Abstract
Non-steroidal anti-inflammatory drugs (NSAIDs) are one of the most commonly prescribed drugs in the world. NSAID-induced lower gastrointestinal (GI) complications are increasing while upper GI complications are decreasing. Lower GI events accounted for 40% of all serious GI events in patients on NSAIDs. Capsule endoscopy and device assisted enteroscopy are available for detection of small intestinal lesions. Capsule endoscopy studies have demonstrated that NSAIDs use in healthy volunteers raised the incidence (55% to 75%) of intestinal damage. It appears that selective cyclooxygenase-2 inhibitors (coxibs) improved upper and lower GI safety based on results of clinical trials. Selective coxibs are still capable of triggering GI adverse events and cardiovascular toxicity issues were the main focus of concerns. Unfortunately, definite strategies are not available to prevent or heal NSAID-induced intestinal injuries. Thus, there is still a strong clinical need for effective drugs with improved safety profiles than the existing NSAIDs.
Keywords: Anti-inflammatory agents, non-steroidal; Lower gastrointestinal tract; Cyclooxygenase 2 inhibitors
INTRODUCTION
Non-steroidal anti-inflammatory drugs (NSAIDs) are one of the most commonly prescribed drugs in the world for their analgesic and anti-inflammatory properties. However, NSAID has limitation in prescribing because of gastrointestinal (GI) toxicity. Recently, NSAID-induced enteropathy has gained much attention due to the introduction of new emerging diagnostic modalities, capsule endoscopy (CE) and device assisted enteroscopy as well as due to the increased use of aspirin and NSAIDs. Recent CE studies have demonstrated NSAID use in healthy volunteers raised the incidence (55% to 75%) of intestinal damage.1-6 NSAID-induced enteropathy has been under-examined or even ignored in clinical situation before the times of CE.
Previous attention or studies have focused primarily on upper GI events but recent researches have shifted to the small bowel and colon during chronic NSAID use. Proton pump inhibitor (PPI) can't protect NSAID-induced intestinal injuries below Treitz ligament while PPI is very helpful to reduce NSAID-induced gastroduodenal damages.7 Until now, no medications are available yet to prevent or heal NSAID-induced intestinal injuries. It is important to extend the understanding about small bowel and colonic injuries associated with NSAID because all clinician, especially gastroenterologists, should have thorough knowledge of the GI toxicity of NSAIDs.
EPIDEMIOLOGY
It has been known that lower GI events accounted for one-third of all clinically relevant GI events. NSAID-induced lower GI complications (perforation, bleeding, or obstruction) are increasing while upper GI complications are decreasing.7 In fact, the ratio of upper/lower was 4.1 in 1996 and it has decreased to only 1.4 in 2005.7,8 Current evidences suggest that NSAIDs increase the risk of lower GI bleeding and perforation to a similar extent to that seen in the upper GI tract.9 Post-hoc analysis of a large-scale clinical outcome trial showed that lower GI events accounted for 40% of all serious GI events in patients on NSAIDs.10
There were notable CE studies in short-term NSAID users as well as chronic NSAID users. Goldstein et al.5 reported that small bowel mucosal breaks were induced in 55% of healthy volunteers who had taken naproxen for 2 weeks. Maiden et al.4 reported that 2-week ingestion of slow release diclofenac resulted in macroscopic injury to the small intestine in 68% to 75% of healthy volunteers. Graham et al.3 performed CEs in arthritic patients who had been using NSAIDs for at least 3 months and reported as high as 71% for the incidence of small intestinal mucosal injury after chronic NSAID administration. The Japanese Study Group for Double-Balloon Endoscopy reported that NSAID-induced enteropathy occurred in half of the patients taking NSAIDs based on the registry of a 2-year period.11 CE and double-balloon enteroscopy (DBE) findings suggest that the small intestinal mucosa is very sensitive to NSAID-induced injuries (Table 1).
Table 1.
Epidemiology of Non-steroidal Anti-inflammatory Drug (NSAID) Enteropathy
GI, gastrointestinal.
