Abstract
As critical regulators of numerous cell signaling pathways, tyrosine kinases are implicated in the pathogenesis of several diseases, including rheumatoid arthritis (RA). In the absence of disease, synoviocytes produce factors that provide nutrition and lubrication for the surrounding cartilage tissue; few cellular infiltrates are seen in the synovium. In RA, however, macrophages, neutrophils, T cells and B cells infiltrate the synovium and produce cytokines, chemokines and degradative enzymes that promote inflammation and joint destruction. In addition, the synovial lining expands owing to the proliferation of synoviocytes and infiltration of inflammatory cells to form a pannus, which invades the surrounding bone and cartilage. Many of these cell responses are regulated by tyrosine kinases that operate in specific signaling pathways, and inhibition of a number of these kinases might be expected to provide benefit in RA.
Introduction
Rheumatoid arthritis (RA) is an autoimmune synovitis that affects 0.5% of the population and can result in disability owing to joint destruction.1 A number of cellular responses are thought to be involved in the pathogenesis of RA. An adaptive autoimmune response mediated by T cells and B cells is important in initiating the inflammatory cascade. Macrophages, neutrophils, T cells and B cells migrate into synovial tissue, where they produce immune mediators and degradative molecules that break down the extra-cellular matrix, in particular that of cartilage. Synoviocytes undergo hyperplasia, and angiogenesis occurs, possibly to support the growth of the synovial lining. Finally, osteoclasts become activated and erode bone. The activation and function of the cell types involved in each of these processes depend on signaling through specific pathways, many of which involve protein tyrosine kinases (Table 1, Figure 1). In support of an important role for tyrosine kinases in RA, proteins from the synovial tissue of RA patients have been found to be extensively phos phorylated by intracellular tyrosine kinases.2 In this article, we discuss the experimental evidence that implicates specific tyrosine kinases in signaling pathways that are central to the pathogenesis of RA, and address the potential to therapeutically target these kinases. Owing to space limitations, we will not discuss other potential tyrosine kinase targets, including focal adhesion kinase, fibroblast growth factor receptor, epidermal growth factor receptor, and discoidin receptor 2.
Table 1.
Expression of select tyrosine kinases in human rheumatoid arthritis patients
| Name | Type | Ligands | Expression in human RA synovium | Roles in RA |
|---|---|---|---|---|
| PDGFRα8 | RTK | PDGFAA, BB, CC, DD, AB | Synovial fuid | FLS proliferation |
| PDGFRβ10 | RTK | PDGFAA, BB, CC, DD, AB | Lining, blood vessels | FLS proliferation, angiogenesis |
| VEGFR117,24,25 | RTK | VEGFA–D | Monocytes/macrophages of sublining, blood vessels, pannus | Angiogenesis |
| VEGFR2 (Flk-1 in mice)23, 24 | RTK | VEGFA–D | Blood vessels, pannus | Angiogenesis |
| VEGFR323 | RTK | VEGFA–D | Blood vessels | Lymphangiogenesis |
| TIE132 | RTK | ANG1–4 | Lining, monocytes/macrophages of sublining, and blood vessels | Angiogenesis |
| TIE232 | RTK | ANG1–4 | Lining, monocytes/macrophages and lymphocytes of sublining and blood vessels | Angiogenesis |
| KIT43 | RTK | SCF | Mast cells of sublining | Production of infammatory cytokines and MMPs |
| CSF1R51 | RTK | M-CSF | Lining, monocytes/macrophages of sublining and blood vessels | Macrophage maturation, osteoclastogenesis |
| Lck38 | Non-RTK | NA | Lymphocytes of sublining | Production of infammatory cytokines |
| Btk39,a | Non-RTK | NA | Lymphocytes and mast cells of sublining | Activation of B cells, monocytes/macrophages and mast cells |
| Syk76 | Non-RTK | NA | Lining and synovial fuid | B-cell activation |
| Src64, 65 | Non-RTK | NA | Lining, monocytes/macrophages and mast cells of sublining and synovial fuid | Migration of monocytes/macrophages and FLSs, osteoclastogenesis |
| JAK373 | Non-RTK | NA | Lining, monocytes/macrophages and lymphocytes | T-cell activation |
Molecule expression examined in normal tissue, but not examined in RA tissue. Abbreviations: ANG, angiopoietin; CSF1R, colony-stimulating factor 1 receptor; FLS, fibroblast-like synoviocyte; JAK, Janus kinase; M-CSF, macrophage colony-stimulating factor; MMP, matrix metalloproteinase; NA, not applicable; PDGF, platelet-derived growth factor; RA, rheumatoid arthritis; RTK, receptor tyrosine kinase; SCF, stromal cell factor; VEGF, vascular endothelial growth factor.
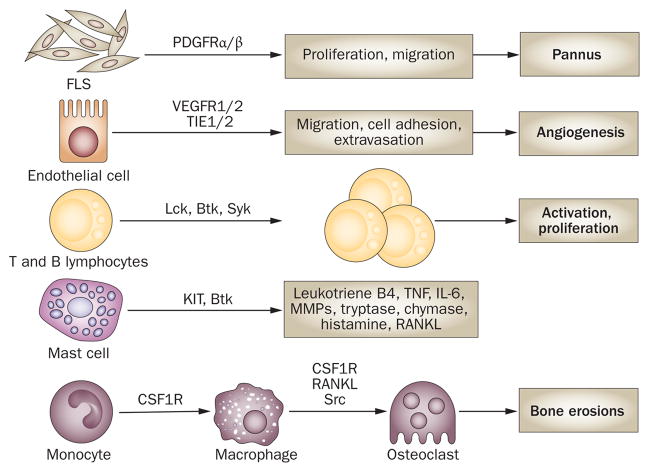
Figure 1.
Cellular responses mediated by tyrosine kinases that contribute to the pathogenesis of rheumatoid arthritis. Signaling through PDGFRs promotes the proliferation and migration of FLSs, contributing to the formation of a pannus. Migration of endothelial cells to form blood vessels through angiogenesis is promoted by signaling through VEGFRs and regulated by TIE1 and TIE2. Activation of T cells and B cells through T-cell receptors and B-cell receptors, respectively, requires a variety of tyrosine kinases, including Lck, Btk and Syk. Mast cells, which produce numerous inflammatory and degradative factors in the synovium, can be activated by several routes, such as binding of SCF to KIT. M-CSF binding CSF1R promotes the maturation of monocytes into macrophages and subsequent osteoclast formation, which results in bone erosion. Abbreviations: CSF1R, colony-stimulating factor 1 receptor; IL-6, interleukin-6; FLS, fibroblast-like synoviocyte; M-CSF, macrophage colony-stimulating factor; MMP, matrix metalloproteinase; PDGFR, platelet-derived growth factor receptor; RANKL, receptor activator for nuclear factor B ligand; SCF, stem cell factor; TNF, tumor necrosis factor.
