Summary
Translocations involving the Mixed Lineage Leukemia (MLL) gene result in human acute leukemias with very poor prognosis. The leukemogenic activity of MLL fusion proteins is critically dependent on their direct interaction with menin, a product of the MEN1 gene. Here, we present the first small molecule inhibitors of the menin-MLL fusion protein interaction that specifically bind to menin with nanomolar affinities. These compounds effectively reverse MLL fusion protein-mediated leukemic transformation by downregulating the expression of target genes required for MLL fusion protein oncogenic activity. They also selectively block proliferation and induce both apoptosis and differentiation of leukemia cells harboring MLL translocations. Identification of these compounds provides a new tool for better understanding MLL-mediated leukemogenesis and represents a new approach for studying the role of menin as an oncogenic cofactor of MLL fusion proteins. Our findings also highlight a new therapeutic strategy for aggressive leukemias with MLL rearrangements.
Introduction
The Mixed Lineage Leukemia (MLL) gene is a common target of chromosomal translocations found in patients with acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) 1,2, affecting both children3 and adults4. Fusion of MLL with one of over 50 different partner genes forms chimeric oncogenes encoding MLL fusion proteins, which preserve the N-terminal 1400 amino acid fragment of MLL fused to distinct protein partners2,5–8. MLL is required for the maintenance of HOX genes expression, which are important regulators of hematopoietic differentiation9,10. Disruption of MLL by translocation upregulates HOX expression, including HOXA9, HOXA7 and the HOX cofactor MEIS1, which enhances proliferation and blocks hematopoietic differentiation, ultimately leading to acute leukemia11–14. MLL fusion proteins play a causative role in MLL-mediated leukemogenesis15,16. The presence of MLL translocations in leukemia patients is generally associated with a very poor prognosis6,17,18, emphasizing the pressing need for developing more effective therapies for the treatment of MLL leukemias.
The leukemogenic activity of MLL fusion proteins is critically dependent on their direct interaction with menin19–21, a protein encoded by the Multiple Endocrine Neoplasia 1 (MEN 1) gene22. Menin is a tumor suppressor protein which directly controls cell growth in endocrine organs23. Menin is also a highly specific partner of MLL, and an essential component of the MLL SET1-like histone methyl transferase complex24,25. Association of menin with MLL fusion proteins upregulates expression of target genes, such as HOXA9 and MEIS1, and is critical for oncogenic transformation induced by MLL fusion oncoproteins19,21. Myeloid cells transformed with oncogenic MLL-AF9 require menin for efficient proliferation21. Loss of menin relieves the differentiation block induced by MLL fusion proteins in transformed leukemic blasts19. Mutations within the N-terminus of MLL fusion proteins that block association with menin abrogate the development of acute leukemia in mice19,20. These findings demonstrate that menin functions as an essential oncogenic co-factor of MLL fusion proteins, and implies that the menin-MLL interaction represents a valuable target for molecular therapy19–21. A small molecule that specifically binds to menin and inhibits the menin-MLL interaction would be a valuable tool to study the molecular mechanism of MLL-mediated leukemogenesis and might lay a foundation for novel targeted therapy for MLL leukemias.
We have recently characterized the molecular basis of the menin interaction with the N-terminal fragment of MLL26. The major objective of the studies presented here was to develop small molecule inhibitors of the protein-protein interaction between menin and MLL fusion proteins and evaluate if such compounds are capable of blocking the leukemogenic activity of MLL fusion proteins. By applying high throughput screening (HTS) we identified small molecule inhibitors of the menin-MLL interaction, which we then optimized by medicinal chemistry to develop inhibitors with nanomolar affinities. These compounds, which represent the first reported small molecule inhibitors of the menin-MLL interaction, reverse the leukemogenic activity of MLL fusion proteins by downregulating the expression of HOXA9 and MEIS1. They also block proliferation and induce both apoptosis and differentiation of MLL leukemia cells. Our results demonstrate the utility of menin-MLL inhibitors as chemical tools to study the mechanism of leukemogenesis mediated by MLL fusion proteins. Furthermore, our work strongly supports the rationale of targeting the menin-MLL interaction with small molecules as a novel therapeutic approach for acute leukemias associated with MLL translocations.
Results
Identification of menin-MLL inhibitors
We employed HTS to identify initial lead compounds targeting menin and inhibiting the menin-MLL interaction. We screened a collection of 49,000 small molecules using a fluorescence polarization (FP) assay with a fluorescein labeled MLL derived peptide comprising the high affinity menin binding motif (MBM1)26, (Supplementary Methods and Supplementary Results, Supplementary Table 1). A step-wise procedure, including two FP assays with fluorescein and Texas Red labeled MBM1 followed by NMR experiments to validate binding of compounds to menin, was applied to identify menin-MLL inhibitors. The most potent compound identified by HTS, MI-1 (1), which belongs to the thienopyrimidine class, reversibly inhibited the menin-MLL interaction with an IC50 value of 1.9 μM (Fig. 1a and Supplementary Fig. 1a). We have also identified two other compounds belonging to the thienopyrimidine class, but they were 20–40 fold weaker than MI-1 (Supplementary Fig. 1b).
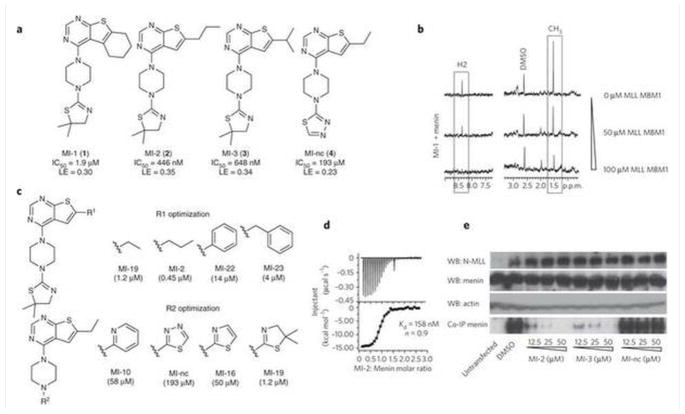
Figure 1. Characterization of the menin-MLL inhibitors.
