Abstract
Previous studies suggest that P-glycoprotein (P-gp) modulates the PK/PD of many compounds including opioid agonists and chemotherapeutic agents. The objective of this study was to assess the P-gp affinity status of oxycodone, the P-gp expression, and the paclitaxel’s tissue distribution in oxycodone-treated rats. P-gp ATPase assay, Caco-2 transepithelial permeability studies, and mdr1a/b (−/−) mice were used to assess the P-gp affinity status of oxycodone. P-gp expression was determined by Western blot analysis while [14C] paclitaxel’s distributions in the liver, kidney, brain, and plasma tissues were determined by liquid scintillation counter. Oxycodone stimulated the P-gp ATPase activity in a concentration-dependant manner. The Caco-2 secretory transport of oxycodone was reduced from 3.64 ×10−5 to 1.96 × 10−5 cm/s (p <0.05) upon preincubation with the P-gp inhibitor, verapamil. The brain levels of oxycodone in mdr1a/b (+/+) were not detectable (<15 ng/mL) while in mdr1a/b (−/−) the average levels were 115 ± 39 ng/mL. The P-gp protein levels were increased by 1.3–4.0 folds while paclitaxel’s tissue distributions were decreased by 38–90% (p <0.05) in oxycodone-treated rats. These findings display that oxycodone is a P-gp substrate, induces overexpression of P-gp, and affects paclitaxel’s tissue distribution in a manner that may influence its chemotherapeutic activity.
Keywords: P-glycoprotein, blood-brain barrier (BBB), Caco-2 cells, distribution, drug transport
INTRODUCTION
Opioid analgesics are by far the most efficacious pharmacotherapeutic agents for the management of moderate to severe pain. Opioids produce analgesia by binding to specific opioid receptors (μ, δ, and κ) both within and outside the CNS. Unfortunately, effective management of chronic cancer pain represents a therapeutic challenge since chronic administration of opioids in most cases is accompanied by undesired effects, such as respiratory depression, nausea, vomiting, constipation, tolerance, and dependence.1–3 Extensive studies have tended to focus on determining the pharmacokinetics as well as the pharmacodynamics of many opioid agonists, attributing differences among opioids to factors like binding affinity to opioid-receptors, lipophilicity, molecular weight, and blood-brain barrier (BBB) permeability.1,3 However, recent studies indicate that the affinity of opioids to P-glycoprotein (P-gp; ABCB1) is a major factor that affects the transport, uptake, and PK/PD of many opioids that are P-gp substrates.4–6
Multidrug resistance (MDR) is a phenomenon where by cells exposed to one cytotoxic agent develop cross resistance to a range of structurally and functionally unrelated compounds.7,8 In humans, the major proteins responsible for the MDR phenotype are the P-gp (ABCB1) and the multidrug resistance associated proteins (MRP; ABCC family).9,10 P-gp is a 170 KDa transmembrane protein belonging to the ATP-binding cassette family.8 P-gp is located in several tissues, including intestinal epithelial cells, the apical surface of epithelial cells of proximal tubules in kidneys, the epithelial cells of placenta, the canalicular membranes of hepatocytes, and the luminal surface of the brain capillary endothelial cells.11 P-gp is considered as an integral component of the BBB, extrudes various xenobiotics out of the brain, and thereby affects their pharmacological activity.
A convincing body of evidence suggests that P-gp modulates opioids’ transport and antinociceptive effects.4,6,12–15 The P-gp inhibitor GF120918 produced an enhancement of morphine accumulation in BBMECs.12 The antinociceptive effect of morphine was significantly enhanced (~twofold) in mdr1a (−/−) mice when compared to mdr1a (+/+) mice.6 Brain P-gp expression in Sprague Dawley rats was approximately twofold higher in morphine tolerant rats compared to saline control rats based on Western blot analysis.16 Also, the brain uptake levels of fentanyl, loperamide, meperidine, methadone, morphine, deltorphin II, DPDPE, naltrindole, SNC 121, bremazocine, and U-69593 were determined in mdr1a (+/+) and mdr1a (−/−) mice. The differences in brain uptake between P-gp competent and P-gp deficient mice ranged from no detectable effect (meperidine) to ≥ eightfold increase in uptake (DPDPE, loperamide, and SNC 121).4 These results suggest that P-gp plays a significant role in the transport, distribution, and the antinociceptive activity of many opioid agonists.
Oxycodone is an opioid agonist used for the management of moderate to severe pain. In 1996, the legal distribution of oxycodone in the United States alone reached two million grams,17 indicating the extensive use of oxycodone in pain management. Oxycodone has been used effectively for over 90 years, but unlike other opioid agonists little is known about its P-gp affinity status (substrate, inducer, or inhibitor). Chronic administration of morphine and other P-gp substrates, e.g., cyclosporin A and dexamethasone resulted in upregulation of P-gp,16,18 whether P-gp is upregulated upon repeated administration of oxycodone is yet to be elucidated. For the management of cancer, oxycodone is usually coadministered with many chemotherapeutic agents, the majority of these agents are P-gp substrates.19–22 Changes in P-gp expression upon repeated administration of oxycodone may affect the uptake, the distribution, and the potency of these chemotherapeutic agents which in turn may lead to ineffective cancer therapy. The goal of this study was to investigate the influence of multiple administration of oxycodone on the level of expression of P-gp in different tissues in Sprague Dawley rats. To examine this, the following objectives were investigated: (1) to determine if oxycodone is a P-gp substrate using in vitro (P-gp ATP assay, Caco-2 transport study) and in vivo (mdr1a/b (−/−) mice) studies, (2) to determine the expression levels of P-gp upon repeated administration of oxycodone, and (3) to examine the influence of P-gp induction in oxycodone-treated rats on the distribution of paclitaxel, a model P-gp substrate.
METHODS
Oxycodone-Stimulated P-gp ATPase Activity
Oxycodone stimulated P-gp ATPase activity was estimated by the P-gp-GIO assay system (Promega, Madison, WI). This method relies on the ATP dependence of the light-generating reaction of firefly luciferase. ATP consumption is detected as a decrease in luminescence. In a 96-well plate, recombinant human P-gp (25 μg) was incubated with P-gp-GIO assay buffer™ (Promega) (20 μL), verapamil (200 μM), sodium orthovanadate (100 μM), or oxycodone (5, 10, 200, 1000, and 2000 μM) (gift from Dr. Coop’s Laboratory, UMB). Verapamil served as a positive control while sodium orthovanadate was used as a P-gp ATPase inhibitor. In the presence of sodium orthovanadate ATP consumption by P-gp is negligible and without sodium orthovanadate, P-gp consumes ATP to a greater or lesser extent than the control, dependent on the effect of the test compound. The reaction was initiated by addition of MgATP (10 mM), stopped 40 min later by addition of 50 μL of firefly luciferase reaction mixture (ATP detection reagent) that initiated an ATP-dependent luminescence reaction. Signals were measured 60 min later, integrated for 10 s using Lmax® luminometer (Molecular Devices Corporation, Sunnyvale, CA) and converted to ATP concentrations by interpolation from a luminescent ATP standard curve. The rate of ATP consumption (pmol/min/μg protein) was determined as the difference between the amount of ATP in the absence and the presence of sodium orthovanadate.
