, Barisich A, Schoengold C, Cherry S, Surico J, DeBarba A, Fabris F, Rabold E. Food deprivation and leptin prioritize ingestive and sex behavior without affecting estrous cycles in Syrian hamsters. Horm Behav. 2007;51:413–27. doi: 10.1016/j.yhbeh.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Gattermann R, Johnston RE, Yigit N, Fritzsche P, Larimer S, Ozkurt S, Neumann K, Song Z, Colak E, Johnston J, McPhee ME. Golden hamsters are nocturnal in captivity but diurnal in nature. Biol Lett. 2008;4:253–5. doi: 10.1098/rsbl.2008.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston RE. The causation of two scent-marking behavior patterns in female hamsters (Mesocricentus auratus) Animal Behaviour. 1977;25:317–327. doi: 10.1016/0003-3472(77)90007-0. [DOI] [PubMed] [Google Scholar]
- 6.Craig W. Appetites and aversions as constituents of instinct. Proc Natl Acad Sci. 1917;3:685–688. doi: 10.1073/pnas.3.12.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev. 1990;14:217–32. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- 8.Sherrington CS. The Integrative Action of the Nervous System. New York: Scribner; 1906. [Google Scholar]
- 9.Lorenz K. The comparative method in studying innate behavior patterns. Symp Soc Exp Biol. 1950;1950:221–268. [Google Scholar]
- 10.Johnston RE. Sexual attraction function of golden hamster vaginal secretion. Behav Biol. 1974;12:111–7. doi: 10.1016/s0091-6773(74)91101-8. [DOI] [PubMed] [Google Scholar]
- 11.Lisk RD, Ciaccio LA, Catanzaro C. Mating behavior of the golden hamster under seminatural conditions. Anim Behav. 1983;31:659–666. [Google Scholar]
- 12.Johnston RE. Scent marking by male Golden Hamsters (Mesocricetus auratus) III. Behavior in a seminatural environment. Z Tierpsychol. 1975;37:213–21. [PubMed] [Google Scholar]
- 13.Meisel RL, Joppa M, ARowe RK. Dopamine receptor antagonists attenuate conditioned place preference following sexual behavior in female Syrian hamsters. Eur J Pharmacol. 1996;309:21–4. doi: 10.1016/0014-2999(96)00389-5. [DOI] [PubMed] [Google Scholar]
- 14.Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell JB, Gratton A. Involvement of mesolimbic dopamine neurons in sexual behaviors: implications for the neurobiology of motivation. Rev Neurosci. 1994;5:317–29. doi: 10.1515/revneuro.1994.5.4.317. [DOI] [PubMed] [Google Scholar]
- 16.Salamone JD. The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behav Brain Res. 1994;61:117–33. doi: 10.1016/0166-4328(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 17.Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience. 1999;89:637–41. doi: 10.1016/s0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- 18.Alcaro A, Huber R, Panksepp J. Behavioral functions of the mesolimbic dopaminergic system: an affective neuroethological perspective. Brain Res Rev. 2007;56:283–321. doi: 10.1016/j.brainresrev.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salamone JD, Correa M, Farrar AM, Nunes EJ, Pardo M. Dopamine, behavioral economics, and effort. Front Behav Neurosci. 2009;3:13. doi: 10.3389/neuro.08.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–28. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 21.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 23.Lajtha A, Sershen H. Heterogeneity of reward mechanisms. Neurochem Res. 2010;35:851–67. doi: 10.1007/s11064-009-0096-4. [DOI] [PubMed] [Google Scholar]
- 24.Meisel RL, Joppa MA. Conditioned place preference in female hamsters following aggressive or sexual encounters. Physiol Behav. 1994;56:1115–8. doi: 10.1016/0031-9384(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 25.Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Kohlert JG, Meisel RL. Sexual experience sensitizes mating-related nucleus accumbens dopamine responses of female Syrian hamsters. Behav Brain Res. 1999;99:45–52. doi: 10.1016/s0166-4328(98)00068-0. [DOI] [PubMed] [Google Scholar]