(a) Structures and IC50 values measured by FP for the inhibitors of the menin-MLL interaction, MI-1, MI-2, MI-3 and MI-nc. LE (ligand efficiency) values were calculated according to the formula: LE=R*T*ln(IC50)/HA; where R is gas constant, T is temperature and HA is a number of non-hydrogen atoms in the compound. (b) NMR saturation transfer difference (STD) experiments. Top spectrum: 1D STD spectrum of MI-1 (200 μM) with menin (5 μM). Boxes show STD effect for MI-1 signals, corresponding to the aromatic proton from pyrimidine ring (H2) and two methyl groups at thiazoline ring (CH3). Middle and bottom spectra represent STD spectra for MI-1 (200 μM) with menin (5 μM) and increasing concentrations of MLL MBM1 peptide (50 μM and 100 μM, respectively). (c) SAR for selected analogues of MI-1 with different R1 and R2 substituents. (d) ITC experiment demonstrating direct binding of MI-2 to menin with 1:1 stoichiometry. (e) Co-IP experiment in HEK293 cells transfected with Flag-MLL-AF9. MI-nc was used as a negative control. Untransfected cells serve as a control for endogenous expression of menin and lack of MLL-AF9 expression. WB, Western Blot; LE, ligand efficiency; ppm – parts per million; MBM1 – menin binding motif 1.
To validate that MI-1 directly binds to menin and competes with MLL, we employed Saturation Transfer Difference (STD) NMR experiments27 (Supplementary Methods). A strong STD effect was observed for MI-1, indicative of its direct binding to menin (Fig. 1b). We then employed a competition STD experiment to assess whether MI-1 competes with MLL for binding to menin. Indeed, addition of the MBM1 peptide to the menin-MI-1 complex significantly decreased the STD effect for MI-1 in a dose dependent manner (Fig. 1b). This demonstrates that binding of MI-1 and MLL to menin is mutually exclusive and confirms the reversible and specific binding of MI-1 to menin.
Development of potent menin-MLL inhibitors
We explored both commercial and synthesized compounds to develop the structure-activity relationship (SAR) for analogues of the MI-1 lead compound with modifications at R1 and R2 positions (Fig. 1c and Supplementary Tables 2 and 3). We introduced several heterocyclic rings at R2 and the dimethyl-thiazoline group represented the best substituent (Fig. 1c and Supplementary Tables 2 and 3). Assessment of diverse hydrophobic groups at R1 led to the development of several compounds with IC50 values in the nanomolar range, including MI-2 (2) (IC50 = 446 ± 28 nM) and MI-3 (3) (IC50 = 648 ± 25 nM) (Fig. 1a, Supplementary Fig. 1c and Supplementary Scheme 1). We validated the specific binding of these compounds to menin by competition STD NMR experiments (Supplementary Fig. 2a,b). The n-propyl represented an optimal substituent at R1 while a larger hydrophobic group or branched aliphatic chains were not well tolerated (Supplementary Table 3). As a negative control compound, we selected a compound sharing the same molecular scaffold and similar functional groups, MI-nc (4), (Fig. 1a and Supplementary Scheme 2), which showed very weak in vitro inhibition of the menin-MLL interaction (IC50 = 193 μM). In order to assess the binding affinities of the most potent compounds, we employed Isothermal Titration Calorimetry (ITC) (Supplementary Methods). The dissociation constants measured for the menin-MLL inhibitors were at the nanomolar level, Kd = 158 nM for MI-2 (Fig. 1d), and Kd = 201 nM for MI-3 (Supplementary Fig. 2c). Importantly, the stoichiometry of binding of both compounds to menin was 1:1, which further supports their specific binding to the protein.
We have recently determined the crystal structure of a menin homolog from Nematostella vectensis and mapped the MLL binding site to a large central cavity on menin28. To assess whether menin inhibitors interact with the same pocket were MLL binds, we employed the thermal shift assay (Supplementary Methods) and tested binding of menin inhibitors to the wild-type menin as well as to two point mutants (M278K and Y323K) which lack binding to MLL28. As expected, we clearly observed binding of MI-2 and MI-3 to the wild-type menin (Supplementary Fig. 3). In contrast, we detected no binding of MI-2 and MI-3 to M278K and Y323K menin mutants (Supplementary Table 4). These data further emphasize the specificity of compound interaction with the protein and also map the compound binding site to the MLL binding site on menin.
Inhibition of the menin-MLL fusion interaction in cells
To test if the most potent compounds disrupt the menin-MLL-AF9 interaction in mammalian cells, we performed co-immunoprecipitation (co-IP) experiments in HEK293 cells transfected with Flag-MLL-AF9 (Supplementary Methods, Fig. 1e and Supplementary Figure 4). Expression of endogenous menin was validated by Western blot. Menin co-IPs with MLL-AF9 in DMSO treated sample (Fig. 1e). Treatment with MI-2 and MI-3, however, very efficiently disrupted the menin-MLL-AF9 complex without affecting the expression level of menin and MLL-AF9 (Fig. 1e). In contrast, we observed no effect in the co-IP experiment for the negative control compound MI-nc (Fig. 1e). These data demonstrate that MI-2 and MI-3 can access the protein target and very effectively inhibit the menin-MLL-AF9 interaction in human cells.
Menin-MLL inhibitors induce cell growth arrest
Disruption of the menin-MLL fusion protein interaction should result in cell growth arrest19,20. To determine whether such an effect can be achieved upon treatment with the menin-MLL inhibitors, we tested MI-2 and MI-3 in mouse bone marrow cells (BMC) transduced with MLL-AF9 and MLL-ENL (Supplementary Fig. 5a). As a control, we used BMC transduced with the E2A-HLF oncogene, which is not dependent on Hoxa9 for transformation. Cell growth inhibition was assessed using the MTT cell viability assay29 (Supplementary Methods). The menin-MLL inhibitors very effectively blocked proliferation of MLL-AF9 and MLL-ENL transduced BMC, with GI50 values of about 5 μM for MI-2 and MI-3 (Fig. 2a and Supplementary Fig. 5b). Substantial inhibition of cell proliferation was also reflected in growth curves (Fig. 2b and Supplementary Fig. 5c). In contrast, MI-nc, which is a very weak inhibitor of the menin-MLL interaction, showed a very little effect on proliferation of MLL fusion transduced BMC (Fig. 2a and 2b). Because MI-nc is chemically very similar to the active compounds (Fig. 1a), the lack of activity of this compound serves as evidence that the observed effects are due to specific targeting of the menin-MLL fusion protein interaction rather than off-target effects. Furthermore, MI-2 and MI-3 showed only a small effect on the cell growth of E2A-HLF transduced BMC (GI50 > 50 μM, Fig. 2a and Supplementary Fig. 5b), which may be due to inhibition of the menin interaction with wild-type MLL. Overall, these results demonstrate high specificity of the menin-MLL inhibitors towards MLL fusion protein transformed cells.