Caco-2 Transport Study
Caco-2 transport studies were performed to examine the role of MDR and MRP on the in vitro permeability of oxycodone. Caco-2 cell lines were obtained from ATCC (Manassas, VA) and used between passages 30–50. Experimental supplies were purchased from Fisher Scientific (Fair Lawn, NJ). P-gp and MRP inhibitors were purchased from Sigma Chemical Co. (St. Louis, MO). Caco-2 cells were seeded at a density of 80000 cells/cm2 onto 12-well, 0.4 μm transwell inserts (Coaster®, Corning, NY) and grown in the presence of Dulbecco’s Modified Eagle Medium (DMEM) with 10% fetal bovine serum (FBS) for 21–28 days at 37°C, 95% humidity, and 5% CO2. Formation of tight junctions was checked by measuring the transepithelial electrical resistance (TEER) using a Millicell®-ERS meter (Millipore Corp., Bedford, MA) and by determining the permeability of the paracellular marker [14C] mannitol (0.5 μCi/mL) obtained from Sigma Chemical Co. Only cell monolayers having TEER values >300 Ω.cm2 were used. The mannitol permeability through three representative transwells was <7.20 ×10−6 cm/s. TEER values >300 Ω.cm2 and mannitol permeability of 7.20 ×10−6 cm/s indicated tight junction formation in these cell monolayers. The functional activity of the P-gp expressed in Caco-2 cells was determined by evaluating the bidirectional transport of the P-gp substrate, [14C] paclitaxel (0.5 μCi/mL), obtained from Sigma Chemical Co. Paclitaxel’s efflux ratio determined in representative transwells (n = 6) was 10.5 indicating expression of functional P-gp in these cell monolayers. Transepithelial permeability studies for oxycodone (1000 μM) were conducted from apical to basolateral side (A–B) and basolateral to apical side (B–A) for 120 min with and without preincubation with P-gp inhibitor (100 μM verapamil) or MRP inhibitor (100 μM probenecid) for 45 min at 37°C. Oxycodone samples were collected from the receiver compartment and analyzed using a validated UV–HPLC method. Radioactive mannitol and paclitaxel samples were analyzed using Beckman Coulter LS 6500 (Fullerton, CA) multipurpose scintillation counter.
Experimental Animals
Male FVB mdr1a/b (+/+) and mdr1a/b (−/−) mice weighing 25 ± 5 g were purchased from Taconic Laboratories (Germantown, NY). Male Sprague Dawley rats weighing 275 ± 15 g were purchased from Harlan Laboratories (Indianapolis, IN). The animals were housed individually and allowed to acclimate at least 2 days before the experiment was conducted. They were fed chow and water “ad libitum” and maintained on a 12-h light/dark cycle. The protocol for the animal studies was approved by the School of Pharmacy, University of Maryland IACUC.
CNS Distribution of Oxycodone in mdr1a/b (−/−) and Wild Type Mice After Multiple Dosing
In vivo studies were performed in P-gp deficient and P-gp competent mice to determine the P-gp affinity status of oxycodone and to elucidate the influence of P-gp on the brain uptake of oxycodone. Oxycodone (5 mg/kg b.i.d. × 6 days) was administered to male mdr1a/b (−/−) (n = 5) and male mdr1a/b (+/+) mice (n = 5) via i.p. injection. On day 6, upon tolerance development, oxycodone-treated mice were decapitated and both plasma and brain tissues were collected and stored at −80°C till analyzed by a validated HPLC method.23
Monitoring of the Antinociceptive Effect After Oxycodone Administration
To determine the appropriate sampling schedule to be used in the multiple oxycodone administration studies, male Sprague Dawley rats (n = 6) were administered an i.p. dose of 5 mg/kg oxycodone. This dose was selected based on previous oxycodone studies.24,25 Antinociceptive effect was monitored at 5, 15, 30, 60, 90, and 120 min postdosing using the hot plate analgesia meter (UGO BASILE Instrument, Varese, Italy).26 Responses were measured in triplicate after placing rats on a 55°C hot plate. Baseline was determined and occurred within 9–13 s. A cutoff time of 35 s was selected to prevent tissue damage. Response latency to the first hind-paw response was recorded. The hind-paw response may be either a paw lick or a foot shake, whichever occurs first. Rats that fail to respond within the respective cut-off times were defined as “analgesic.”
Sprague Dawley rats were assigned to one of two groups administered either oxycodone 5 mg/ kg i.p. dose twice daily (n = 6) or saline 1 mL/kg i.p. twice daily (n = 6) for 8 days. Antinociceptive effect was measured each day 30 min after administration of the first dose by means of the hot plate analgesia meter as described above.
Determination of the Level of Expression of P-gp by Western Blot Analysis After Repeated Oxycodone Administration
To determine whether repeated oxycodone administration is accompanied by changes in P-gp expression levels, Western blot analysis was performed. Oxycodone- and saline-treated rats were sacrificed by decapitation and intestine, liver, kidney, and brain tissues were isolated and homogenized according to the procedure described elsewhere.16 Briefly, tissues were removed, rinsed in 4°C isotonic phosphate buffered saline, and homogenized in hypotonic lysis buffer (10 mM Tris HCl, 10 mM NaCl, 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 1 mM phenylmethylsulphonyl fluoride, pH 7.4). Tissue homogenates were centrifuged at 3000×g (4°C) for 15 min to remove nuclear debris and particulate matter. The supernatants were collected and centrifuged at 40000×g (4°C) for 30 min and the resulting pellets were resuspended in 1 mL of lysis buffer each. The protein concentration was determined using DC protein assay kit (Bio-Rad, Hercules, CA). Bovine serum albumin (BSA) was used as the standard and P-gp levels were quantitated by Western blot analysis.
Plasma membrane preparations (n = 6/tissue/ group) were resuspended in a sample buffer. Aliquots (20 μL) containing 60 μg of pooled protein were loaded onto a 10% SDS–polyacrylamide gel. Electrophoresis was conducted for 2 h at 30 mA. Following electrophoresis, the samples were transferred at 25 V (constant) and 160 mA (initial) for 2 h onto polyvinylidene membrane (PVDF). Nonspecific binding sites were blocked overnight with 5% nonfat dry milk in TBS-Tween 0.1%. PVDF membrane was incubated with P-gp C219 antibody (1:1500) (ID Laboratory, ON, Canada) for 1 h, then the membrane was washed with TBS-T (3 × I5 min) and incubated with a horseradish peroxidase-linked secondary antibody (1:10000) (KPL Laboratories, Gaithersburg, MD) for 1 h followed by rinsing with TBS-T (3 × 15 min). β-actin was detected using monoclonal β-actin antibody (Sigma Chemical Co). Detection was made using ECL reagents (Amersham, Piscataway, NJ). P-gp band intensity was quantified by densitometric analysis software and normalized to β-actin band intensity.
Distribution of a Model P-gp Substrate, Paclitaxel, in Oxycodone-Treated Rats
To determine the functionality of the upregulated P-gp in oxycodone-treated rats, the distribution of paclitaxel, a model P-gp substrate,27,28 was determined in the brain, liver, kidney, and plasma samples. Sprague Dawley rats were assigned to one of two groups (n = 5) for one of the following regimens: oxycodone 5 mg/kg i.p. twice daily for 8 days or saline 1 mL/kg i.p. twice daily for 8 days. On day 9, all rats in both groups received a single i.p. dose of [14C] paclitaxel (30 μCi/kg) (0.5 mg/kg). Sixty minutes later, rats were sacrificed by CO2 asphyxiation and the brain, liver, kidney, and plasma samples were collected and analyzed for [14C] paclitaxel using Beckman multipurpose scintillation counter.