Tyrosine kinases
Cell surface and cytoplasmic tyrosine kinases
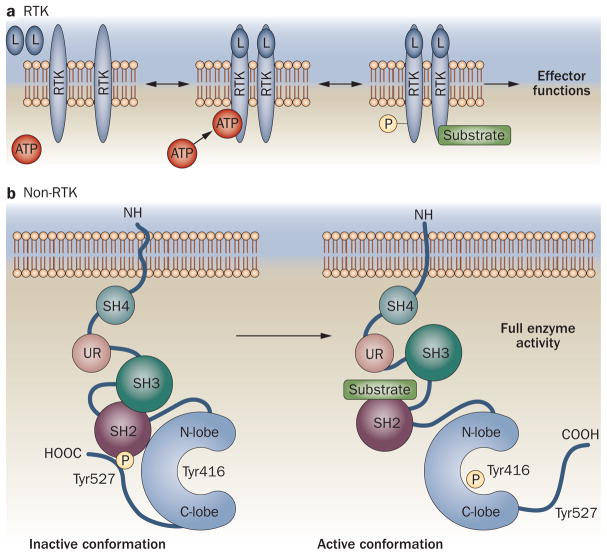
Tyrosine kinases control many fundamental cell processes, and comprise two general classes of molecules: receptor tyrosine kinases (RTKs) and non-receptor tyrosine kinases (non-RTKs).3 In addition to an extracellular ligand-binding domain and a membrane-spanning domain, RTKs usually possess an intracellular cytoplasmic domain that contains a kinase core and regulatory sequences. In the absence of ligands (growth factors, cytokines, etc.), RTKs are thought to exist in an equilibrium of monomers and dimers on the cell surface; ligand binding, however, increases the stability of dimers. Typically, on dimerization, motifs within the intracellular portion of the receptor undergo autophosphorylation, which induces a conformational change that allows the receptor to bind ATP and substrate.3 The active kinase can then catalyze the transfer of the phosphate from ATP to the hydroxyl groups of tyrosine residues on the receptor itself or on substrate proteins. Tyrosine phosphorylation creates docking sites on the receptor for downstream signaling molecules or activates substrate proteins, both of which promote signal transduction (Figure 2).3
Figure 2.
Activation of tyrosine kinases. There are two main types of tyrosine kinase: RTKs and non-RTKs. a | Ligand binding increases the stability of transmembrane RTK dimers; motifs within the intracellular domain undergo autophosphorylation, inducing a conformational change that allows ATP and substrate binding.3 The active kinase catalyzes the transfer of γ-phosphate from ATP to hydroxyl groups of tyrosine residues on the receptor itself or on substrate proteins, creating binding sites or activating substrate proteins, respectively, to promote signal transduction. b | Src-family kinases normally adopt an inactive conformation: the molecule is folded upon itself, such that the activation loop, containing tyrosine 416, is buried between the N-lobe and C-lobe of the kinase domain. This configuration is maintained by interactions between the SH3 domain and the linker region connecting the SH2 with the catalytic domain, and by binding of the SH2 domain to the C-terminal tail following phosphorylation of tyrosine 527. Activation occurs when interactions between the SH2 and SH3 regions and high-affinity binding ligands disrupt these intramolecular interactions, allowing unfolding and revealing tyrosine 416 for autophosphorylation. The mechanism of Src activation is outlined here. Abbreviations: L, ligand; RTK, receptor tyrosine kinase; SH2, SH3 and SH4, Src-homology 2, 3 and 4 domains; UR, unique region.
Similarly, activation of non-RTKs, which lack ligand-binding and transmembrane domains, occurs following phosphorylation of tyrosine residues on proteins by kinases within cytoplasmic complexes. As we will be discussing two members of the Src kinase family—Src and Lck—we will use Src-family kinases to outline non-RTK activation. Members of the Src family have six common structural regions, which, from the N-terminal end, include a Src-homology (SH)-4 domain, a unique region, an SH3 domain, an SH2 domain, a catalytic domain (which comprises an N-lobe and a C-lobe), and a C-terminal tail. Lipid moieties can attach to the SH4 domain to promote plasma membrane localization; the unique region of each family member mediates specific interactions with the cyto plasmic regions of RTKs and other non-RTKs; and the SH3 domain mediates interactions with target signaling molecules. The remaining domains, together with the SH3 domain, modulate the activation of Src-family kinases using two critical tyrosine residues—tyrosine 416 in the catalytic domain and tyrosine 527 in the C-terminal tail (the numbering refers to Src)—with opposing effects. Normally, Src-family kinases reside in an inactive conformation, in which the molecule is folded upon itself such that the activation loop (which contains tyrosine 416) is buried between the two lobes of the catalytic domain. This folded configuration is maintained by interactions between the SH3 domain and the linker region between the SH2 and catalytic domains, and by the binding of the SH2 domain to the C-terminal tail following phosphorylation of tyrosine 527. Activation occurs when interactions between the SH2 and SH3 regions and high-affinity binding ligands disrupt the intramolecular interactions, allowing the molecule to unfold to expose tyrosine 416 on the activation loop for autophosphorylation. Phosphorylation of this residue activates the kinase.4
Inhibitors of tyrosine kinases
A large number of small-molecule tyrosine kinase inhibitors (TKIs) have been developed. These inhibitors typically, but not always, bind to the nucleotide-binding pocket of the catalytic domain, and can thereby modulate changes in the conformation of the molecule that are necessary for kinase activation. For instance, imatinib is a potent inhibitor of several tyrosine kinases, including KIT (also known as CD117), platelet-derived growth factor receptors α and β (PDGFRα and β), breakpoint cluster region (Bcr)–Abelson murine leukemia viral oncogene homolog 1 (ABL1), colony-stimulating factor 1 receptor (CSF1R), and leukocyte-specific protein tyrosine kinase (Lck). Imatinib blocks the function of Bcr–ABL, and possibly these other tyrosine kinases, by stabilizing their inactive conformation.5
FLS hyperplasia
RA is characterized by hyperplasia of the synovial lining.1 Synovial tissues derived from RA patients exhibit a marked increase in the number of macrophage-like and fibroblast-like synoviocytes (FLSs).1,6
PDGFRs in FLS proliferation
Key molecules that are involved in FLS proliferation are PDGFRs, of which there are two (PDGFRα and β),7 and their ligands, which are dimers formed from PDGFs A–D.2,8 Increased levels of PDGFRa transcripts and proteins are seen in FLSs cultured from RA patients compared with those taken from control patients; PDGFRβ is expressed on stromal cells in the synovial lining, and in smooth muscle cells and capillary cells in RA synovium (Table 1).8–10
PDGFRs as potential targets
In vitro, PDGF induces proliferation of synovial fibroblasts derived from patients with RA more potently than synovial fibroblasts derived from healthy individuals.9 In rodent models of RA, imatinib reduced the severity of symptoms when administered before the development of the disease and inhibited disease progression when given to mice that had already developed the disease.11–13 In addition, nilotinib, which inhibits Bcr–ABL, PDGFRa and β and KIT, was effective in treating arthritis in the K/BxN serum-induced model (C. D’Aura Swanson et al., unpublished data). The efficacy of imatinib (and nilotinib) in models of arthritis is probably attributable, at least in part, to inhibition of PDGFR, as several groups have shown that imatinib inhibits the proliferation of FLSs from RA patients by blocking the phosphorylation of PDGFR and the activation of downstream mediators of the PDGFR signaling pathway.14,15 Evidence to date, therefore, indicates that PDGFRα and β have a central role in the proliferation of FLSs, and thereby represent potential therapeutic targets in RA.