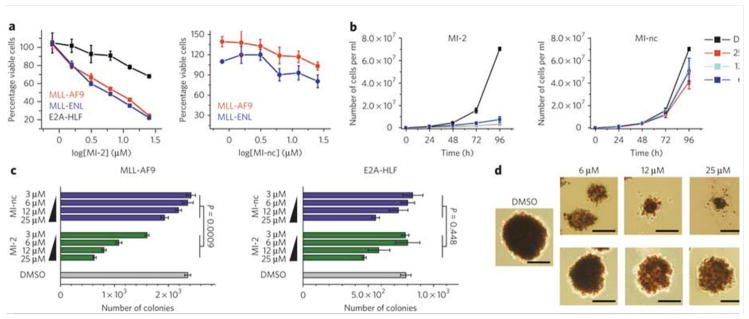
Figure 2. MI-2 induces growth arrest and inhibits transformation in MLL fusion-transduced bone marrow cells.
(a) MI-2 inhibits proliferation of mouse BMC transduced with MLL-AF9 or MLL-ENL but not E2A-HLF (left panel) as detected by MTT cell viability assay. MI-nc is a negative control (right panel). Data represent mean values for quadruplicates ± s.d. Experiments were performed three times. (b) Growth curves for MI-2 (left panel) and MI-nc (right panel) treated MLL-AF9 transduced BMC grown in liquid culture. Data represent mean values for duplicate samples ± s.d. (c) Colony counts for methylcellulose colony assay performed with MLL-AF9 transduced BMC (left panel) and E2A-HLF transduced BMC (right panel) treated for 7 days with MI-2 and MI-nc. Error bars indicate SD from duplicate experiments; two-way ANOVA analysis demonstrates statistical significance of the effect observed for MI-2 as compared to MI-nc (P<0.001) in MLL-AF9 transduced BMC, and no significant difference between MI-2 or MI-nc treated E2A-HLF transduced BMC (P=0.448). Experiments were performed three times. (d) Representative colonies shown for DMSO, MI-2 and MI-nc treated MLL-AF9 transduced BMC plated on methylcellulose. Scale bars are 200 μm.
Menin-MLL inhibitors block transformation by MLL fusions
To assess the effect of menin-MLL inhibitors on transforming potential of MLL fusion proteins, we utilized colony formation assays with MLL-AF9 transformed BMC. The E2A-HLF transduced BMC served as a negative control. MI-2 and MI-3 substantially reduced colony formation in MLL-AF9 transformed cells (Fig. 2c and Supplementary Fig. 5d). After replating and culturing for an additional 7 days, we observed a greater inhibition of colony formation with MI-2 and MI-3 (Supplementary Fig. 5e). In contrast, we detected a very weak effect on colony formation for the MI-nc (Fig. 2c). Importantly, the morphology and size of the colonies were also dramatically different upon treatment with MI-2 and MI-3 (Fig. 2d and Supplementary Fig. 5f). Colonies for DMSO or MI-nc treated cells were large and displayed a dense, compact, blast-like morphology, indicative of transformation30. In contrast, treatment with MI-2 and MI-3 resulted in much smaller, diffuse colonies, indicative of differentiation (Fig. 2d and Supplementary Fig. 5f). Very similar effects were observed upon acute loss of menin19 and after overexpression of a dominant negative fragment of MLL20. Importantly, we observed only a limited effect of MI-2 on the number, size and morphology of colonies formed with E2A-HLF transformed BMC (Fig. 2c and Supplementary Fig. 5g), with no statistically significant difference between treatment with MI-2 and MI-nc. Overall, these data demonstrate that MI-2 and MI-3 substantially and specifically reduce the immortalization potential of cells transformed with MLL fusion oncoproteins.
Menin-MLL inhibitors induce hematopoietic differentiation
Myeloid blasts transformed by MLL oncoproteins are critically dependent on interaction with menin to effectively maintain their undifferentiated state 19,20. Therefore, we tested whether MI-2 and MI-3 can induce differentiation in MLL fusion protein transformed cells (Supplementary Methods). Indeed, we observed that the MLL-AF9 transformed BMC that remained viable after 7 days of treatment with MI-2 and MI-3 showed substantial changes in morphology, indicative of monocytic differentiation, as evidenced by increased cell size, lower nuclear to cytoplasmic ratio and highly vacuolated cytoplasm (Fig. 3a and Supplementary Fig. 6a). By 10 days, the effect was more dramatic as a majority of the cells treated with MI-2 and MI-3 differentiated into macrophages (Fig. 3a and Supplementary Fig. 6b). In contrast, cells treated with DMSO or MI-nc failed to differentiate and maintained a blast-like morphology (Fig. 3a and Supplementary Fig. 6b). Likewise, we also observed differentiation in MLL-ENL transformed BMC treated with MI-2 and MI-3, while no effect was detected after treatment with MI-nc (Supplementary Fig. 6b). Furthermore, the E2A-HLF transformed BMC did not differentiate under treatment with MI-2, MI-3 or MI-nc (Supplementary Fig. 6c), validating the specificity of the menin-MLL inhibitors for the MLL fusion protein transformed cells.
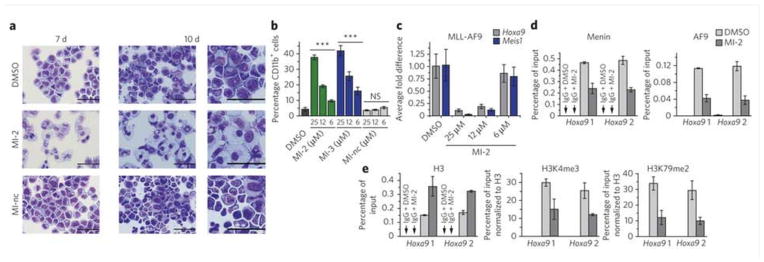
Figure 3. MI-2 induces hematopoietic differentiation and affects expression of MLL fusion protein target genes.
(a) Wright-Giemsa stained cytospins on MLL-AF9 transformed BMC after 7 or 10 days of treatment with DMSO, MI-2 (25 μM) and MI-nc (25 μM). Last column represents zoom for cells after 10 days of treatment. (b) Quantification of CD11b expression in MLL-AF9 transduced BMC treated for 7 days with the menin-MLL inhibitors as detected by flow cytometry. Data represent mean values for triplicates ± s.d. Statistical analysis was performed using one way ANOVA relative to DMSO treated control (*** indicates P < 0.001, ns – not significant). Experiment was performed three times. (c) Quantitative real-time PCR showing the expression of Hoxa9 and Meis1 in MLL-AF9 transduced BMC upon 6 days of treatment with MI-2. Expression of Hoxa9 and Meis1 was normalized to β-actin and referenced to DMSO treated cells. Data represent mean values for triplicates ± s.d. Experiment was performed three times. (d, e) ChIP experiments with antibodies against menin, AF9 and IgG (d), or H3, H3K4me3, H3K79me2 and IgG (e) in the MLL-AF9 transduced BMC after treatment with MI-2 at 12.5 μM or DMSO. Binding was assessed at two sites (Hoxa9 1 and Hoxa9 2) in the promoter region. The signal for H3K4me3 and H3K79me2 was normalized relatively to H3 input. Data represent mean values for duplicate samples ± s.d. Experiment was performed two times.