UV-HPLC and LC/MS Analysis of Oxycodone in Various Biological Matrices
Oxycodone samples obtained from the in vitro transport studies were analyzed using HPLC with UV detection. The chromatographic system included the following: (1) Waters 510 solvent delivery system (Waters-Millipore, Milford, MA), (2) Waters 717 autosampler, (3) Waters 486 Ultraviolet detector (λ = 285 nm), (4) 3390A Hewlett Packard Integrator Plotter (Hewlett Packard, Avondale, PA), and (5) Waters Symmetry SB C-18 (4.6 × 150 mm) (Waters-Millipore). The mobile phase was prepared using 70% methanol and 30% 50 mM phosphate buffer (pH 7.4). The mobile phase was delivered at a flow rate of 1 mL/min and oxycodone was eluted at 2.7 min. The assay was linear (r ≥ 0.999) over the tested concentrations (0.5–50 μg/mL).
Oxycodone samples obtained from in vivo studies were analyzed using LC/MS detection. Oxycodone brain and plasma concentrations were determined using a previously described solid-phase extraction (SPE) method.23 Briefly, brain tissues were homogenized with a fivefold volume (w/v) of 0.1 M perchloric acid and centrifuged at 3000 rpm for 20 min. The clear supernatants were transferred to another tube and centrifuged again for 20 min. One hundred microliter of the brain homogenate supernatant or 100 μL of the plasma were subjected to SPE as follows: Vac C18, 3 cc (300 mg) SPE columns (Waters-Millipore) were placed on a vacuum manifold (PrepTorr) and preconditioned with 5 mL methanol, 3 mL of 0.1 M phosphoric acid:acetonitrile (9:1), 5 mL of deionized water, and then the columns were briefly dried under vacuum. Standard samples were prepared combining 100 μL brain supernatant or plasma, internal standard (codeine 50 ng/mL), and varying concentrations of oxycodone (0–1200 ng/mL). Samples were then diluted to 3 mL with 0.5 M ammonium sulfate (pH 9.5). Mice samples from treated rats were prepared analogously without the addition of oxycodone. Samples (3 mL) were drawn through the SPE columns, following which the columns were washed with 20 mL of 5 mM ammonium sulfate (pH 9.5), 0.5 mL of water, and 0.1 mL of methanol. Oxycodone and codeine were eluted from the column with 6 mL of methanol. The collected elute was evaporated under a stream of nitrogen at 45°C to a white residue, reconstituted in 150 μL of mobile phase, transferred to a centrifugal filter (Millipore Corp.), and filtered at 12500 rpm over 20 min. Ten microliter of clear filtrate was subjected to LC/MS analysis. Liquid chromatography conditions were optimized through modification of a previously described method.29 The analytical system consisted of a (1) Finnigan MAT Spectra System coupled to a Finnigan LCQ quadrupole ion trap mass spectrometer, (2) Zorbax SB C-18 (2.1 × 50 mm) (Agilent, Palo Alto, CA), and (3) the mobile phase consisted of acetonitrile:water (0.1% acetic acid) 15:85, while the column temperature was maintained at 60°C, and the flow rate was maintained at 0.2 mL/min. The ions monitored included MH+ 316.4 for oxycodone and MH+ 300.4 for codeine. The assay was linear (r ≥ 0.999) over the tested concentrations (15–1200 ng/mL). The mass retention times for the ions corresponding to codeine and oxycodone were approximately 1.8 and 2.3 min, respectively.
Analysis of Radiolabelled Paclitaxel
Brain, liver, and kidney tissues were weighed and homogenized with an equal volume (1 g/1 mL) of PBS. Tissue solubilizer, Solvable®, Perkin-Elmer, Waltham, MA (1 mL) was added to the tissue homogenate (200 μL), then incubated at 50°C for 3 h. Glacial acetic acid (50 μL) was added to the homogenate and incubated at 50°C for 1 h for decolorization and then left to cool down to room temperature. Ultima Gold scintillation cocktail (10 mL) was then added to the homogenate and kept at room temp for 1 h before counting by Beckman Coulter LS 6500 multipurpose scintillation counter. For plasma samples, 100 μL of plasma was added directly to 10 mL of Ultima Gold scintillation cocktail and then analyzed as described above.
Data Analysis
ATPase Assay
Basal P-gp activity, test compound stimulated P-gp activity, and fold stimulation by a test compound were calculated according to the following equations:
Basal P-gp activity (pmol ATP consumed/μg P-gp/min)
Test compound stimulated P-gp activity (pmol ATP consumed/μg P-gp/min)
where ATPvanadate is the number of nonconsumed (total) pmols of ATP in the presence of sodium orthovanadate. ATPcontrol is the number of nonconsumed pmols of ATP in the presence of the assay buffer. ATPcompound is the number of nonconsumed pmols of ATP in the presence of a test compound.
Transport Study
The calculation of the apparent permeability, Papp, for transport studies across cell monolayers was determined from the following equation,
where Papp is the apparent permeability, Vr is the receiver compartment volume, dCr is the change in the receiver compartment concentration with time, A is the area of the filter, and Cd is the initial donor compartment concentration. The efflux ratio, ER, for MDR substrates with/without the P-gp/MRP inhibitors was calculated by the formula,
Antinociceptive Study
The hot plate latency values were converted to percentages of the maximum possible effect (%MPE) and plotted against time.24,25
All data were presented as mean ± SEM. Student’s t-test, analysis of variance (ANOVA) with repeated measures, or ANOVA followed by either Dunnett’s or Bonferroni’s posthoc tests (SigmaStat™ V2.03 statistical package, San Jose, CA), where appropriate, were used to determine the statistical significance between groups. SEM of ratios was calculated by the delta method.30 The 0.05 level of probability was used as the criterion of significance.
RESULTS
Effect of Oxycodone on P-gp ATPase Activity
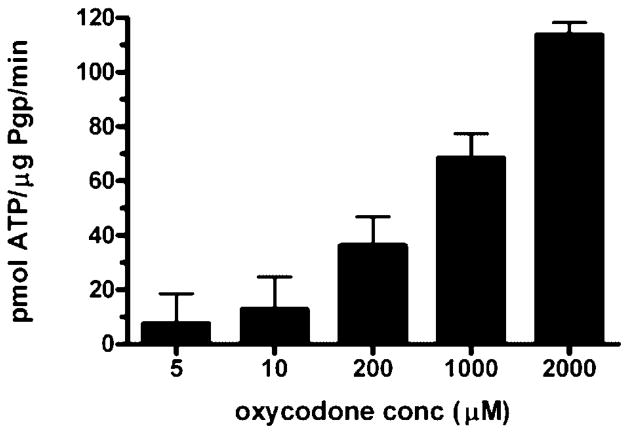
Wide range of oxycodone concentrations were examined for their effects on P-gp ATPase activity. Each oxycodone concentration together with a known excess of ATP was incubated with recombinant human P-gp for 40 min. ATP consumption was detected as a decrease in luminescence i.e., the higher the stimulation of the P-gp ATPase activity, the lower the luminescence signal. The rate of ATP consumption increased in a concentration-dependant manner and ranged from 7.60 to 113.74 pmol ATP/μg P-gp/min (Fig. 1). At lower concentrations of oxycodone, 5 μM and 10 μM, the rates of ATP consumption were not significantly different ( p >0.05) from the control (drug-free membranes). However, at higher concentrations of oxycodone 200 μM, 1000 μM, and 2000 μM, the rate of ATP consumption was significantly different (p <0.05) from the control. The known P-gp substrate, verapamil (200 μM), stimulated the rate of ATP consumption by 4.23 fold and consumed ATP at the rate of 65.73 ± 2.96 pmol ATP/μg P-gp/min (p <0.05) indicating that oxycodone at higher concentrations is capable of stimulating the P-gp ATPase activity at rates higher than that of verapamil, the model P-gp substrate.