Angiogenesis
Vascular density is higher in the synovia of patients with RA and osteoarthritis (OA) than in healthy synovium.16 Increased vascularity might support the growth of the synovial lining, so inhibiting angiogenesis is an attractive therapeutic approach for the treatment of arthritis. Such inhibition might be achieved by targeting vascular endothelial growth factor receptors 1 and 2 (VEGFR1 and 2) and TIE1 and TIE2 (also known as TEK).
VEGFs and VEGFRs as potential targets
VEGFRs comprise the most-studied family of RTKs in RA. The family includes VEGFR1 (also known as Flt-1 or Flk-2), VEGFR2 (known as KDR in humans and Flk-1 in mice), VEGFR3 (Flt-4), and two receptors that lack kinase domains: neuropilin (NRP)1 and NRP2.17 Ligands for the VEGFRs include VEGFs A–F and placental growth factor.17 Several studies have demonstrated an increase in the expression of VEGFA protein in the synovial fluid, lymph, serum and synovial tissue of patients with RA. The levels of serum VEGFA positively correlate with RA disease activity.18–20 VEGFA activates VEGFR1 and VEGFR2, which, in turn, induces endothelial cell proliferation, sprouting, migration and tube formation, thereby promoting the generation of blood vessels.17 VEGFA also supports osteoclastogenesis by mimicking the actions of macrophage colony-stimulating factor (M-CSF; also known as CSF1).17,21 Certain VEGFA isoforms and NRP1 induce the upregulation in synoviocytes of B-cell leukemia/lymphoma 2 (Bcl-2), which can protect the cells against apoptosis and thereby promote hyperplasia.22 VEGFC and VEGFD increase vascular permeability and angiogenesis by signaling through VEGFR2.17 VEGFC is present in many cells in the thickened synovial lining in RA, especially in blood vessel pericytes and smooth muscle cells.23 Endothelial cells, monocytes/macrophages and osteoblasts in the synovial tissue of RA patients express VEGFR1 (Table 1), but the levels of this protein do not correlate with vascularity.17,24,25 VEGFR2 can be detected in endothelial cells, especially in small blood vessels in pannus tissue, and its expression is upregulated in the synovial tissue of RA patients compared to that of OA patients (Table 1).22,23,25
Several groups have shown that inhibition of VEGFA or VEGFR1 delays the onset and reduces the severity of murine arthritis.26–29 VEGFR2 is thought to mediate VEGFA-induced endothelial cell migration and proliferation, but the value of inhibiting VEGFR2 or VEGFR3 in arthritis models remains unclear.17 Inhibition of VEGFR2 has yielded mixed results, whereas pan-VEGF inhibitors have shown only moderate efficacy.26,28,29 Sorafenib, which inhibits multiple tyrosine kinases, including VEGFR2 and VEGFR3, showed moderate therapeutic efficacy against murine K/BxN serum-induced arthritis, but failed to reduce disease scores or paw swelling in murine collagen-induced arthritis (CIA) (C. D’Aura Swanson et al., unpublished data). Sunitinib, which inhibits VEGFR1, VEGFR2 and VEGFR3, as well as several other kinases,30 reduced the incidence and severity of both K/BxN serum-induced arthritis and CIA (C. D’Aura Swanson et al., unpublished data). However, because sorafenib and sunitinib inhibit more than one form of VEGFR, more-specific inhibitors or genetic manipulation of the individual VEGFRs is required to determine the importance of the different VEGFRs in arthritis models; thus, further research is needed to clarify the roles of individual VEGFRs in RA. Nevertheless, findings to date indicate that VEGFR1 and VEGFA are prime candidates for therapeutic intervention in RA.
TIE1 and TIE2 as potential targets
The RTKs TIE1 and TIE2 regulate angiogenesis and are expressed on endothelial cells.31 Their ligands are the angiopoietins 1–4:31 angiopoietin 1 is a potent TIE2 agonist, angiopoietin 2 can function as a TIE2 agonist or antagonist, depending on cell context, and TIE1 regulates TIE2 activity.31 TIE1, TIE2, angiopoietin 1 and angiopoietin 2 are all expressed in synovial tissue from RA patients,32,33 and inhibition of TIE1 or TIE2 signaling was shown to be beneficial in CIA (Table 1). Adenovirus-mediated overexpression of a soluble form of TIE2, which functions as an antagonist of TIE2 signaling, significantly decreased disease incidence and severity in a CIA model; these results were associated with a reduction in angiogenesis, synovial cell infiltration and radiographic paw scores.34 TIE1-751, a naturally occurring splice variant of TIE1, also reduced arthritis severity in CIA.28 TIE1 and TIE2, therefore, represent potential targets in RA therapy.
T-cell and B-cell activation
The importance of T cells and B cells in the patho genesis of RA is underscored by several findings: the genetic linkage of RA to HLA genes; the association of RA with autoantibodies; the success of a fusion protein that inhibits T-cell costimulation (CTLA4–Ig; abatacept) in an RA clinical trial; and the efficacy of B-cell depletion by rituximab in the treatment of RA patients.35,36
Kinase involvement
Activation of T cells and B cells through T-cell receptors (TCRs) and B-cell receptors (BCRs), respectively, requires a variety of tyrosine kinases, including Lck, spleen tyrosine kinase (Syk), and Bruton’s tyrosine kinase (Btk).37 Inhibition of Lck disrupts TCR signaling, and thereby reduces T-cell activation, proliferation, and cytokine production.38 Btk is primarily expressed in B cells, mast cells, platelets and myeloid cells.39 This tyrosine kinase mediates calcium signaling following BCR engagement, mast cell activation following FceRI crosslinking, and possibly monocyte activation following engagement of Toll-like receptor 4.39,40
Lck, Syk and Btk as potential targets
Two Btk inhibitors, Compound 4 (Celera Genomics, Alameda, CA) and cgi1746 (CGI Pharmaceuticals, Branford, CT), have shown efficacy in collagen antibody-induced arthritis (CAIA) and in CIA, respectively (Table 2).39,41 Preventive treatment with dasatinib, which potently inhibits Lck and Btk in addition to other tyrosine kinases,42 reduced the incidence and severity of both CIA and K/BxN serum-induced arthritis (C. D’Aura Swanson et al., unpublished data). Syk will be discussed in further detail below.