We also monitored differentiation of MLL-AF9 transformed BMC by detecting the expression level of CD11b, a differentiation marker of myeloid cells. Consistent with the change in cell morphology, the expression of CD11b was substantially increased on MLL-AF9 transformed BMC after 7 days of treatment with MI-2 and MI-3 (Fig. 3b and Supplementary Fig. 7a). No increase in CD11b expression was detected for MI-nc, (Fig. 3b). Collectively, these findings reveal that inhibition of the menin-MLL fusion protein interaction by small molecule inhibitors relieves the differentiation block in MLL fusion protein transformed leukemic blasts, closely recapitulating the effects observed after acute loss of menin in leukemic cells19.
Menin-MLL inhibitors downregulate MLL fusion target genes
Interaction between menin and MLL fusion proteins is required for the maintenance of Hox gene expression in MLL fusion protein transformed cells19,20. Therefore, we tested whether inhibition of the menin-MLL fusion protein interaction by small molecules leads to downregulation of target genes important for transformation. The expression level of Hoxa9 and Meis1 measured by quantitative RT-PCR was substantially reduced in MLL-AF9 transduced BMC, with more than an 80% decrease after 6 days of treatment with 25 μM and 12.5 μM of MI-2 (Fig. 3c). We also observed a similar effect in cells transformed with MLL-ENL (Supplementary Fig. 7b). These results show that disruption of the menin-MLL fusion protein interaction by small molecules effectively downregulates expression of target genes required for leukemogenic activity of MLL fusion proteins. These data are in agreement with the results obtained after acute loss of menin19, as well as, upon expression of the dominant negative MLL fragment20.
MI-2 reduces menin-MLL-AF9 occupancy on the Hoxa9 locus
Menin associates with MLL oncoproteins on Hox gene promoters19,20. To establish whether menin-MLL inhibitors deplete menin at the Hoxa9 locus, we performed chromatin immunoprecipitation (ChIP) assays in MLL-AF9 transduced BMC (Supplementary Methods). Using the anti-menin and anti-AF9 antibody, which recognizes the C-terminus of AF9 retained in the fusion protein31, we demonstrated that both menin and MLL-AF9 were present at the Hoxa9 promoter in the control samples treated with DMSO (Fig. 3d). As expected, treatment with MI-2 decreased binding of menin and MLL-AF9 to the Hoxa9 locus (Fig. 3d). This remains in agreement with the previous studies emphasizing cooperative localization of menin and MLL fusion proteins to the Hoxa9 locus19,20,31. Treatment with MI-2 also resulted in elevated levels of histone H3 reflecting an increase in chromatin condensation after reduction of MLL-AF9 and menin occupancy on the Hoxa9 locus (Fig. 3e). This was also accompanied by a decrease in H3K4 tri-methylation and H3K79 di-methylation (Fig. 3e), consistent with a decrease in transcriptional activity at the Hoxa9 locus after treatment with MI-2. Importantly, our results closely recapitulate the epigenetic changes observed upon excision of Men1 in MLL-AF9 transformed cells31.
Effect of menin-MLL inhibitors in human leukemia cells
Next, we have tested MI-2 and MI-3 in a panel of human MLL leukemia cell lines harboring different MLL translocations. Both compounds showed an effective and dose-dependent growth inhibition of several cell lines with different MLL translocations (Fig. 4a and Supplementary Figure 8a). Treatment with MI-2 resulted in GI50 values below 10 μM in MV4;11 (harboring MLL-AF4; GI50 = 9.5 μM), KOPN-8 (MLL-ENL; GI50 = 7.2 μM) and ML-2 (MLL-AF6; GI50 = 8.7 μM), and in MonoMac6 (MLL-AF9; GI50 = 18 μM) (Fig. 4a and Supplementary Fig. 8a). Very similar results were obtained for MI-3 (Fig. 4a). A pronounced effect of MI-2 and MI-3 on cell growth was also seen in other MLL leukemia cells (Supplementary Fig. 8a). In contrast, MI-nc displayed a significantly weaker effect on proliferation of MLL leukemia cells (Fig. 4a and Supplementary Fig. 8a). The small effect observed upon treatment of MV4;11 and ML-2 cells with MI-nc may result from higher sensitivity of these cells to the menin-MLL inhibitors or a small cytotoxic effect of this compound when used at higher doses.
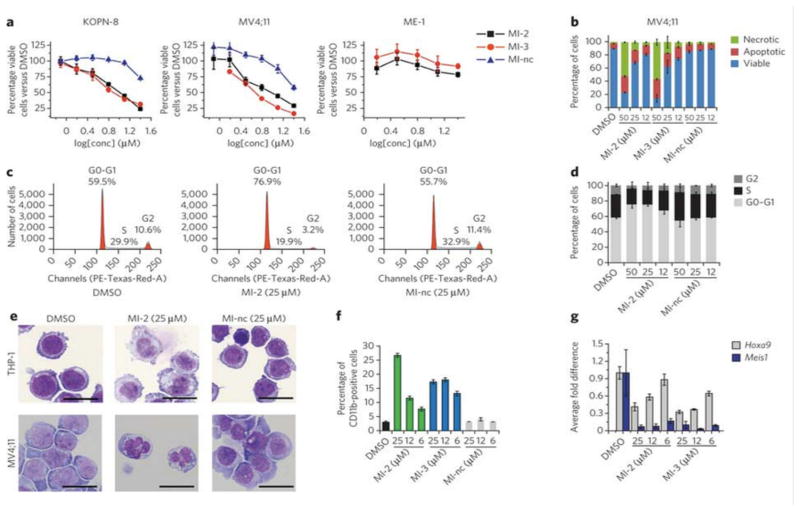
Figure 4. Effect of MI-2 and MI-3 in human MLL leukemia cells.