Figure 1.
Rate of ATP consumption by different oxycodone concentrations. Recombinant human P-gp were incubated with P-gp-GIO assay buffer™ (20 μL) (control), verapamil (200 μM) (positive control), sodium orthovanadate (100 μM) (P-gp inhibitor), and oxycodone (5 μM, 10 μM, 200 μM, 1000 μM, and 2000 μM). Verapamil stimulated the basal activity by 4.23-fold and consumed ATP at a rate of 65.73 ±2.96 pmol ATP/μg P-gp/min. Data are expressed as the mean ± SEM (n = 3–4).
Transepithelial Transport Studies for Oxycodone
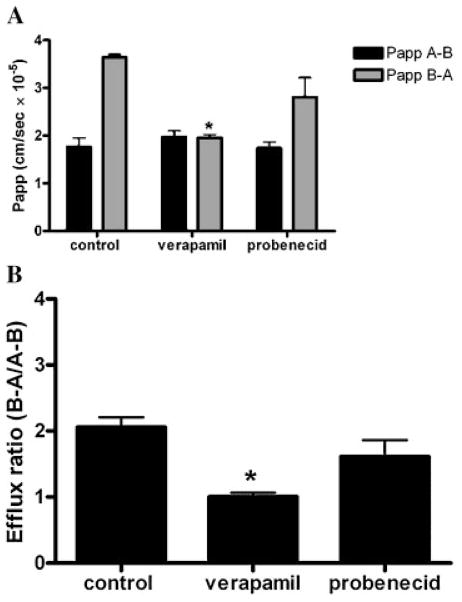
To assess the role of P-gp and MRP on the transport of oxycodone across Caco-2 monolayers, bidirectional transport studies from apical to basolateral side and basolateral to apical side were conducted for 120 min in the absence and presence of P-gp inhibitor (100 μM verapamil) or MRP inhibitor (100 μM probenecid). Figure 2A presents the apparent permeability of oxycodone in the absence and presence of P-gp and MRP inhibitors. Absorptive transport of oxycodone was not altered significantly in the presence of P-gp or MRP inhibitors. Apparent permeabilities (Papp A–B) were 1.77 × 10−5, 1.97 × 10−5, and 1.73 × 10−5 cm/s for oxycodone alone, oxycodone in the presence of 100 μM verapamil, and oxycodone in the presence of 100 μM probenecid, respectively. Secretory transport of oxycodone from (B–A) was reduced significantly in the presence of verapamil (p <0.05) but not in the presence of probenecid. Apparent permeabilities (Papp B–A) were 3.64 × 10−5, 1.96 × 10−5 (p <0.05), and 2.80 ×10−5 cm/s for oxycodone alone and oxycodone in the presence of either 100 μM verapamil or 100 μM probenecid, respectively. Efflux ratios of oxycodone were 2.06 ± 0.15, 1.01 ± 0.06 (p <0.05), and 1.62 ± 0.25 (p >0.05) for oxycodone alone, oxycodone plus 100 μM verapamil, and oxycodone plus 100 μM probenecid, respectively (Fig. 2B).
Figure 2.
(A) Transepithelial permeabilities of oxycodone in the presence and absence of P-gp inhibitor (100 μM verapamil, or MRP inhibitor (100 μM probenecid) (black columns) apical to basolateral permeability (A–B), (gray columns) basolateral to apical permeability (B–A). (B) Efflux ratios for oxycodone alone, oxycodone plus 100 μM verapamil, and oxycodone plus 100 μM probenecid. Data are expressed as the mean ± SEM (n = 3). * indicates significant difference from the control (p <0.05).
CNS Distribution of Oxycodone in mdr1a/b (−/−) and Wild Type Mice After Repeated Administration
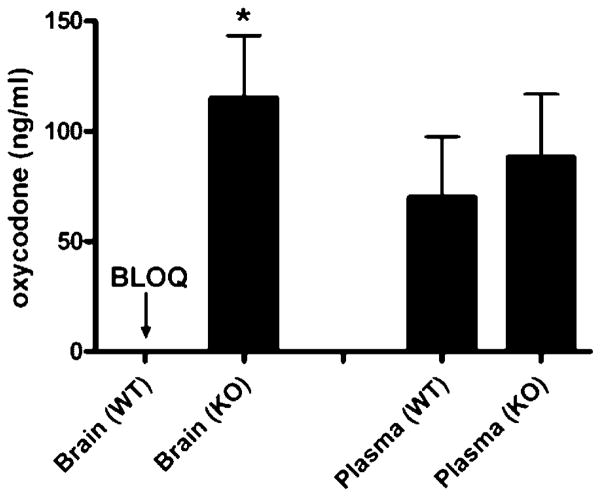
To determine the P-gp affinity status of oxycodone and the influence of P-gp on the brain distribution of oxycodone after repeated administration, in vivo studies were conducted using mdr1a/b (−/−) and mdr1a/b (+/+) mice. After 6 days of repeated oxycodone administration, mice were sacrificed and oxycodone brain levels were determined in both groups. We observed a significant (p <0.05) difference in the brain plasma levels of oxycodone in mdr1a/b (−/−) mice (115 ± 39 ng/mL) versus mdr1a/b (+/+) mice (levels below quantitation limit <15 ng/mL) (Fig. 3). In addition, the oxycodone plasma levels were not significantly different (p >0.05) between the two groups, mdr1a/b (−/−) mice (88.33 ± 28.48 ng/mL) versus mdr1a/b (+/+) mice (70 ± 27.5 ng/mL) (Fig. 3).
Figure 3.
Oxycodone concentration in brain and plasma tissues of wild type (WT) and P-gp knockout (KO) mice that received i.p. dose of 5 mg/kg oxycodone twice daily for 6 days. (BLOQ: below limit of quantitation <15 ng/mL). Data are expressed as the mean ± SEM (n = 3–5). 3 indicates significant difference at (p <0.05).
Multiple Dose Administration of Oxycodone and Tolerance Development
After single i.p. dose of oxycodone (5 mg/kg), the antinociceptive response was monitored by hot plate analgesia meter for 120 min. The antinociceptive effect peaked at 30 min with 99.4 %MPE (data not shown). As a result, the 30 min time point was selected to monitor the latency responses in the multiple oxycodone administration study. Rats treated with oxycodone developed tolerance after 8 days of treatment. On day 1, the %MPE was 90.9% (p <0.05) and decreased gradually to 2.9% (p >0.05) below baseline on day 8, indicating development of tolerance to the analgesic effect of oxycodone (Fig. 4A).