Table 2.
Small-molecule inhibitors of select tyrosine kinases
| Generic name | Described kinase targets | Stage of clinical development | Primary disease indication |
|---|---|---|---|
| Imatinib11,14,46 | KIT, PDGFRα, PDGFRβ, Bcr–ABL, CSF1R, Lck | Approved | CML, GIST |
| Dasatinib38,42 | FAK, Fyn, Yes, Lck, Src, Bcr–ABL, Lyn, EphB4, Btk, DDR1, KIT, PDGFRβ, DDR2, Tec | Approved | CML |
| Sorafenib30 | PGFRβ, VEGFR2, VEGFR3, KIT, CSF1R, Flt-3, Raf1, B-Raf | Approved | Renal cell carcinoma and hepatocellular cancer |
| Sunitinib30 | VEGFR1, VEGFR2, VEGFR3, PDGFRβ, PDGFRα, KIT, Flt-3, CSF1R | Approved | Renal cell carcinoma |
| Nilotinib30 | Bcr–ABL, PDGFRs, KIT, Lck | Approved | CML |
| CP-690550 (P3zer Inc.)73 | JAK3 | Phase II | RA |
| INCB18424 (Incyte Inc.)74 | JAK1, JAK2 | Phase II | RA |
| Fostamatinib disodium (R406/R788; Rigel Pharmaceuticals Inc.)76 | Syk | Phase II | RA |
| Compound 4 (Celera Genomics)39 | Btk | Preclinical | NA |
| Cgi1746 (CGI Pharmaceuticals Inc.)41 | Btk | Preclinical | NA |
| GW2580 (GlaxoSmithKline plc)59 | CSF1R, TrkA | Preclinical | NA |
| Ki20227 (Kirin Pharma Company Ltd.)60 | CSF1R, VEGFR2, KIT, PDGFRβ | Preclinical | NA |
Abbreviations: Bcr–ABL, breakpoint cluster region–Abelson; CML, chronic myelogenous leukemia; CSF1R, colony-stimulating factor-1 receptor; DDR, discoidin domain receptor; FAK, focal adhesion kinase; JAK, Janus kinase; GIST, gastrointestinal stromal tumor; NA, not applicable; PDGF, platelet-derived growth factor; RA, rheumatoid arthritis; VEGFR, vascular endothelial growth factor receptor.
Mast cells
Activation and function
Mast cells produce factors that regulate a number of functions, including lymphocyte migration, angiogenesis, inflammation, and cartilage and bone destruction.43,44 Mast cells enhance vascular permeability through the release of bradykinin, and produce leukotriene B4, which might recruit CD8+ effector cells.43,44 Through the production of heparin, basic fibroblast growth factor, tumor necrosis factor (TNF), interleukin (IL)-13, IL-1β, IL-8, VEGF and PDGF, mast cells promote angiogenesis; by producing tryptase, chymase, histamine and receptor activator for nuclear factor B ligand (RANKL; also known as TNF ligand superfamily, member 11), these cells also promote the destruction of extracellular matrix and bone.43,44 Mast cells constitute up to 3% of cells in healthy synovium, but this figure can increase to 5% in murine and human arthritic synovial tissue.43,44 In the synovial tissue of RA patients, mast cells are observed at the cartilage–pannus junction, near blood vessels, and in areas of fibrosis.44 Molecules that inhibit mast cell degranulation decrease joint swelling in murine models of arthritis;44 W/Wv mice, which are deficient in mast cells, are resistant to K/BxN serum-induced arthritis.45
Targeting KIT
Signaling through FceRI, Fc RIII, KIT and complement receptors probably activates mast cells in RA.43,44 Because mast cell activation can occur through several distinct signaling pathways, blocking this activation can be challenging. One strategy to reduce the production of mast cell effector molecules is to induce apoptosis in these cells; indeed, imatinib has been shown to reduce the number of cultured mast cells via apoptosis.46 The KIT ligand (also known as stem-cell factor [SCF]) is thought to be one of the most important growth factors for mast cells.43,44 SCF is found in increased quantities in the synovial fluid of RA patients compared to that of OA patients,47 is expressed on multiple cells in the synovium,48 and induces a strong chemotactic response in mast cells from RA patients.49 KIT represents, therefore, a logical target for RA therapy, especially given its restricted expression in mast cells.
Osteoclast-mediated destruction
Osteoclasts erode periarticular bone in RA.21 Their formation from monocytes and dendritic cells is regulated by RANKL and M-CSF. Monocytes from synovial fluid from the joints of RA patients form large, multinucleated, tartrate-resistant and acid phosphatase-positive cells (which is indicative of the presence of osteoclasts) on culture with M-CSF and RANKL.50 M-CSF, through its ability to induce Bcl-2 and activate Ras-related C3 botulinum toxin substrate 1, also promotes osteoclast survival and the formation of resorptive pits.21
CSF1R as a potential target
M-CSF and its receptor CSF1R (a product of the fms gene) are expressed in the synovium of RA patients. CSF1R protein expression is observed in the synovial lining layer (predominantly on CD68+ macrophages) and around vessels in the sublining, as well as in osteoclasts (Table 1).51 Endothelial cells and fibroblasts from RA patients express high levels of M-CSF,52 and M-CSF levels are increased in synovial tissues and fluid derived from RA patients.53
Findings from rodent models of arthritis indicate that M-CSF and CSF1R have crucial roles in disease pathogenesis and progression. M-CSF exacerbates arthritis induced by submaximal levels of collagen or methylated bovine serum albumin in mice,54,55 and in rat arthritis.56 Mice deficient in M-CSF (op/op mice) are resistant to the development of arthritis,57 whereas antibodies against M-CSF reduced the clinical severity of CIA,54 and neutralizing antibodies targeting CSF1R inhibited inflammatory osteolysis.58 Furthermore, the small- molecule CSF1R inhibitors GW2580 (GlaxoSmithKline, Uxbridge, UK) and Ki20227 (Kirin Pharma Company Ltd, Takasaki, Japan) show therapeutic efficacy in rodent models of arthritis.59,60 Several pharmaceutical companies are developing CSF1R-specific inhibitors.