(a) MTT cell viability assay in the MLL leukemia cells KOPN-8 and MV4;11 induced by MI-2, MI-3 and MI-nc after 72h treatment. Non-MLL leukemia cell line ME-1 is shown for comparison. Data represent mean values for four samples ± s.d. Experiment was performed three times. (b) Apoptosis and cell death induced by MI-2, MI-3 and MI-nc in MV4;11 cells as detected by flow cytometry using AnnexinV/propidium iodide (PI) staining. Data represent mean values for triplicates ± s.d. (c) Selected histograms from cell cycle analysis performed by FACS after PI staining in MV4;11 cells treated with DMSO, MI-2 or MI-nc. (d) Dose-dependent effect of MI-2 on cell cycle progression measured by FACS in MV4;11 cells after PI staining, with MI-nc as a negative control. Data represent mean values for triplicates ± s.d. (e) Wright-Giemsa stained cytospins on THP-1 and MV4;11 cells after 10 days of treatment with DMSO, MI-2 or MI-nc. (f) Detection of CD11b expression in THP-1 cells assessed by flow cytometry after 6 days of treatment with DMSO, MI-2 or MI-nc. Data represent mean values for triplicates ± s.d. (g) Expression of the HOXA9 and MEIS1 genes normalized to 18S rRNA determined by qRT-PCR in THP-1 cells treated for 6 days with MI-2 and MI-3. Data represent mean values for duplicates ± s.d. Experiment was performed three times.
To assess specificity, we assayed the effects of MI-2 and MI-3 on the growth of several human acute leukemia cell lines lacking MLL fusions and expressing a low level of HOXA9, such as Kasumi-1, ME-1, HAL-01 and REH32,33. Both compounds, as well as MI-nc, showed a very limited effect on proliferation of these cells (Fig. 4a and Supplementary Fig. 8b). Interestingly, we detected some effect of MI-2 and MI-3, but less pronounced for MI-nc, on the cell growth of leukemic cells lacking MLL fusions but expressing high or moderate levels of HOXA (HOXA9, HOXA7, HOXA10) and/or MEIS1, such as U937, HL-60 and K562 cell lines33–35, Supplementary Fig. 8c. This effect most likely results from inhibition of the menin interaction with wild type MLL, suggesting that these compounds could potentially be utilized in other acute leukemias with elevated level of HOXA genes32,36,37. Together, these results demonstrate that MI-2 and MI-3 can selectivity target MLL leukemia cells resulting in inhibition of cell growth, emphasizing that these effects should result from on-target effects of these compounds.
We assessed the effect of compounds on apoptosis in two MLL leukemia cell lines, MV4;11 and MonoMac6, by flow cytometry analysis of Annexin V/Propiduim Iodide (PI) stained cells. Treatment with MI-2 and MI-3 for 48h resulted in a substantial, and dose-dependent increase in Annexin V (+) and Annexin V/PI (+)/(+) cells (Fig. 4b and Supplementary Fig. 9a), demonstrating an increase in the number of cells undergoing apoptosis. On the contrary, we did not observe such effect for MI-nc (Fig. 4b). Increased incubation time with MI-2 and MI-3 resulted in more pronounced apoptosis (Supplementary Fig. 9b). The increased apoptosis seen with the menin-MLL inhibitors is consistent with the data reported for the siRNA treatment of the RS4;11 leukemia cells harboring the MLL-AF4 translocation38. In contrast, we detected very limited or no apoptosis in the non-MLL leukemia cells after treatment with MI-2 (Supplementary Fig. 9c), further demonstrating specificity of this compound towards MLL leukemia cells.
We also assessed the effect of menin-MLL inhibitors on cell cycle progression (Supplementary Methods) and observed a consistent increase in the number of cells in G0/G1 phase following treatment of MV4;11 leukemia cells with MI-2 (Fig. 4c,d). Concomitantly, we detected a significant decrease in the population of cells in S and G phases of the cell cycle. These data are consistent with the G0/G1 cell cycle arrest observed after acute loss of menin in the MLL-ENL transformed bone marrow cells19. In contrast, we observed no significant changes in cell cycle for the negative control compound MI-nc (Fig. 4c,d).
Differentiation and HOX downregulation in leukemia cells
We determined the effect of the menin-MLL inhibitors on differentiation of human MLL leukemia cells harboring different MLL translocations, and detected a marked morphology change after treatment with MI-2 and MI-3 indicating that these cells undergo hematopoietic differentiation (Fig. 4e and Supplementary Figs. 10–13). Cell morphology did not change under treatment with MI-nc (Fig. 4e and Supplementary Figs. 10–13). Furthermore, we detected a substantial increase in CD11b positive cells upon 6 days of treatment of THP-1 cells with MI-2 and MI-3 (Fig. 4f and Supplementary Fig. 10b,c). In contrast, treatment with MI-nc did not result in an increase of CD11b expression (Fig. 4f). Together, these results demonstrate that inhibition of the menin-MLL fusion protein interaction by MI-2 and MI-3 relieves differentiation block induced by MLL fusion proteins in human leukemia cells.
HOX cluster genes, particularly HOXA9 and HOXA7, and the HOX cofactor MEIS1, are highly expressed in MLL-associated leukemias11,12,39. We found that treatment of THP-1 cells with MI-2 and MI-3 resulted in substantially reduced expression of HOXA9 and MEIS1 (Fig. 4g). In addition, we observed downregulation of other genes dependent on the menin-MLL interaction (HOXA7, HOXA10 and p27Kip1)19,40 (Supplementary Fig. 14). These results demonstrate that small-molecule inhibitors of the menin-MLL fusion protein interaction effectively downregulate expression of downstream targets of MLL-fusion proteins required for their leukemogenicity in human leukemia cells. These findings are in agreement with the data reported for the menin conditional knock-out19 and upon expression of a dominant negative MLL fragment20.
Discussion
Menin plays a critical role as an oncogenic cofactor of MLL fusion proteins, and the menin-MLL interaction was validated as a potential therapeutic target in acute leukemias with MLL rearrangements 19,20. Inhibition of the menin-MLL fusion protein interaction by small molecules could represent a novel therapeutic strategy for leukemia patients with MLL translocations. Here we report development of small molecules, MI-2 and MI-3, that specifically bind to menin with nanomolar affinities and are potent and reversible inhibitors of the menin-MLL interaction in vitro and in human cells. The in vitro inhibition of the menin-MLL interaction and the cellular response to treatment with these compounds, such as inhibition of proliferation, apoptosis and differentiation of MLL fusion protein transformed cells, are well correlated. Downregulation of HOXA9 and MEIS1 expression, inhibition of transforming properties of MLL fusion proteins, and reduced occupancy of the menin-MLL fusion protein complex on the Hoxa9 promoter induced by these compounds strongly supports specificity in their mechanism of action. These results imply that the cellular activities of MI-2 and MI-3 are through on-target effects. Overall, our results demonstrate for the first time that development of small-molecule inhibitors targeting the menin-MLL interaction is feasible and that such compounds are capable of reversing the leukemogenic activity of MLL fusion proteins. The effects observed upon treatment with these compounds closely recapitulate the effects of disruption of the menin-MLL fusion protein interaction achieved by excision of the menin binding motif from the N-terminus of MLL-GAS7 and MLL-ENL fusion proteins19. They also recapitulate the effects observed in MLL fusion protein transduced bone marrow progenitor cells following acute loss of menin19 or the expression of a dominant negative N-terminal fragment of MLL20.