Figure 4.
(A) Hot plate latencies expressed as %MPE versus time for Sprague Dawley rats that received i.p. doses of 5 mg/kg oxycodone (○) or saline (●) twice daily for 8 days. Data are expressed as mean ± SEM (n = 6). (B) Representative immunodetection of P-gp (170 KDa) in liver, kidney, brain, and intestine tissues of Sprague Dawley rats that were subjected to 8 days of (S) saline 1 mL/kg or (O) oxycodone (5 mg/kg) treatment. Aliquots (20 μL) containing 60 μg of protein were loaded onto a 10% SDS–polyacrylamide gel. Electrophoresis was conducted for 2 h at 30 mA. Samples were then transferred onto PVDF membrane, incubated with P-gp C219 antibody (1:1500) for 1 h, then the membrane was washed and incubated with a horseradish peroxidase-linked secondary antibody. Detection was made using ECL reagents and P-gp band intensity was quantified by densitometric analysis software. 3 indicates significant difference at (p <0.05).
P-gp Induction in Sprague Dawley Rats
Figure 4B is a representative immunoblot of P-gp detected in the intestine, liver, kidney, and brain tissues of both saline and oxycodone-treated rats. The P-gp monoclonal antibody mAB C219 recognized a protein of approximately 170 kDa, corresponding to P-gp. Densitometric quantitation of the P-gp bands indicated that there was an upregulation of P-gp in all four tissues, where P-gp was induced in oxycodone-treated rats by approximately 2.0-fold, 4.0-fold, 1.6-fold, and 1.3-fold in the intestine, liver, kidney, and brain tissue, respectively.
Distribution of a Model P-gp Substrate, Paclitaxel, in Oxycodone-Treated Rats
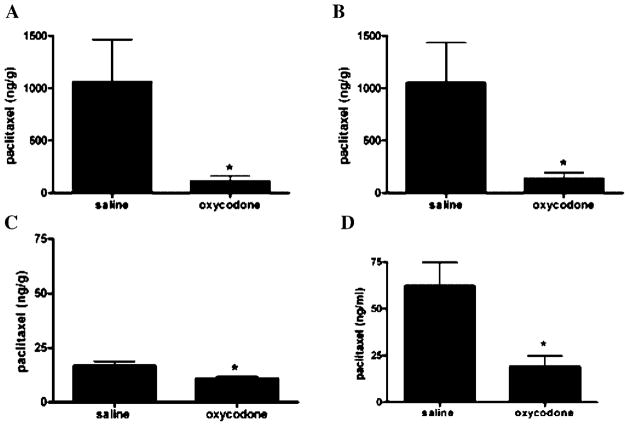
To determine if the increase in P-gp expression observed modulates the in vivo function of P-gp, the liver, kidney, brain, and plasma distribution of paclitaxel was evaluated in oxycodone tolerant and control rats. Upregulation of P-gp in the liver, kidney, and brain of oxycodone-treated rats significantly (p <0.05) hindered the accumulation of paclitaxel in these tissues. The levels of paclitaxel were decreased by 90% (p <0.05) in liver (Fig. 5A), 87% (p <0.05) in kidney (Fig. 5B), 38% (p <0.05) in brain (Fig. 5C), and 70% (p <0.05) in plasma (Fig. 5D), 60 min after dosing with the chemotherapeutic agent. The tissues to plasma ratios for saline-treated rats were 17.07, 16.91, 0.27; however, for the oxycodone-treated rats the ratios were 5.79, 7.14, and 0.57 for liver, kidney, and brain tissues, respectively. These results indicate that the upregulation of P-gp upon repeated oxycodone treatment adversely affects the distribution of paclitaxel (P-gp substrate) in tissues expressing this efflux transporter.
Figure 5.
Paclitaxel concentration in (A) liver, (B) kidney, (C) brain, and (D) plasma samples of Sprague Dawley rats that were sacrificed 60 min after a single i.p. dose of [14C] paclitaxel (30 μCi/kg) (0.5 mg/kg). Rats received a pretreatment of either oxycodone (5 mg/kg) or saline (1 mL/kg) twice daily for 8 days. Data are expressed as the mean ± SEM (n = 5). * indicates significant difference at (p <0.05).
DISCUSSION
Oxycodone has been used effectively for pain management but little is known about its P-gp affinity status. In addition, there are no data reported on the level of expression of P-gp upon repeated oxycodone administration. Changes in the level of P-gp expression may modulate the pharmacokinetics as well as the pharmacodynamics of the concomitantly administered therapeutic agents that are P-gp substrates. The present study was designed to investigate the P-gp affinity status of oxycodone, the influence of repeated oxycodone administration on the level of expression of P-gp, and the tissue distribution of paclitaxel, the well-known P-gp substrate in oxycodone-treated rats.
Many methods have been used to evaluate the P-gp affinity status of different compounds. These methods are either in vitro assays, e.g., P-gp ATPase activity assay, and monolayer efflux assay, and calcein-AM fluorescence assay,31 or in vivo assays involving transgenic P-gp knockout mice.4 Classification of opioids or any other compounds as P-gp substrates or inhibitors based on only one of the aforementioned methods may be misleading.31 As a result, both in vitro and in vivo approaches were used herein to evaluate the P-gp affinity status of oxycodone. For the in vitro studies, both the P-gp ATPase assay and the monolayer efflux assay were performed. The P-gp ATPase assay indicated that the tested oxycodone concentrations stimulated the P-gp ATPase activity in a concentration-dependant manner suggesting that oxycodone is most likely a P-gp substrate. At low concentrations the rate of ATP consumption was not significantly different from the drug-free control (basal activity; p >0.05); however at higher concentrations ≥ 200 μM, dramatic increases in the rates of ATP consumption were observed (p <0.05) (Fig. 1). This biphasic response for P-gp ATPase activation observed for oxycodone has also been reported for a variety of compounds, e.g., promethazine, propafenone, quinidine, dipridamole, and digoxin, and indicates that these compounds require threshold concentrations for measurable ATPase activation.32,33 It should be noted that certain compounds stimulate P-gp ATPase activity, but are not transported by P-gp across cell membranes.31 For this reason, a series of bidirectional transport studies were conducted using Caco-2 cells, a cell line known to express both P-gp and MRP.34,35 These studies were performed to determine whether the permeabilities of oxycodone were modulated in the presence of P-gp or MRP inhibitors. Our results showed that absorptive transport from (A–B) was not significantly altered in the presence of either P-gp or MRP inhibitors (Fig. 2A). However, the secretory transport of oxycodone from (B–A) was reduced by 50% (p <0.05) with the P-gp inhibitor (100 μM verapamil) but was not significantly reduced in the presence of the MRP inhibitor (100 μM probenecid) (Fig. 2A). These observations suggest that only the P-gp located in the apical layer of the Caco-2 cells affects the secretory transport of oxycodone and indicates that oxycodone is a P-gp substrate. Similarly, in a Caco-2 transport study,34 P-gp was also found to only affect the secretory transport of morphine and the authors reported that the efflux ratio of morphine was 2.07 comparable to that of oxycodone (efflux ratio = 2.06).