PATHOPHYSIOLOGY
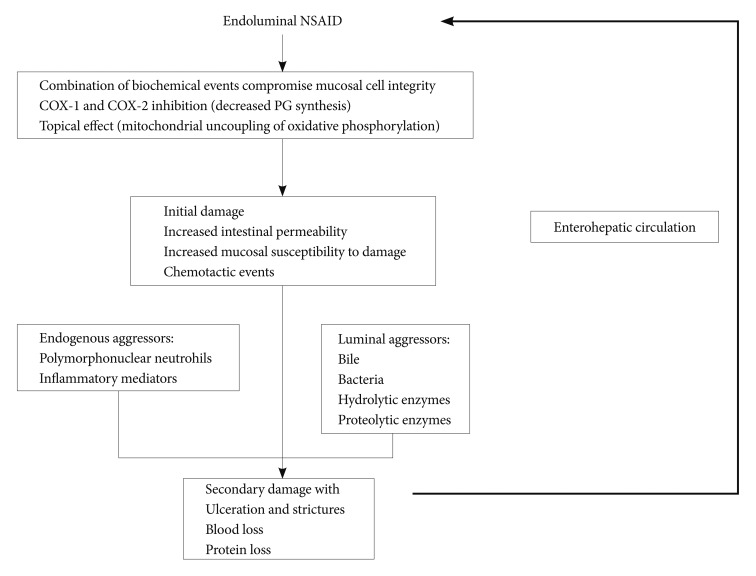
The pathogenesis of NSAID-induced enteropathy is distinct from that of NSAID-induced gastropathy. Unlike stomach, NSAID-induced lower GI injuries are not caused by sup-pression of prostaglandin synthesis due to inhibition of cyclo-oxygenase (COX) activity12,13 but, most of the time, by gram negative bacteria and bile.4-22 Bjarnason et al.15 proposed a "three hit" hypothesis. First, NSAIDs solubilize lipids of phospholipids on the mucosal surface, so the epithelial mitochondria are directly damaged. Second, the mitochondrial damage depletes intercellular energy and leads to calcium efflux and to induction of free radicals, a disruption of intercellular junctions occurs, and mucosal permeability increases in the small intestinal mucosa. Third, the mucosal barrier becomes weakened, so bile acid, proteolytic enzymes, intestinal bacteria, or toxins can easily penetrate into the epithelial cells, resulting in mucosal injury. NSAID-induced small intestinal injuries augment through the enterohepatic circulation of the NSAID (Fig. 1).14 NSAIDs with no enterohepatic circulation did not cause significant intestinal damage in animal models.14
Fig. 1.
Pathophysiology of non-steroidal anti-inflammatory drug (NSAID)-induced enteropathy. NSAIDs suppress cyclooxygenase isoenzymes and directly damage intestinal epithelium. Intercellular junctions weaken and then intraluminal aggressors including polymorphonuclear neutrophils, inflammatory mediators, bile, bacteria, hydrolytic, or proteolytic enzymes attack the intestinal epithelial cells. Secondary damage with ulceration, stricture and blood, or protein loss is augmented by the enterohepatic circulation of NSAID. PG: prostaglandin.