MI-2 and MI-3 are valuable chemical probes that can be used to study the biology and mechanism of MLL-fusion protein mediated leukemogenesis and to further validate the role of menin as an oncogenic co-factor of MLL fusion proteins. These compounds also represent a valuable pharmacophore that can be applied to address the potential therapeutic benefit of inhibiting the menin-MLL interaction and might result in development of therapeutically useful compounds for leukemia patients with rearrangements of the MLL gene.
The menin binding motif is located at the N-terminus of MLL19,20,26 and is preserved in wild-type MLL and in all MLL fusion proteins. Therefore, targeting the menin-MLL interaction represents a general approach to inhibit the leukemogenic activity of all MLL fusion proteins regardless of the fusion partner. Due to inhibition of the menin-MLL wild-type interaction, MI-2 and MI-3 may also serve as valuable chemical probes to improve our understanding of menin’s precise role in complex with MLL during hematopoiesis and in transcriptional regulation. Furthermore, the MLL-AF9 fusion protein requires the expression of wild-type MLL to induce and maintain leukemogenic transformation31. Therefore, simultaneous targeting of the menin-MLL and menin-MLL fusion protein interactions by small-molecule inhibitors reported here could effectively block oncogenesis by MLL fusion proteins. Future efforts will be undertaken to improve the therapeutic potential of the compounds identified in this study with the goal of developing targeted therapy for acute leukemia patients with translocations of the MLL gene and possibly other acute leukemias with upregulated HOX genes 32,36,37. These compounds possess desirable properties, such as high target potency, selectivity, low molecular weight (below 400 Da), synthetic accessibility and utility in experimental biology. This establishes the plausibility of developing both drug-like derivatives for therapeutic application and chemical probes for further understanding the biology and oncogenic activity of MLL fusion proteins.
Methods
Protein expression
The expression and purification of menin was done as described previously26.
High Throughput Screening
FITC-MBM1 at 15 nM and menin at 150 nM in the FP buffer (Supplementary Table 1) were mixed and incubated for 1h in the dark at room temperature. A collection of 49,000 compounds from the Center for Chemical Genomics, University of Michigan, was used for HTS. For point screening, the 0.2 μL of each compound (20 μM final concentration, 1% DMSO) was added to 20 μL of the aliquot of the protein-peptide mixture and incubated on 384-well plates in the dark at room temperature for 1h. In confirmation screening, the serial dilution plates with compounds in DMSO were prepared and used to titrate the menin-FITC-MBM1 complex. Change in fluorescence polarization was monitored at 525 nm after excitations at 495 nm using the PHERAstar microplate reader (BMG) and applied to determine IC50 values with the Origin 7.0 program.
Cell lines and cell culture
HEK 293 cells were cultured in Dulbecco’s modified Eagle’s medium, DMEM, (Invitrogen) supplemented with 10% fetal bovine serum, FBS, (Invitrogen), and 1× non-essential amino acids, NEAA, (Invitrogen). KOPN-8, ML-2, Kasumi-1 and ME-1 cells were cultured in Roswell Park Memorial Institute (RPMI-1640) medium (Invitrogen) supplemented with 10% FBS. MV4;11 and THP-1 cells were cultured in RPMI-1640 medium with 10% FBS, 1% penicillin/streptomycin and NEAA. MonoMac-6 cells were cultured in RPMI-1640 medium with 15% FBS, human insulin, 1% penicillin/streptomycin (Invitrogen) and NEAA.
Mouse bone marrow cells
MLL-AF9, MLL-ENL and E2A-HLF transduced mouse bone marrow cells were prepared as described previously 41. These cells were cultured in IMDM media supplemented with 15% FBS, 1% penicillin/streptomycine and IL-3.
Vector construction
The expression vector for Flag-MLL-AF9 was prepared as described previously41.
Chromatin immunoprecipitation assay (ChiP)
ChiP was performed in MLL-AF9 transduced BMC treated with MI-2 or DMSO using the procedure described previously 42. Primary antibodies specific for AF9, Menin (Bethyl), histone H3, H3K4 trimethylation, and H3K79 dimethylation (Abcam) were used, see Supplementary Methods for details.
Annexin V/PI assay of inhibitor effects on apoptosis
5×105 cells/ml were plated in 12-well plates (1ml/well) and treated with compounds (0.25% final concentration of DMSO for each condition) or 0.25% DMSO control and incubated for 48h at 37 °C in a 5% CO2 incubator. After incubation, 1.5×105 cells were harvested and resuspended in 100 μl 1× Annexin V binding buffer from the Annexin V-FITC Apoptosis kit (BD Biosciences Pharmingen), incubated with 4 μl of AnnexinV-FITC and 6 μl of Propidium iodide (Sigma-Aldrich) at room temperature in the dark for 10 minutes and analyzed by flow cytometry on a LSR II instrument. Data analysis was performed using WinList software. The experiments were performed three times in triplicates with calculation of mean and standard deviation for each condition.
Inhibitor effects on expression of CD11b
THP-1 cells or MLL-AF9 transduced bone marrow cells were plated in 12-well plates at initial concentration of 5×105 cells/ml and treated with compounds or 0.25% DMSO. Media were changed every 48h with viable cell concentration restored to 5×105 cells/ml and compounds resupplied. Six days after the experiment was set-up, the 1.5×105 cells were harvested and washed with FACS buffer (PBS, 1% FBS, 0.1% NaN3). Cells were resuspended in 100 μl FACS buffer and incubated with 2 μl Pacific Blue mouse anti-human CD11b antibody (BD Biosciences) or 1 μl Pacific Blue rat anti-mouse CD11b antibody (BioLegend) at 4°C for 30 min. Cells were then washed, resuspended in 100 μl Annexin V binding buffer, and incubated with 4 μl Annexin V-FITC (BD Biosciences) and 6 μl Propidium iodide (1mg/ml, Sigma-Aldrich) at room temperature for 10 min before being analyzed by flow cytometry.