Although in vitro assays are commonly used to characterize the P-gp affinity status of several compounds including opioids, results based on in vitro studies alone must be evaluated with caution. Fentanyl was thought to be a P-gp inhibitor based on in vitro studies;36 however, in vivo antinociceptive studies indicated that fentanyl is a P-gp substrate.37 Quite the opposite was evident for meperidine, where in vitro studies indicated that meperidine is a P-gp substrate5 while in vivo brain uptake studies and antinociceptive studies indicated that meperidine is not a P-gp substrate.4,37 As such, it is clear that results based on one assay whether it is in vitro or in vivo must be interpreted with caution. In this regard, we expanded our investigation to elucidate the P-gp affinity status of oxycodone in P-gp deficient and P-gp competent mice. Our in vivo studies were in a good agreement with the in vitro studies’ findings and further confirmed that oxycodone is a P-gp substrate. The CNS levels of oxycodone were significantly higher (p <0.05) in the mdr1a/ b (−/−) mice (115 ± 39 ng/mL) versus nondetectable levels (<15 ng/mL) in the P-gp competent mice. The plasma levels of oxycodone were not significantly different between the two groups indicating that the higher brain uptake of oxycodone in mdr1a/b (−/−) mice is due to the lack of P-gp in the BBB. Similarly, the brain uptake of many opioid agonists, e.g., morphine, methadone, DPDPE, and loperamide were reported to be enhanced in P-gp deficient mice in comparison to P-gp competent mice.4,6,15,38
P-gp substrates such as morphine, cyclosporin A, vincristine, dexamethasone, and rhodamine 123 are known to induce the level of expression of P-gp upon chronic administration.16,18 As such, it would be expected that repeated administration of P-gp substrates that are opioid agonists will markedly increase the P-gp expression. In fact, when morphine was administered to Sprague Dawley rats, a twofold increase in brain P-gp expression was observed in morphine-treated rats when compared to saline-treated rats.16 To evaluate the possible effect of repeated oxycodone administration on the level of expression of P-gp in various tissues, Sprague Dawley rats were subjected to multiple doses of oxycodone (5 mg/kg i.p.). Western blot analysis after 8 days of treatment was associated with upregulation in the level of expression of P-gp in the intestine, liver, kidney, and brain tissues in oxycodone-treated rats when compared to saline-treated rats (Fig. 4B). The upregulation of P-gp observed in oxycodone tolerant rats in our studies and in morphine tolerant rats reported elsewhere16 may contribute to the development of tolerance. Upregulation of P-gp at the BBB may hinder oxycodone or morphine uptake into the CNS and hence minimize interaction of these agents with opioid receptors resulting in decreased antinociceptive activity. Further, we observed upregulation of P-gp in the intestine, liver, and kidney. This apparent change in P-gp expression may lead to a decrease in absorption, and an increase in the elimination of oxycodone, hence leading to a reduction in its systemic exposure and possibly contribute to tolerance development.
It should be noted that the enhanced expression of P-gp will have physiological relevance only if there is a change in the functionality of the protein, e.g., altered drug distribution or drug uptake in different tissues that express P-gp. In consideration of this point, we expanded our work to determine the influence of P-gp induction on the tissue distribution of paclitaxel, the P-gp substrate. Paclitaxel was selected for the following reasons: (1) paclitaxel is a well-known P-gp substrate28 and induction of P-gp will have an impact on its tissue distribution, and (2) oxycodone is concomitantly administered with paclitaxel (antineoplastic agent) for alleviating pain in cancer patients. There was a significant (p <0.05) decrease in paclitaxel concentration in the brain, liver, kidney, and plasma samples of oxycodone tolerant rats when compared to saline-treated rats (Fig. 5). The lower level of paclitaxel observed in the plasma may suggest a higher rate of systemic clearance in oxycodone tolerant rats which can be attributed to the significant upregulation of P-gp in both liver and kidney. P-gp is expressed in the canalicular membranes of the hepatocytes and the apical surface of epithelial cells of the proximal tubules in the kidney. An enhanced expression of P-gp at these sites facilitates the elimination of P-gp substrates, e.g., paclitaxel.8 Our results suggest that repeated administration of oxycodone not only causes upregulation of P-gp, but also significantly affects the transport of paclitaxel across the BBB, liver, and kidney. These findings have potentially important implications on the therapeutic activity of paclitaxel especially in the treatment of brain, hepatic and renal tumors19–21,39,40 when coadministered with oxycodone. It should be noted that oxycodone levels would have been quantified in the oxycodone tolerant animals if radiolabelled oxycodone was available. The evaluation of nonlabeled oxycodone would yield confounding results to which conclusions concerning the functionality of P-gp expression on oxycodone tissue distribution could not be determined. Hence, we used a known P-gp substrate, paclitaxel to display the functional changes in P-gp in oxycodone tolerant rats.
Both our in vitro and in vivo studies are supportive of oxycodone’s status as a P-gp substrate. The P-gp inhibitor, PSC833 (cyclosporin A analogue), was reported to have no effect on the brain disposition of oxycodone in Sprague Dawley rats.23 PSC833 although known to be a potent inhibitor for the efflux transporter, P-gp, lacks specificity, as it is also a potent inhibitor for the influx transporter, Oatp2 located in the BBB.41,42 In addition, the authors tested only a single, very low dose of oxycodone (0.3 mg/kg; 1-h i.v. infusion). The limitations of defining substrate status based on a low dose are illustrated in a study involving the administration of three different doses of morphine (1, 3, 5 mg/kg) to P-gp deficient and P-gp competent mice.37 In this study, the authors concluded that morphine was a substrate for P-gp only at the higher doses (3 and 5 mg/kg) and not at the low dose (1 mg/kg).37 The 3 and 5 mg/kg morphine doses resulted in significant increases in the AUECs for the P-gp deficient mice versus the P-gp competent mice. However, no significant difference in AUEC was observed for the two groups after the 1 mg/kg dose. If this study was limited to the low dose (1 mg/kg), morphine would not have been considered as a P-gp substrate. Even our own in vitro P-gp ATPase data indicated that oxycodone behaves as a P-gp substrate only at higher concentrations.
In general, for a compound to be considered a P-gp substrate, it should exhibit one or more of the following characteristics: (1) has an efflux ratio more than 1.5 that can be decreased to 1 by P-gp inhibitors,31 (2) shows significantly higher accumulation in brain or other tissues of mdr1a/b (−/−) mice in comparison to mdr1a/b (+/+) mice,6,15,43 or (3) results in upregulation of P-gp upon multiple administration.16,18 In this study, oxycodone had an efflux ratio of 2.06 that was decreased to 1.01 by verapamil, and significantly higher oxycodone brain levels were observed for mdr1a/b (−/−) versus mdr1a/b (+/+) mice. Finally repeated administration of oxycodone resulted in upregulation of P-gp and also affected the accumulation of the well-known P-gp substrate, paclitaxel in the liver, kidney, and brain tissues of oxycodone tolerant rats.