Src as a potential target
Src is a ubiquitously expressed non-RTK that is activated, among other means, by binding to protein tyrosine kinase 2β (PTK2β; also known as Pyk2) following integrin activation.61 Integrins, such as αvβ3, are critical for bone resorption, as they are thought to mediate macrophage and osteoclast migration and osteoclast adhesion to bone,62 and they promote osteoclast survival.63 Src is also important in RANK signaling, as interaction of Src with TNF receptor-associated factor 6 (TRAF6) following RANK receptor engagement leads to the phosphorylation of downstream signaling molecules.64 In RA patients, cells of the synovial lining and subsynovial macrophages express phosphorylated (activated) Src.65 Targeted disruption of Src in mice induces osteopetrosis, which is characterized by decreased bone resorption.62 In addition, overexpression in vivo of C-terminal Src kinase (Csk), which negatively regulates Src and other Src-family kinases, reduced the expression of these kinases and decreased arthritis severity in rats.66
Chemokine and cytokine production
TNF and IL-1 have key roles in RA as evidenced by the efficacy of inhibitors of both of these molecules in the treatment of RA.67,68 Moreover, TNF and IL-1 signaling pathways can synergize with, or activate, tyrosine kinases. Specifically, PDGF can synergize with IL-1 and TNF to promote fibroblast proliferation and prostaglandin E2 production.69 Similarly, IL-1 and TNF stimulate the production of M-CSF by cartilage and fibroblasts.70 Blockade of TNF and IL-1 might, therefore, provide benefit in RA by reducing the production of synovial M-CSF and effects of PDGF.
TKIs and RA
Eight small-molecule TKIs have so far been approved by the FDA for the treatment of different types of cancer; five of these TKIs might provide benefit in the treatment of RA (Table 2). Several TKIs are also in clinical development for the treatment of RA (Table 2). A number of case reports and a small case series indicate that imatinib, which is approved for the treatment of cancer, might provide benefit in RA and other inflammatory diseases. These reports include studies involving patients with long-standing RA who developed Bcr–ABL-positive chronic myelogenous leukemia71 or KIT-positive gastrointestinal stromal tumor.72 These patients received imatinib (300–400 mg daily) for their malignancy and showed improvement in RA symptoms, as reflected by reductions in the levels of inflammatory markers and improvements in disease activity indices.72 On the basis of these results, Eklund and Joensuu72 administered open-label imatinib treatment to three patients who had treatment-refractory RA. All three patients experienced some degree of clinical improvement, as assessed by measurement of inflammatory markers and swollen and tender joint counts; however, one patient discontinued therapy owing to the development of a rash.72
Inhibitors that target other tyrosine kinases with potential involvement in RA, such as members of the Janus kinase (JAK) family73 (CP-690550 [Pfizer Inc., New York, NY], INCB18424 [Incyte Inc., Wilmington, DE])74,75 or Syk76 (fostamatinib disodium [R788; Rigel Pharmaceuticals Inc., San Francisco, CA]),77 have undergone or are currently undergoing phase II clinical trials for the treatment of this disease (Table 2). The Syk inhibitor fostamatinib disodium showed significant improvements in ACR response and 28-joint disease activity score (DAS28) in a phase II study.77 Nevertheless, the use of Syk inhibitors might be limited by toxicity-related adverse effects that result in hepatic inflammation and hypertension. It remains unclear whether these toxicities are the result of a class effect or a molecule effect.77
Conclusions
Scientists and clinical researchers have begun to investigate small-molecule TKIs as a novel therapeutic approach to RA and other inflammatory diseases. Of the tyrosine kinases discussed in this Review, those that currently show the greatest potential as therapeutic targets in RA are CSF1R and KIT. The expression of these kinases is restricted to specific cell types within the synovium, and cumulative evidence from studies on tissues from RA patients and from animal models, as well as the efficacy of imatinib in treating RA, point to a central role for CSF1R and KIT in RA pathogenesis. However, as our knowledge regarding the roles of other tyrosine kinases in RA increases, their importance as targets might also be fully appreciated.
In vitro and in vivo observations involving both rodent models and samples derived from RA patients indicate that targeting the kinases discussed herein might provide benefit in the treatment of RA. However, several issues remain. First, although each of these molecules seems to have an important role in the pathogenesis of RA, their relative contributions need to be further defined. Second, the therapeutic effect (or lack thereof) of TKIs in animal models cannot reliably be extrapolated to human RA patients.78 Third, TKIs such as imatinib and dasatinib are pleiotropic inhibitors—they might additionally inhibit tyrosine kinases that are not involved in RA, and thus be more likely to cause unrelated tissue damage. Tyrosine kinases have central roles in many physiologic processes: for example, PDGFR regulates fibroblast proliferation and wound healing,79 and CSF1R regulates monocyte lineage cell survival and differen tiation.80 Small-molecule TKIs will partly block several of these physiologic responses, and might thereby cause target-based toxicities that limit their therapeutic use, as illustrated by recent clinical trials involving Syk inhibitors.77 It is difficult to fully anticipate the toxicities and therapeutic benefits that might arise from the inhibition of a particular tyrosine kinase or set of tyrosine kinases.
Although we focus on small-molecule inhibitors in this Review, other methods exist to target tyrosine kinases in RA. For example, antibodies targeting the extracellular domains of RTKs could compete with ligand binding or interfere with the adoption of molecular conformations necessary for activation. In addition, small interfering RNAs might be used to downregulate the expression of specific tyrosine kinases.
Specific targeting of tyrosine kinases that have central roles in the pathogenesis of RA will need to be carried out in clinical trials. The severity of disease and the adverse effects of a given TKI must be carefully considered. Given the breadth of therapeutics that are already available for the treatment of RA, it will be essential to identify TKIs with therapeutic indices that are sufficient for their use in RA.
Key points.