Colony formation assay
The MLL-AF9 and E2A-HLF transduced murine BMC were plated in 12-well plates at the concentration of 5×103 cells/ml with 1 ml methylcellulose medium M3234 (StemCell Technologies) containing 20% IMDM medium, 1% penicillin/streptomycin, IL-3 and 0.25% DMSO or compounds. 6 days later colonies were stained with 100 μl iodonitrotetrazolium chloride (Sigma-Aldrich) at final concentration of 1mg/ml, incubated at 37°C for 30 min and counted. To replate for the 2nd round, colonies were counted at day 6 without staining and cells were washed out by 1×PBS buffer and resuspended in IMDM medium containing 15% FBS, 1% penicillin/streptomycin and IL-3. 5×103 cells were plated in 12-well plates with 1ml methycellulose medium M3234 (StemCell Technologies, Inc.) containing 20% IMDM medium, 1% penicillin/streptomycin, IL-3 and 0.25% DMSO or compounds. 6 days later colonies were stained and counted.
Real-Time PCR
Total RNA was extracted from cells using RNeasy mini kit (Qiagen). 100 ng – 1000 ng of total RNA was reverse transcribed using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) according to the manufacturer’s protocol. Real-time PCR was performed using the ABI Prism 7700 sequence detection system. Taqman Gene Expression Master Mix and Taqman Gene Expression Assays for mouse Hoxa9 (Mm00439364_m1), Meis1 (Mm00487664_m1), β-Actin (4352933), human HOXA9 (Hs00365956_m1), MEIS1 (Hs00180020_m1) and 18S RNA (Hs99999901_s1) were purchased from Applied Biosystems. Relative quantification of each gene transcript was carried out using the comparative Ct method as described in the Applied Biosystems User Bulletin No. 2.
Statistical analysis
Mean values and standard deviations were calculated to estimate the degree of data variation, as specified for each experiment in Figure legend. GraphPad Prism software was used for statistical analysis. Two-way ANOVA analysis was applied to calculate the statistical significance of the data (P values).
Supplementary Material
Acknowledgments
This work was supported by grants from the Leukemia and Lymphoma Society (TRP grant 6070-09 to J.G. and LLS SCOR grant to J.L.H.), NIH R01 (1R01 CA-160467-01 to J.G.), Cancer Center (University of Virginia to J.G. and T.C.), Children’s Leukemia Research Association (to J.G.), American Cancer Society (RSG-11-082-01-DMC to TC) and startup funds to J.G. and T.C. provided by the Department of Pathology (University of Michigan). The authors greatly appreciate support from Dr. John Bushweller (University of Virginia) for providing the research environment for carrying out part of these studies. We are grateful to Martha Larsen from the Center for Chemical Genomics, University of Michigan, for technical expertise during HTS. We would like to thank Jiaying Tan (University of Michigan) for discussion of experimental procedures and Dr. Ronald Craig from the Department of Pathology (University of Michigan) for technical support with flow cytometry experiments.
Footnotes
Author contributions
J.G. and T.C. initiated the project, lead the project team, designed experiments and analyzed results. S.H., T.P. and A.G.M. performed cellular assays. A.S., R.J.S., H.D.S. synthesized compounds. M.M. and T.H. expressed and purified proteins, run ITC and thermal shift assays. A.B. performed biochemical assays. J.L.H. designed experiments and analyzed results. J.G. and T.C. wrote the paper with input from all authors.
Competing Financial Interests Statement
The authors declare no competing financial interests.
Supplementary information, chemical compound and chemical probe information is available online at http://www.nature.com/naturechemicalbiology. Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/.
References
- 1.Pui CH, et al. Outcome of treatment in childhood acute lymphoblastic leukaemia with rearrangements of the 11q23 chromosomal region. Lancet. 2002;359:1909–15. doi: 10.1016/S0140-6736(02)08782-2. [DOI] [PubMed] [Google Scholar]
- 2.Liu H, Cheng EH, Hsieh JJ. MLL fusions: pathways to leukemia. Cancer Biol Ther. 2009;8:1204–11. doi: 10.4161/cbt.8.13.8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorensen PH, et al. Molecular rearrangements of the MLL gene are present in most cases of infant acute myeloid leukemia and are strongly correlated with monocytic or myelomonocytic phenotypes. J Clin Invest. 1994;93:429–37. doi: 10.1172/JCI116978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox MC, et al. Chromosomal aberration of the 11q23 locus in acute leukemia and frequency of MLL gene translocation: results in 378 adult patients. Am J Clin Pathol. 2004;122:298–306. doi: 10.1309/RX27-R8GJ-QM33-0C22. [DOI] [PubMed] [Google Scholar]
- 5.Hess JL. MLL: a histone methyltransferase disrupted in leukemia. Trends Mol Med. 2004;10:500–7. doi: 10.1016/j.molmed.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Popovic R, Zeleznik-Le NJ. MLL: how complex does it get? J Cell Biochem. 2005;95:234–42. doi: 10.1002/jcb.20430. [DOI] [PubMed] [Google Scholar]
- 7.Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nat Rev Cancer. 2007;7:823–33. doi: 10.1038/nrc2253. [DOI] [PubMed] [Google Scholar]
- 8.Slany RK. The molecular biology of mixed lineage leukemia. Haematologica. 2009;94:984–93. doi: 10.3324/haematol.2008.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hess JL, Yu BD, Li B, Hanson R, Korsmeyer SJ. Defects in yolk sac hematopoiesis in Mll-null embryos. Blood. 1997;90:1799–806. [PubMed] [Google Scholar]
- 10.Lawrence HJ, et al. Mice bearing a targeted interruption of the homeobox gene HOXA9 have defects in myeloid, erythroid, and lymphoid hematopoiesis. Blood. 1997;89:1922–30. [PubMed] [Google Scholar]
- 11.Rozovskaia T, et al. Upregulation of Meis1 and HoxA9 in acute lymphocytic leukemias with the t(4:11) abnormality. Oncogene. 2001;20:874–8. doi: 10.1038/sj.onc.1204174. [DOI] [PubMed] [Google Scholar]