Recently, Bostrom et al., (2006)23 reported that oxycodone has a net steady state flux between brain and plasma above unity and concluded that the brain uptake of oxycodone is mediated by an unknown influx transporter. Although possible, these results can also be explained by the relatively high passive permeability of oxycodone (177 nm/s, Fig. 2A) that is more than the suggested critical values for CNS distribution (>150 nm/s).44 In addition, many compounds were shown to have high affinity to P-gp while maintaining brain to plasma ratios above unity. In our laboratory, many CNS acting compounds, e.g., cocaine and benzotropine analogs were found to be P-gp substrates (efflux ratio = 4.2–8.0) and have flux values more than unity (2.0–6.6).45
In summary, these findings display that oxycodone is a P-gp substrate and that repeated treatment with oxycodone produces an induction of P-gp expression that significantly minimizes the distribution of paclitaxel into the liver, the kidney, and the CNS. This upregulation of P-gp can lead to a time-dependant exclusion of oxycodone from the brain, affecting oxycodone brain accumulation, leading to subtherapeutic levels in the CNS, and thus can contribute to tolerance development to its analgesic effect. These findings can also in part explain cross tolerance development among oxycodone and possibly other opioid agonists that are P-gp substrates, e.g., morphine and methadone. Where, tolerance development to oxycodone can cause upregulation of P-gp that in turn will hinder other opioid agonists from reaching the needed therapeutic levels. Further studies involving drug–drug interactions and cross tolerance studies between oxycodone and other opioid agonists are warranted.
Acknowledgments
This study was supported in part by University of Maryland Intramural Research grant, a Predoctoral Fellowship from the Egyptian Ministry of Higher Education (H.E.H.). Part of this study was selected to receive the Eli Lilly PPDM Graduate Student Award H.E.H. at the Annual Meeting of the American Association of Pharmaceutical Scientists (AAPS), San Antonio, Texas, October 29th–November 2nd, 2006.
Abbreviations used
- MDR
multidrug resistance proteins
- P-gp
P-glycoprotein
- MRP
multidrug resistance-associated proteins
- Caco-2
epithelial human colon adenocarcinoma cell line
- %MPE
percentage of maximal possible effect
- AUEC
area under the percentage maximum possible effect versus time curve
- BBMECs
bovine brain microvessel endothelial cells
References
- 1.Smith H. Drugs for Pain. 1. Philadelphia: Hanely and Belfus; 2003. pp. 83–155. [Google Scholar]
- 2.Stimmel B. Pain and Its Relief Without Addiction. 2. New York: The Haworth Medical Press; 1997. pp. 123–172. [Google Scholar]
- 3.Ronold M, Patrick W. Hand Book of Pain Managment. 1. New York: Churchill Livingstone; 2003. pp. 377–396. [Google Scholar]
- 4.Dagenais C, Graff CL, Pollack GM. Variable modulation of opioid brain uptake by P-glycoprotein in mice. Biochem Pharmacol. 2004;67:269–276. doi: 10.1016/j.bcp.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 5.Callaghan R, Riordan JR. Synthetic and natural opiates interact with P-glycoprotein in multidrug-resistant cells. J Biol Chem. 1993;268:16059–16064. [PubMed] [Google Scholar]
- 6.Zong J, Pollack GM. Morphine antinociception is enhanced in mdr1a gene-deficient mice. Pharm Res. 2000;17:749–753. doi: 10.1023/a:1007546719287. [DOI] [PubMed] [Google Scholar]
- 7.Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 8.Pastan I, Gottesman MM. Multidrug resistance. Annu Rev Med. 1991;42:277–286. doi: 10.1146/annurev.me.42.020191.001425. [DOI] [PubMed] [Google Scholar]
- 9.Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: The multidrug resistance-associated proteins. J Natl Cancer Inst. 2000;92:1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- 10.Zaman GJ, Flens MJ, van Leusden MR, de Haas M, Mulder HS, Lankelma J, Pinedo HM, Scheper RJ, Baas F, Broxterman HJ, Borst P. The human multidrug resistance-associated protein MRP is a plasma membrane drug-efflux pump. Proc Natl Acad Sci U S A. 1994;91:8822–8826. doi: 10.1073/pnas.91.19.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kharasch ED, Hoffer C, Whittington D, Sheffels P. Role of P-glycoprotein in the intestinal absorption and clinical effects of morphine. Clin Pharmacol Ther. 2003;74:543–554. doi: 10.1016/j.clpt.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Letrent SP, Polli JW, Humphreys JE, Pollack GM, Brouwer KR, Brouwer KL. P-glycoprotein-mediated transport of morphine in brain capillary endothelial cells. Biochem Pharmacol. 1999;58:951–957. doi: 10.1016/s0006-2952(99)00180-x. [DOI] [PubMed] [Google Scholar]
- 13.Letrent SP, Pollack GM, Brouwer KR, Brouwer KL. Effects of a potent and specific P-glycoprotein inhibitor on the blood-brain barrier distribution and antinociceptive effect of morphine in the rat. Drug Metab Dispos. 1999;27:827–834. [PubMed] [Google Scholar]
- 14.Letrent SP, Pollack GM, Brouwer KR, Brouwer KL. Effect of GF120918, a potent P-glycoprotein inhibitor, on morphine pharmacokinetics and pharmacodynamics in the rat. Pharm Res. 1998;15:599–605. doi: 10.1023/a:1011938112599. [DOI] [PubMed] [Google Scholar]
- 15.Dagenais C, Zong J, Ducharme J, Pollack GM. Effect of mdr1a P-glycoprotein gene disruption, gender, and substrate concentration on brain uptake of selected compounds. Pharm Res. 2001;18:957–963. doi: 10.1023/a:1010984110732. [DOI] [PubMed] [Google Scholar]
- 16.Aquilante CL, Letrent SP, Pollack GM, Brouwer KL. Increased brain P-glycoprotein in morphine tolerant rats. Life Sci. 2000;66:PL47–PL51. doi: 10.1016/s0024-3205(99)00599-8. [DOI] [PubMed] [Google Scholar]
- 17.Joranson DE, Ryan KM, Gilson AM, Dahl JL. Trends in medical use and abuse of opioid analgesics. Jama. 2000;283:1710–1714. doi: 10.1001/jama.283.13.1710. [DOI] [PubMed] [Google Scholar]
- 18.Jette L, Beaulieu E, Leclerc JM, Beliveau R. Cyclosporin A treatment induces overexpression of P-glycoprotein in the kidney and other tissues. Am J Physiol. 1996;270:F756–F765. doi: 10.1152/ajprenal.1996.270.5.F756. [DOI] [PubMed] [Google Scholar]
- 19.Fellner S, Bauer B, Miller DS, Schaffrik M, Fankhanel M, Spruss T, Bernhardt G, Graeff C, Farber L, Gschaidmeier H, Buschauer A, Fricker G. Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J Clin Invest. 2002;110:1309–1318. doi: 10.1172/JCI15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glantz MJ, Choy H, Kearns CM, Mills PC, Wahlberg LU, Zuhowski EG, Calabresi P, Egorin MJ. Paclitaxel disposition in plasma and central nervous systems of humans and rats with brain tumors. J Natl Cancer Inst. 1995;87:1077–1081. doi: 10.1093/jnci/87.14.1077. [DOI] [PubMed] [Google Scholar]