Rheumatoid arthritis (RA) is characterized by leukocyte infiltration, synoviocyte hyperplasia and osteoclastogenesis, and tyrosine kinases have key roles in the signaling pathways that regulate these processes
nhibition of platelet-derived growth factor receptors, vascular endothelial growth factor receptors and TIE receptors might reduce synovial hyperplasia and angiogenesis
Inhibition of colony-stimulating factor receptor-1 and Src might reduce monocyte maturation and osteoclastogenesis
Blocking signaling through Bruton’s tyrosine kinase might reduce B-cell and T-cell activation
Blocking KIT activation might induce mast cell apoptosis, thereby reducing the production of inflammatory cytokines and degradative molecules in the synovium
Imatinib, which inhibits several tyrosine kinases, and more-specific inhibitors of Janus kinases and Syk, have already shown efficacy in the treatment of RA; however, toxicity remains an issue
Acknowledgments
We thank members of the Robinson laboratory for their scientific input. This work was funded by NIH NHLBI contract N01 HV 28183, NIH NIAMS R01 AR-054822, and Veterans Affairs Health Care System funding to W. H. Robinson; Stanford University Program in Immunology training grant support to C. D’Aura Swanson; and an NIH F31 Fellowship Award and a Lieberman Fellowship Award to R. T. Paniagua.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.Sano H, et al. Coexpression of phosphotyrosine-containing proteins, platelet-derived growth factor-B, and fibroblast growth factor-1 in situ in synovial tissues of patients with rheumatoid arthritis and Lewis rats with adjuvant or streptococcal cell wall arthritis. J Clin Invest. 1993;91:553–565. doi: 10.1172/JCI116235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 4.Benati D, Baldari CT. SRC family kinases as potential therapeutic targets for malignancies and immunological disorders. Curr Med Chem. 2008;15:1154–1165. doi: 10.2174/092986708784310404. [DOI] [PubMed] [Google Scholar]
- 5.Schindler T, et al. Structural mechanism for STI-571 inhibition of Abelson tyrosine kinase. Science. 2000;289:1938–1942. doi: 10.1126/science.289.5486.1938. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda M, Hosoda Y, Hirose S, Okada Y, Ikeda E. Expression of vascular endothelial growth factor isoforms and their receptors Flt-1, KDR, and neuropilin-1 in synovial tissues of rheumatoid arthritis. J Pathol. 2000;191:426–433. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH649>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 7.Betsholtz C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004;15:215–228. doi: 10.1016/j.cytogfr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Pohlers D, et al. Expression of platelet-derived growth factors C and D in the synovial membrane of patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 2006;54:788–794. doi: 10.1002/art.21670. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe N, et al. Gene expression profile analysis of rheumatoid synovial fibroblast cultures revealing the overexpression of genes responsible for tumor-like growth of rheumatoid synovium. Biochem Biophys Res Commun. 2002;294:1121–1129. doi: 10.1016/S0006-291X(02)00608-3. [DOI] [PubMed] [Google Scholar]
- 10.Reuterdahl C, et al. Characterization of platelet-derived growth factor beta-receptor expressing cells in the vasculature of human rheumatoid synovium. Lab Invest. 1991;64:321–329. [PubMed] [Google Scholar]
- 11.Paniagua RT, et al. Selective tyrosine kinase inhibition by imatinib mesylate for the treatment of autoimmune arthritis. J Clin Invest. 2006;116:2633–2642. doi: 10.1172/JCI28546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ando W, et al. Imatinib mesylate inhibits osteoclastogenesis and joint destruction in rats with collagen-induced arthritis (CIA) J Bone Miner Metab. 2006;24:274–282. doi: 10.1007/s00774-006-0684-1. [DOI] [PubMed] [Google Scholar]
- 13.Koyama K, et al. Imatinib mesylate both prevents and treats the arthritis induced by type II collagen antibody in mice. Mod Rheumatol. 2007;17:306–310. doi: 10.1007/s10165-007-0592-9. [DOI] [PubMed] [Google Scholar]
- 14.Sandler C, et al. Imatinib mesylate inhibits platelet derived growth factor stimulated proliferation of rheumatoid synovial fibroblasts. Biochem Biophys Res Commun. 2006;347:31–35. doi: 10.1016/j.bbrc.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 15.Kameda H, et al. Imatinib mesylate inhibits proliferation of rheumatoid synovial fibroblast-like cells and phosphorylation of Gab adapter proteins activated by platelet-derived growth factor. Clin Exp Immunol. 2006;144:335–341. doi: 10.1111/j.1365-2249.2006.03067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giatromanolaki A, et al. The angiogenic pathway “vascular endothelial growth factor/flk-1(KDR)-receptor” in rheumatoid arthritis and osteoarthritis. J Pathol. 2001;194:101–108. doi: 10.1002/path.842. [DOI] [PubMed] [Google Scholar]
- 17.Roy H, Bhardwaj S, Ylä-Herttuala S. Biology of vascular endothelial growth factors. FEBS Lett. 2006;580:2879–2887. doi: 10.1016/j.febslet.2006.03.087. [DOI] [PubMed] [Google Scholar]
- 18.Ballara S, et al. Raised serum vascular endothelial growth factor levels are associated with destructive change in inflammatory arthritis. Arthritis Rheum. 2001;44:2055–2064. doi: 10.1002/1529-0131(200109)44:9<2055::AID-ART355>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Sone H, et al. Elevated levels of vascular endothelial growth factor in the sera of patients with rheumatoid arthritis correlation with disease activity. Life Sci. 2001;69:1861–1869. doi: 10.1016/s0024-3205(01)01264-4. [DOI] [PubMed] [Google Scholar]
- 20.Olszewski WL, et al. Lymph draining from foot joints in rheumatoid arthritis provides insight into local cytokine and chemokine production and transport to lymph nodes. Arthritis Rheum. 2001;44:541–549. doi: 10.1002/1529-0131(200103)44:3<541::AID-ANR102>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka S. Signaling axis in osteoclast biology and therapeutic targeting in the RANKL/RANK/OPG system. Am J Nephrol. 2007;27:466–478. doi: 10.1159/000106484. [DOI] [PubMed] [Google Scholar]
- 22.Kim WU, et al. Interaction of vascular endothelial growth factor 165 with neuropilin-1 protects rheumatoid synoviocytes from apoptotic death by regulating Bcl-2 expression and Bax translocation. J Immunol. 2006;177:5727–5735. doi: 10.4049/jimmunol.177.8.5727. [DOI] [PubMed] [Google Scholar]