- 27.Bradley KC, Meisel RL. Sexual behavior induction of c-Fos in the nucleus accumbens and amphetamine-stimulated locomotor activity are sensitized by previous sexual experience in female Syrian hamsters. J Neurosci. 2001;21:2123–30. doi: 10.1523/JNEUROSCI.21-06-02123.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol. 2003;464:220–37. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- 29.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Woolf EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 30.Hakansson ML, Brown H, Ghilardi N, Skoda RC, Meister B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J Neurosci. 1998;18:559–72. doi: 10.1523/JNEUROSCI.18-01-00559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mercer JG, Beck B, Burlet A, Moar KM, Hoggard N, Atkinson T, Barrett P. Leptin (ob) mRNA and hypothalamic NPY in food-deprived/refed Syrian hamsters. Physiol Behav. 1998;64:191–5. doi: 10.1016/s0031-9384(98)00039-0. [DOI] [PubMed] [Google Scholar]
- 32.Backholer K, Smith J, Clarke IJ. Melanocortins may stimulate reproduction by activating orexin neurons in the dorsomedial hypothalamus and kisspeptin neurons in the preoptic area of the ewe. Endocrinology. 2009;150:5488–97. doi: 10.1210/en.2009-0604. [DOI] [PubMed] [Google Scholar]
- 33.Qi Y, Oldfield BJ, Clarke IJ. Projections of RFamide-related peptide-3 neurones in the ovine hypothalamus, with special reference to regions regulating energy balance and reproduction. J Neuroendocrinol. 2009;21:690–7. doi: 10.1111/j.1365-2826.2009.01886.x. [DOI] [PubMed] [Google Scholar]
- 34.Bentley GE, Jensen JP, Kaur GJ, Wacker DW, Tsutsui K, Wingfield JC. Rapid inhibition of female sexual behavior by gonadotropin-inhibitory hormone (GnIH) Horm Behav. 2006;49:550–5. doi: 10.1016/j.yhbeh.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Seymour PL, Dettloff SL, Jones JE, Wade GN. Corticotropin-releasing factor receptor subtypes mediating nutritional suppression of estrous behavior in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol. 2005;289:R418–R423. doi: 10.1152/ajpregu.00168.2005. [DOI] [PubMed] [Google Scholar]
- 36.Jones JE, Pick RR, Dettloff SL, Wade GN. Metabolic fuels, neuropeptide Y, and estrous behavior in Syrian hamsters. Brain Res. 2004;1007:78–85. doi: 10.1016/j.brainres.2004.01.069. [DOI] [PubMed] [Google Scholar]
- 37.Morin LP. Effects of various feeding regimens and photoperiod or pinealectomy on ovulation in the hamster. Biology of Reproduction. 1975;13:99–103. doi: 10.1095/biolreprod13.1.99. [DOI] [PubMed] [Google Scholar]
- 38.Morin LP. Environment and hamster reproduction: responses to phase-specific starvation during estrous cycle. American Physiological Society. 1986:R663–R669. doi: 10.1152/ajpregu.1986.251.4.R663. [DOI] [PubMed] [Google Scholar]
- 39.Schneider JE, Wade GN. Availability of metabolic fuels controls estrous cyclicity of Syrian hamsters. Science. 1989;244:1326–8. doi: 10.1126/science.2734610. [DOI] [PubMed] [Google Scholar]
- 40.Li H-Y, Wade GN, Blaustein JD. Manipulations of metabolic fuel availability alter estrous behavior and neural estrogen-receptor immunoreactivity in syrian hamsters. Endocrinology. 1994;135:240–247. doi: 10.1210/endo.135.1.8013358. [DOI] [PubMed] [Google Scholar]
- 41.Bronson FH. Effect of food manipulation on the GnRH-LH-estradiol axis of young female rats. American Journal of Physiology. 1988;254:R616–R621. doi: 10.1152/ajpregu.1988.254.4.R616. [DOI] [PubMed] [Google Scholar]
- 42.I’Anson H, Starer CA, Bonnema KR. Glucoprivic regulation of estrous cycles in the rat. Horm Behav. 2003;43:388–93. doi: 10.1016/s0018-506x(03)00007-2. [DOI] [PubMed] [Google Scholar]
- 43.I’Anson H, Manning JM, Herbosa CG, Pelt J, Friedman CR, Wood RI, Bucholtz DC, Foster DL. Central inhibition of gonadotropin-releasing hormone secretion in the growth-restricted hypogonadotropic female sheep. Endocrinology. 2000;141:520–7. doi: 10.1210/endo.141.2.7308. [DOI] [PubMed] [Google Scholar]
- 44.Hedges VL, Chakravarty S, Nestler EJ, Meisel RL. Delta FosB overexpression in the nucleus accumbens enhances sexual reward in female Syrian hamsters. Genes Brain Behav. 2009;8:442–9. doi: 10.1111/j.1601-183X.2009.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav. 2002;76:353–64. doi: 10.1016/s0031-9384(02)00759-x. [DOI] [PubMed] [Google Scholar]
- 46.Pan Y, Berman Y, Haberny S, Meller E, Carr KD. Synthesis, protein levels, activity, and phosphorylation state of tyrosine hydroxylase in mesoaccumbens and nigrostriatal dopamine pathways of chronically food-restricted rats. Brain Res. 2006;1122:135–42. doi: 10.1016/j.brainres.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