- 21.Heimans JJ, Vermorken JB, Wolbers JG, Eeltink CM, Meijer OW, Taphoorn MJ, Beijnen JH. Paclitaxel (Taxol) concentrations in brain tumor tissue. Ann Oncol. 1994;5:951–953. doi: 10.1093/oxfordjournals.annonc.a058736. [DOI] [PubMed] [Google Scholar]
- 22.Loscher W, Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol. 2005;76:22–76. doi: 10.1016/j.pneurobio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Bostrom E, Simonsson US, Hammarlund-Udenaes M. Oxycodone pharmacokinetics and pharmacodynamics in the rat in the presence of the P-glycoprotein inhibitor P SC833. J Pharm Sci. 2005;94:1060–1066. doi: 10.1002/jps.20327. [DOI] [PubMed] [Google Scholar]
- 24.Ross FB, Wallis SC, Smith MT. Co-administration of sub-antinociceptive doses of oxycodone and morphine produces marked antinociceptive synergy with reduced CNS side-effects in rats. Pain. 2000;84:421–428. doi: 10.1016/s0304-3959(99)00230-4. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen CK, Ross FB, Smith MT. Incomplete, asymmetric, and route-dependent cross-tolerance between oxycodone and morphine in the Dark Agouti rat. J Pharmacol Exp Ther. 2000;295:91–99. [PubMed] [Google Scholar]
- 26.Woofle G, Macdonald A. The evaluation of the analgesic action of pethidine hydrochloride (demerol) J Pharmcol Exp Ther. 1944;80:300–307. [Google Scholar]
- 27.Gallo JM, Li S, Guo P, Reed K, Ma J. The effect of P-glycoprotein on paclitaxel brain and brain tumor distribution in mice. Cancer Res. 2003;63:5114–5117. [PubMed] [Google Scholar]
- 28.Kemper EM, Cleypool C, Boogerd W, Beijnen JH, van Tellingen O. The influence of the P-glycoprotein inhibitor zosuquidar trihydrochloride (LY335979) on the brain penetration of paclitaxel in mice. Cancer Chemother Pharmacol. 2004;53:173–178. doi: 10.1007/s00280-003-0720-y. [DOI] [PubMed] [Google Scholar]
- 29.Dawson M, Fryirs B, Kelly T, Keegan J, Mather LE. A rapid and sensitive high-performance liquid chromatography-electrospray ionization-triple quadrupole mass spectrometry method for the quantitation of oxycodone in human plasma. J Chromatogr Sci. 2002;40:40–44. doi: 10.1093/chromsci/40.1.40. [DOI] [PubMed] [Google Scholar]
- 30.Polli JE, Rekhi GS, Augsburger LL, Shah VP. Methods to compare dissolution profiles and a rationale for wide dissolution specifications for metoprolol tartrate tablets. J Pharm Sci. 1997;86:690–700. doi: 10.1021/js960473x. [DOI] [PubMed] [Google Scholar]
- 31.Polli JW, Wring SA, Humphreys JE, Huang L, Morgan JB, Webster LO, Serabjit-Singh CS. Rational use of in vitro P-glycoprotein assays in drug discovery. J Pharmacol Exp Ther. 2001;299:620–628. [PubMed] [Google Scholar]
- 32.Matsunaga T, Kose E, Yasuda S, Ise H, Ikeda U, Ohmori S. Determination of p-glycoprotein ATPase activity using luciferase. Biol Pharm Bull. 2006;29:560–564. doi: 10.1248/bpb.29.560. [DOI] [PubMed] [Google Scholar]
- 33.Litman T, Zeuthen T, Skovsgaard T, Stein WD. Structure-activity relationships of P-glyco-protein interacting drugs: Kinetic characterization of their effects on ATPase activity. Biochim Biophys Acta. 1997;1361:159–168. doi: 10.1016/s0925-4439(97)00026-4. [DOI] [PubMed] [Google Scholar]
- 34.Crowe A. The influence of P-glycoprotein on morphine transport in Caco-2 cells. Comparison with paclitaxel. Eur J Pharmacol. 2002;440:7–16. doi: 10.1016/s0014-2999(02)01366-3. [DOI] [PubMed] [Google Scholar]
- 35.Crowe A, Lemaire M. In vitro and in situ absorption of SDZ-RAD using a human intestinal cell line (Caco-2) and a single pass perfusion model in rats: Comparison with rapamycin. Pharm Res. 1998;15:1666–1672. doi: 10.1023/a:1011940108365. [DOI] [PubMed] [Google Scholar]
- 36.Wandel C, Kim R, Wood M, Wood A. Interaction of morphine, fentanyl, sufentanil, alfentanil, and loperamide with the efflux drug transporter P-glycoprotein. Anesthesiology. 2002;96:913–920. doi: 10.1097/00000542-200204000-00019. [DOI] [PubMed] [Google Scholar]
- 37.Thompson SJ, Koszdin K, Bernards CM. Opiate-induced analgesia is increased and prolonged in mice lacking P-glycoprotein. Anesthesiology. 2000;92:1392–1399. doi: 10.1097/00000542-200005000-00030. [DOI] [PubMed] [Google Scholar]
- 38.Xie R, Hammarlund-Udenaes M, de Boer AG, de Lange EC. The role of P-glycoprotein in blood-brain barrier transport of morphine: Trans-cortical microdialysis studies in mdr1a (−/−) and mdr1a (+/+) mice. Br J Pharmacol. 1999;128:563–568. doi: 10.1038/sj.bjp.0702804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bell MD. Response to paclitaxel, gemcitabine, and cisplatin in renal medullary carcinoma. Pediatr Blood Cancer. 2006;47:228. doi: 10.1002/pbc.20780. [DOI] [PubMed] [Google Scholar]
- 40.Tono T, Iwazawa T, Matsui S, Yano H, Kimura Y, Kanoh T, Ohnishi T, Nakano Y, Okamura J, Monden T. Hepatic arterial infusion of paclitaxel for liver metastasis from gastric cancer. Cancer Invest. 2004;22:550–554. doi: 10.1081/cnv-200026526. [DOI] [PubMed] [Google Scholar]
- 41.Dagenais C, Ducharme J, Pollack GM. Uptake and efflux of the peptidic delta-opioid receptor agonist. Neurosci Lett. 2001;301:155–158. doi: 10.1016/s0304-3940(01)01640-8. [DOI] [PubMed] [Google Scholar]
- 42.Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, Meier PJ. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J Pharmacol Exp Ther. 2000;294:73–79. [PubMed] [Google Scholar]
- 43.King M, Su W, Chang A, Zuckerman A, Pasternak GW. Transport of opioids from the brain to the periphery by P-glycoprotein: Peripheral actions of central drugs. Nat Neurosci. 2001;4:268–274. doi: 10.1038/85115. [DOI] [PubMed] [Google Scholar]
- 44.Mahar Doan KM, Humphreys JE, Webster LO, Wring SA, Shampine LJ, Serabjit-Singh CJ, Adkison KK, Polli JW. Passive permeability and P-glycoprotein-mediated efflux differentiate central nervous system (CNS) and non-CNS marketed drugs. J Pharmacol Exp Ther. 2002;303:1029–1037. doi: 10.1124/jpet.102.039255. [DOI] [PubMed] [Google Scholar]
- 45.Raje S, Cao J, Newman AH, Gao H, Eddington ND. Evaluation of the blood-brain barrier transport, population pharmacokinetics, and brain distribution of benztropine analogs and cocaine using in vitro and in vivo techniques. J Pharmacol Exp Ther. 2003;307:801–808. doi: 10.1124/jpet.103.053504. [DOI] [PubMed] [Google Scholar]