CLINICAL PICTURES
NSAIDs affect the entire GI system and can often cause various abdominal symptoms such as epigastric pain, nausea, indigestion, constipation, and abdominal distension.13 Multiple ulcers and erosions are common NSAID-induced small intestinal lesions.14 Ulceration often occurs in the GI tract without symptoms because of analgesic effect of NSAIDs. Aspirin itself can also cause stomach and duodenal ulcers, but it is generally believed that aspirin does not usually cause small bowel damage according to intestinal permeability and fecal inflammatory marker studies and aspirin does not have enterohepatic circulation.14 Enteric coated aspirin was originally designed to decrease adverse effects on the stomach, but the use of enteric coated aspirin may have shifted the damage to the distal small bowel. Low dose aspirin is not safe, and chronic low dose aspirin use induced small bowel enteropathy that has many striking similarities with NSAID-induced enteropathy.23
NSAIDs were found to induce obscure overt GI bleeding which manifests as hematochezia or melena, or occult obscure GI bleeding, as positive guaiac fecal occult blood test or anemia of unknown etiology.24 NSAID-induced enteropathy is one of the most common causes of obscure GI bleeding.24
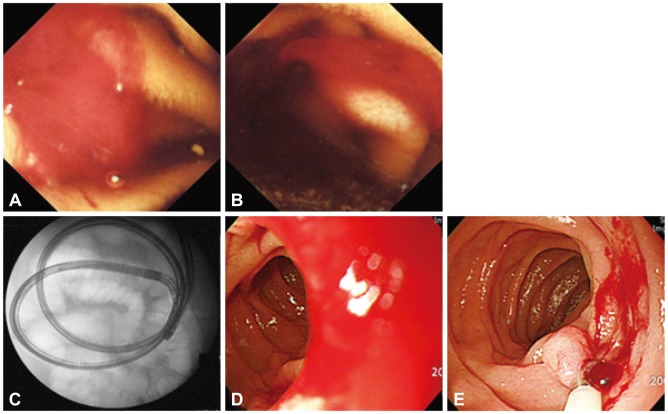
NSAID-induced enteropathy might be suspected strongly in patients with obscure GI bleeding and recent history of taking aspirin or NSAIDs. CE sensitively detects NSAID-induced small intestinal injuries such as red spot, erosion, and ulcer, while NSAID-induced small intestinal injuries are not specific in endoscopic findings and NSAID-induced ulcer is hard to differentiate from other causes of ulcer such as Crohn's disease. NSAID-induced small intestinal bleeding can be effectively controlled by a device assisted enteroscopy (Fig. 2).
Fig. 2.
A 40-year-old man with recurrent obscure gastrointestinal bleeding (Hb=5.0 mg/dL). He had intermittently taken non-steroidal anti-inflammatory drugs for intractable back pain. Capsule endoscopic finding (A, B) showed ongoing mid-jejunal bleeding without definite mucosal lesion and this bleeding was controlled by clipping through single balloon endoscopy (C, D, E).
Concentric diaphragmatic stricture is thought to be the pathognomonic symptom of NSAID injury.25 Clinical presentation of diaphragm disease is nonspecific and may be presented with obstructive symptoms. It develops from scarring reaction secondary to ulcerative injury during long-term NSAID use. The histological features of the diaphragm-like stricture include fibrosis in the submucosa and thickening of the muscularis mucosa.26 Since the muscularis propria layer is intact, the risk of intestinal perforation is low with endoscopic balloon dilation, which is why it is a preferred treatment modality than surgical intervention.27 Capsule can be retrieved successfully by enteroscopy in case of the capsule entrapment.
In randomized trials in patients with rheumatoid or osteoarthritis, diverticulitis and diverticular bleeding are the most common etiologies of NSAID-induced lower GI injuries, accounting for 30% to 50% of all serious GI events associated with NSAIDs.28 Case control studies have shown a significantly higher prevalence of NSAID use with complications of diverticular diseases (diverticulitis and bleeding) compared to controls - their risk estimates varied widely with odds ratios ranging from 1.8 to 16.7 Regular use of aspirin or NSAIDs was associated with increased risks of diverticulitis and diverticular bleeding.7,29
DIAGNOSIS
NSAIDs can induce a variety of abnormalities including ulcerations, perforations, bleeding, and diaphragm-like strictures in the human small intestine.7 Difficulties in accessing the entire small intestine prevented comprehensive evaluation of the extent and localization of NSAID-induced small intestinal injury, however. Thus, prior to the advent of CE and small bowel enteroscopy, most studies had to rely on surrogate markers such as urinary excretion of chromium-51-labeled ethylenediaminetetraacetic acid, which shows intestinal permeability and fecal level of calprotectin, a proposed marker of intestinal inflammation.30,31 Calprotectin is a 36 kDa calcium-binding protein constituting up to 60% of the cytosolic proteins in neutrophil granulocytes and plays an important role in inflammatory processes.32 It is excreted in feces and remains stable against bacterial degradation.33 The presence of calprotectin in feces is a consequence of neutrophils' migration into the GI tissue.33 But now, with CE, we can easily locate the site of injury and evaluate the incidence rates and types of injuries.