- 12.Ayton PM, Cleary ML. Transformation of myeloid progenitors by MLL oncoproteins is dependent on Hoxa7 and Hoxa9. Genes Dev. 2003;17:2298–307. doi: 10.1101/gad.1111603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeisig BB, et al. Hoxa9 and Meis1 are key targets for MLL-ENL-mediated cellular immortalization. Mol Cell Biol. 2004;24:617–28. doi: 10.1128/MCB.24.2.617-628.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong P, Iwasaki M, Somervaille TC, So CW, Cleary ML. Meis1 is an essential and rate-limiting regulator of MLL leukemia stem cell potential. Genes Dev. 2007;21:2762–74. doi: 10.1101/gad.1602107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Argiropoulos B, Humphries RK. Hox genes in hematopoiesis and leukemogenesis. Oncogene. 2007;26:6766–76. doi: 10.1038/sj.onc.1210760. [DOI] [PubMed] [Google Scholar]
- 16.Rice KL, Licht JD. HOX deregulation in acute myeloid leukemia. J Clin Invest. 2007;117:865–8. doi: 10.1172/JCI31861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ernst P, Wang J, Korsmeyer SJ. The role of MLL in hematopoiesis and leukemia. Curr Opin Hematol. 2002;9:282–7. doi: 10.1097/00062752-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Slany RK. When epigenetics kills: MLL fusion proteins in leukemia. Hematol Oncol. 2005;23:1–9. doi: 10.1002/hon.739. [DOI] [PubMed] [Google Scholar]
- 19.Yokoyama A, et al. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell. 2005;123:207–18. doi: 10.1016/j.cell.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 20.Caslini C, et al. Interaction of MLL amino terminal sequences with menin is required for transformation. Cancer Res. 2007;67:7275–83. doi: 10.1158/0008-5472.CAN-06-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen YX, et al. The tumor suppressor menin regulates hematopoiesis and myeloid transformation by influencing Hox gene expression. Proc Natl Acad Sci U S A. 2006;103:1018–23. doi: 10.1073/pnas.0510347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandrasekharappa SC, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276:404–7. doi: 10.1126/science.276.5311.404. [DOI] [PubMed] [Google Scholar]
- 23.Marx SJ. Molecular genetics of multiple endocrine neoplasia types 1 and 2. Nat Rev Cancer. 2005;5:367–75. doi: 10.1038/nrc1610. [DOI] [PubMed] [Google Scholar]
- 24.Hughes CM, et al. Menin associates with a trithorax family histone methyltransferase complex and with the hoxc8 locus. Mol Cell. 2004;13:587–97. doi: 10.1016/s1097-2765(04)00081-4. [DOI] [PubMed] [Google Scholar]
- 25.Yokoyama A, et al. Leukemia proto-oncoprotein MLL forms a SET1-like histone methyltransferase complex with menin to regulate Hox gene expression. Mol Cell Biol. 2004;24:5639–49. doi: 10.1128/MCB.24.13.5639-5649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grembecka J, Belcher AM, Hartley T, Cierpicki T. Molecular basis of the mixed lineage leukemia-menin interaction: implications for targeting mixed lineage leukemias. J Biol Chem. 2010;285:40690–8. doi: 10.1074/jbc.M110.172783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer M, Meyer B. Group epitope mapping by saturation transfer difference NMR to identify segments of a ligand in direct contact with a protein receptor. J Am Chem Soc. 2001;123:6108–17. doi: 10.1021/ja0100120. [DOI] [PubMed] [Google Scholar]
- 28.Murai MJ, Chruszcz M, Reddy G, Grembecka J, Cierpicki T. Crystal structure of Menin reveals the binding site for mixed lineage Leukemia (MLL) protein. J Biol Chem. 2011 doi: 10.1074/jbc.M111.258186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 30.Lavau C, Szilvassy SJ, Slany R, Cleary ML. Immortalization and leukemic transformation of a myelomonocytic precursor by retrovirally transduced HRX-ENL. EMBO J. 1997;16:4226–37. doi: 10.1093/emboj/16.14.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thiel AT, et al. MLL-AF9-induced leukemogenesis requires coexpression of the wild-type Mll allele. Cancer Cell. 2010;17:148–59. doi: 10.1016/j.ccr.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drabkin HA, et al. Quantitative HOX expression in chromosomally defined subsets of acute myelogenous leukemia. Leukemia. 2002;16:186–95. doi: 10.1038/sj.leu.2402354. [DOI] [PubMed] [Google Scholar]
- 33.Yokoyama A, Lin M, Naresh A, Kitabayashi I, Cleary ML. A higher-order complex containing AF4 and ENL family proteins with P-TEFb facilitates oncogenic and physiologic MLL-dependent transcription. Cancer Cell. 2010;17:198–212. doi: 10.1016/j.ccr.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson A, et al. Global down-regulation of HOX gene expression in PML-RARalpha + acute promyelocytic leukemia identified by small-array real-time PCR. Blood. 2003;101:1558–65. doi: 10.1182/blood.V101.4.1558. [DOI] [PubMed] [Google Scholar]
- 35.Fiskus W, et al. Histone deacetylase inhibitors deplete enhancer of zeste 2 and associated polycomb repressive complex 2 proteins in human acute leukemia cells. Mol Cancer Ther. 2006;5:3096–104. doi: 10.1158/1535-7163.MCT-06-0418. [DOI] [PubMed] [Google Scholar]
- 36.Golub TR, et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science. 1999;286:531–7. doi: 10.1126/science.286.5439.531. [DOI] [PubMed] [Google Scholar]
- 37.Quentmeier H, et al. Expression of HOX genes in acute leukemia cell lines with and without MLL translocations. Leuk Lymphoma. 2004;45:567–74. doi: 10.1080/10428190310001609942. [DOI] [PubMed] [Google Scholar]
- 38.Thomas M, et al. Targeting MLL-AF4 with short interfering RNAs inhibits clonogenicity and engraftment of t(4;11)-positive human leukemic cells. Blood. 2005;106:3559–66. doi: 10.1182/blood-2005-03-1283. [DOI] [PubMed] [Google Scholar]
- 39.Armstrong SA, et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet. 2002;30:41–7. doi: 10.1038/ng765. [DOI] [PubMed] [Google Scholar]
- 40.Milne TA, et al. Menin and MLL cooperatively regulate expression of cyclin-dependent kinase inhibitors. Proc Natl Acad Sci U S A. 2005;102:749–54. doi: 10.1073/pnas.0408836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muntean AG, et al. The PAF complex synergizes with MLL fusion proteins at HOX loci to promote leukemogenesis. Cancer Cell. 2010;17:609–21. doi: 10.1016/j.ccr.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milne TA, et al. MLL associates specifically with a subset of transcriptionally active target genes. Proc Natl Acad Sci U S A. 2005;102:14765–70. doi: 10.1073/pnas.0503630102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.