- 23.Paavonen K, et al. Vascular endothelial growth factors C and D and their VEGFR-2 and 3 receptors in blood and lymphatic vessels in healthy and arthritic synovium. J Rheumatol. 2002;29:39–45. [PubMed] [Google Scholar]
- 24.Fava RA, et al. Vascular permeability factor/endothelial growth factor (VPF/VEGF): accumulation and expression in human synovial fluids and rheumatoid synovial tissue. J Exp Med. 1994;180:341–346. doi: 10.1084/jem.180.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pufe T, et al. Splice variants VEGF121 and VEGF165 of the angiogenic peptide vascular endothelial cell growth factor are expressed in the synovial tissue of patients with rheumatoid arthritis. J Rheumatol. 2001;28:1482–1485. [PubMed] [Google Scholar]
- 26.De Bandt M, et al. Blockade of vascular endothelial growth factor receptor I (VEGF-RI), but not VEGF-RII, suppresses joint destruction in the K/BxN model of rheumatoid arthritis. J Immunol. 2003;171:4853–4859. doi: 10.4049/jimmunol.171.9.4853. [DOI] [PubMed] [Google Scholar]
- 27.Lu J, et al. Vascular endothelial growth factor expression and regulation of murine collagen-induced arthritis. J Immunol. 2000;164:5922–5927. doi: 10.4049/jimmunol.164.11.5922. [DOI] [PubMed] [Google Scholar]
- 28.Jin P, et al. Novel splice variants derived from the receptor tyrosine kinase superfamily are potential therapeutics for rheumatoid arthritis. Arthritis Res Ther. 2008;10:R73. doi: 10.1186/ar2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murakami M, et al. Signaling of vascular endothelial growth factor receptor-1 tyrosine kinase promotes rheumatoid arthritis through activation of monocytes/macrophages. Blood. 2006;108:1849–1856. doi: 10.1182/blood-2006-04-016030. [DOI] [PubMed] [Google Scholar]
- 30.Faivre S, et al. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov. 2007;6:734–745. doi: 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- 31.Makinde T, Agrawal DK. Intra and extravascular transmembrane signalling of angiopoietin-1-Tie2 receptor in health and disease. J Cell Mol Med. 2008;12:810–828. doi: 10.1111/j.1582-4934.2008.00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahrara S, et al. Differential expression of the angiogenic Tie receptor family in arthritic and normal synovial tissue. Arthritis Res. 2002;4:201–208. doi: 10.1186/ar407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uchida T, et al. Immunohistochemical localisation of protein tyrosine kinase receptors Tie-1 and Tie-2 in synovial tissue of rheumatoid arthritis: correlation with angiogenesis and synovial proliferation. Ann Rheum Dis. 2000;59:607–614. doi: 10.1136/ard.59.8.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Donnelly E, Kobayashi H, Debusk LM, Lin PC. Gene therapy targeting the Tie2 function ameliorates collagen-induced arthritis and protects against bone destruction. Arthritis Rheum. 2005;52:1585–1594. doi: 10.1002/art.21016. [DOI] [PubMed] [Google Scholar]
- 35.Orozco G, et al. Genetic basis of rheumatoid arthritis. Biomed Pharmacother. 2006;60:656–662. doi: 10.1016/j.biopha.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Yanaba K, et al. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008;223:284–299. doi: 10.1111/j.1600-065X.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 37.Jiang A, et al. Different protein tyrosine kinases are required for B cell antigen receptor-mediated activation of extracellular signal-regulated kinase, c-Jun NH2-terminal kinase 1, and p38 mitogen-activated protein kinase. J Exp Med. 1998;188:1297–1306. doi: 10.1084/jem.188.7.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blake S, et al. The Src/ABL kinase inhibitor dasatinib (BMS-354825) inhibits function of normal human T-lymphocytes in vitro. Clin Immunol. 2008;127:330–339. doi: 10.1016/j.clim.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Rokosz LL, et al. Kinase inhibitors as drugs for chronic inflammatory and immunological diseases: progress and challenges. Expert Opin Ther Targets. 2008;12:883–903. doi: 10.1517/14728222.12.7.883. [DOI] [PubMed] [Google Scholar]
- 40.Felices M, et al. Tec kinases in T cell and mast cell signaling. Adv Immunol. 2007;93:145–184. doi: 10.1016/S0065-2776(06)93004-1. [DOI] [PubMed] [Google Scholar]
- 41.Pan Z, et al. Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. ChemMedChem. 2007;2:58–61. doi: 10.1002/cmdc.200600221. [DOI] [PubMed] [Google Scholar]
- 42.Hantschel O, et al. The Btk tyrosine kinase is a major target of the Bcr-Abl inhibitor dasatinib. Proc Natl Acad Sci USA. 2007;104:13283–13288. doi: 10.1073/pnas.0702654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eklund KK. Mast cells in the pathogenesis of rheumatic diseases and as potential targets for anti-rheumatic therapy. Immunol Rev. 2007;217:38–52. doi: 10.1111/j.1600-065X.2007.00504.x. [DOI] [PubMed] [Google Scholar]
- 44.Bakharevski O, Ryan PF. Mast cells as a target in the treatment of rheumatoid arthritis. Inflammopharmacology. 1999;7:351–362. doi: 10.1007/s10787-999-0029-5. [DOI] [PubMed] [Google Scholar]
- 45.Lee DM, et al. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 46.Juurikivi A, et al. Inhibition of c-kit tyrosine kinase by imatinib mesylate induces apoptosis in mast cells in rheumatoid synovia: a potential approach to the treatment of arthritis. Ann Rheum Dis. 2005;64:1126–1131. doi: 10.1136/ard.2004.029835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carsons SE, et al. Detection and quantitation of stem cell factor (kit ligand) in the synovial fluid of patients with rheumatic disease. J Rheumatol. 2000;27:2798–2800. [PubMed] [Google Scholar]
- 48.Ceponis A, et al. Expression of stem cell factor (SCF) and SCF receptor (c-kit) in synovial membrane in arthritis: correlation with synovial mast cell hyperplasia and inflammation. J Rheumatol. 1998;25:2304–2314. [PubMed] [Google Scholar]
- 49.Olsson N, et al. Demonstration of mast cell chemotactic activity in synovial fluid from rheumatoid patients. Ann Rheum Dis. 2001;60:187–193. doi: 10.1136/ard.60.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adamopoulos IE, et al. Synovial fluid macrophages are capable of osteoclast formation and resorption. J Pathol. 2006;208:35–43. doi: 10.1002/path.1891. [DOI] [PubMed] [Google Scholar]
- 51.Takasugi K, et al. Induction of tumour necrosis factor receptor-expressing macrophages by interleukin-10 and macrophage colony-stimulating factor in rheumatoid arthritis. Arthritis Res Ther. 2006;8:R126. doi: 10.1186/ar2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakano K, et al. Rheumatoid synovial endothelial cells produce macrophage colony-stimulating factor leading to osteoclastogenesis in rheumatoid arthritis. Rheumatology (Oxford) 2007;46:597–603. doi: 10.1093/rheumatology/kel356. [DOI] [PubMed] [Google Scholar]
- 53.Seitz M, et al. Constitutive mRNA and protein production of macrophage colony-stimulating factor but not of other cytokines by synovial fibroblasts from rheumatoid arthritis and osteoarthritis patients. Br J Rheumatol. 1994;33:613–619. doi: 10.1093/rheumatology/33.7.613. [DOI] [PubMed] [Google Scholar]
- 54.Campbell IK, et al. The colony-stimulating factors and collagen-induced arthritis: exacerbation of disease by M-CSF and G-CSF and requirement for endogenous M-CSF. J Leukoc Biol. 2000;68:144–150. [PubMed] [Google Scholar]
- 55.Bischof RJ, et al. Exacerbation of acute inflammatory arthritis by the colony-stimulating factors CSF-1 and granulocyte macrophage (GM)-CSF: evidence of macrophage infiltration and local proliferation. Clin Exp Immunol. 2000;119:361–367. doi: 10.1046/j.1365-2249.2000.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abd AH, et al. The role of macrophages in experimental arthritis induced by Streptococcus agalactiae sonicate: actions of macrophage colony-stimulating factor (CSF-1) and other macrophage-modulating agents. Lymphokine Cytokine Res. 1991;10:43–50. [PubMed] [Google Scholar]
- 57.Yang YH, Hamilton JA. Dependence of interleukin-1-induced arthritis on granulocyte-macrophage colony-stimulating factor. Arthritis Rheum. 2001;44:111–119. doi: 10.1002/1529-0131(200101)44:1<111::AID-ANR15>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 58.Kitaura H, et al. M-CSF mediates TNF-induced inflammatory osteolysis. J Clin Invest. 2005;115:3418–3427. doi: 10.1172/JCI26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Conway JG, et al. Effects of the cFMS kinase inhibitor 5-(3-methoxy-4-[{4-methoxybenzyl}oxy] benzyl)pyrimidine-2, 4-diamine (GW2580) in normal and arthritic rats. J Pharmacol Exp Ther. 2008;326:41–50. doi: 10.1124/jpet.107.129429. [DOI] [PubMed] [Google Scholar]
- 60.Ohno H, et al. The orally-active and selective c-Fms tyrosine kinase inhibitor Ki20227 inhibits disease progression in a collagen-induced arthritis mouse model. Eur J Immunol. 2008;38:283–291. doi: 10.1002/eji.200737199. [DOI] [PubMed] [Google Scholar]
- 61.van Nimwegen MJ, van de Water B. Focal adhesion kinase: a potential target in cancer therapy. Biochem Pharmacol. 2007;73:597–609. doi: 10.1016/j.bcp.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 62.Miyazaki T, et al. The role of c-Src kinase in the regulation of osteoclast function. Mod Rheumatol. 2006;16:68–74. doi: 10.1007/s10165-006-0460-z. [DOI] [PubMed] [Google Scholar]
- 63.Del Fattore A, et al. Osteoclast receptors and signaling. Arch Biochem Biophys. 2008;473:147–160. doi: 10.1016/j.abb.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 64.Wada T, et al. RANKL–RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 65.Shahrara S, et al. Differential expression of the FAK family kinases in rheumatoid arthritis and osteoarthritis synovial tissues. Arthritis Res Ther. 2007;9:R112. doi: 10.1186/ar2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takayanagi H, et al. Suppression of arthritic bone destruction by adenovirus-mediated csk gene transfer to synoviocytes and osteoclasts. J Clin Invest. 1999;104:137–146. doi: 10.1172/JCI6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kay J, Calabrese L. The role of interleukin-1 in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 2004;43(Suppl 3):iii2–iii9. doi: 10.1093/rheumatology/keh201. [DOI] [PubMed] [Google Scholar]
- 68.Segal B, et al. Tumor necrosis factor (TNF) inhibitor therapy for rheumatoid arthritis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:778–787. doi: 10.1016/j.tripleo.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 69.Kumkumian GK, et al. Platelet-derived growth factor and IL-1 interactions in rheumatoid arthritis. Regulation of synoviocyte proliferation, prostaglandin production, and collagenase transcription. J Immunol. 1989;143:833–837. [PubMed] [Google Scholar]
- 70.Campbell IK, et al. Production of macrophage colony-stimulating factor (M-CSF) by human articular cartilage and chondrocytes. Modulation by interleukin-1 and tumor necrosis factor alpha. Biochim Biophys Acta. 1993;1182:57–63. doi: 10.1016/0925-4439(93)90153-r. [DOI] [PubMed] [Google Scholar]
- 71.Miyachi K, et al. Efficacy of imatinib mesylate (STI571) treatment for a patient with rheumatoid arthritis developing chronic myelogenous leukemia. Clin Rheumatol. 2003;22:329–332. doi: 10.1007/s10067-003-0716-3. [DOI] [PubMed] [Google Scholar]
- 72.Eklund KK, Joensuu H. Treatment of rheumatoid arthritis with imatinib mesylate: clinical improvement in three refractory cases. Ann Med. 2003;35:362–367. doi: 10.1080/07853890310001339. [DOI] [PubMed] [Google Scholar]
- 73.Walker JG, et al. Changes in synovial tissue Jak–STAT expression in rheumatoid arthritis in response to successful DMARD treatment. Ann Rheum Dis. 2006;65:1558–1564. doi: 10.1136/ard.2005.050385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pfizer Pipeline: as of September 30, 2008. 2008 http://media.pfizer.com/files/research/pipeline/2008_0930/pipeline_2008_0930.pdf.
- 75.Incyte Corporation. Press Release: Incyte’s JAK inhibitor demonstrates rapid and marked clinical improvement in rheumatoid arthritis patients. 2008 http://investor.incyte.com/phoenix.zhtml?c=69764&p=IROL-NewsText&t=Regular&id=1217246&.
- 76.Cha HS, et al. A novel spleen tyrosine kinase inhibitor blocks c-Jun N-terminal kinase-mediated gene expression in synoviocytes. J Pharmacol Exp Ther. 2006;317:571–578. doi: 10.1124/jpet.105.097436. [DOI] [PubMed] [Google Scholar]
- 77.Weinblatt ME, et al. Treatment of rheumatoid arthritis with a syk kinase inhibitor: a twelve-week, randomized, placebo-controlled trial. Arthritis Rheum. 2008;58:3309–3318. doi: 10.1002/art.23992. [DOI] [PubMed] [Google Scholar]
- 78.Firestein GS. Rheumatoid arthritis in a mouse? Nat Clin Pract Rheumatol. 2009;5:1. doi: 10.1038/ncprheum0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alvarez RH, et al. Biology of platelet-derived growth factor and its involvement in disease. Mayo Clin Proc. 2006:81–1257. doi: 10.4065/81.9.1241. [DOI] [PubMed] [Google Scholar]
- 80.Naito M. Macrophage differentiation and function in health and disease. Pathol Int. 2008;58:143–155. doi: 10.1111/j.1440-1827.2007.02203.x. [DOI] [PubMed] [Google Scholar]