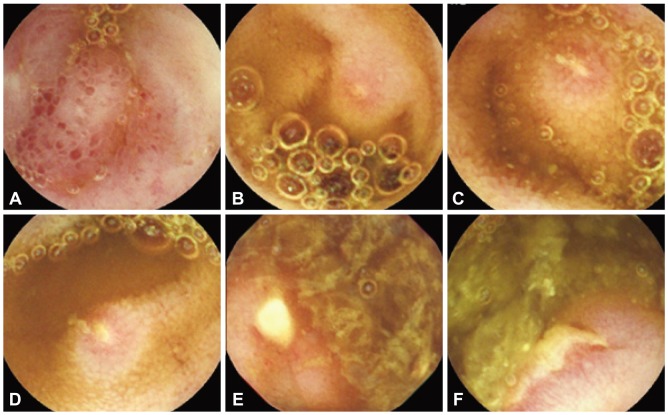
Presently, CE and DBE are available for direct detection of small intestinal lesions. Endoscopic findings of NSAID-induced enteropathy include reddish erosion, multiple sharply demarcated ulcer and concentric stenosis. Multiple discrete ulcers are the most frequent finding. NSAID-induced small intestinal lesions on CE include denuded areas, erosions and ulcers (Fig. 3). A denuded area is defined as a reddened area without villi.4 Maiden et al.4 classified CE findings into five categories: reddened folds, denuded area, red spot, mucosal break, and blood. Graham et al.3 divided CE findings into red spots, small erosions, large erosions, and ulcers, and they found mucosal lesions in 13 out of 21 patients (62%) with chronic NSAID use. Small lesions such as erosions and red spots are detected more often by CE than DBE.
Fig. 3.
Capsule endoscopic findings of non-steroidal anti-inflammatory drug enteropathy. There were denuded areas (A), erosions (B, C, D), and multiple variously-shaped well-demarcated ulcers (E, F).
TREATMENT AND FUTURE DIRECTION
Previous efforts have focused primarily on GI events in the upper GI tract, but recently, efforts have been made to reduce NSAID-induced small bowel and colon injuries including mucosal damage, ulceration, overt bleeding, obstruction/perforation, protein-loss and occult blood loss (with an associated decrease in hemoglobin).
Fujimori et al.34 demonstrated the benefit of treatment with misoprostol in a small pilot study in which small intestinal damage was assessed by CE. Misoprostol co-therapy reduced the incidence of small intestinal lesions induced by a 2-week administration of diclofenac sodium in healthy subjects. Watanabe et al.35 examined therapeutic effect of misoprostol against aspirin induced injury. Their subjects were gastric ulcer patients who were orally taking low dose, enteric coated aspirin. They were treated with PPI for 8 weeks, but all patients had redness and erosions in the small intestine on CE at 8 weeks. When misoprostol was administered instead of PPI for additional 8 weeks, small intestinal lesions were improved on the follow-up CE. Misoprostol appeared to be capable of healing aspirin induced small bowel injury.
Selective COX-2 inhibitors (coxibs) were introduced to the marketplace in the mid-1990s with a promise of improved GI safety. Goldstein et al.5 reported that 2-week regimen with celecoxib, a selective coxib, caused less small intestinal injury than a regimen using naproxen. Selective coxibs were believed to be less injurious than nonselective NSAIDs in the small bowel as well as in the stomach, but Maiden et al.1 found no difference in the incidence of small intestinal injury between chronic users of nonselective NSAIDs and selective coxibs. Selective coxibs may not provide complete protection against GI toxicity. Selective coxibs are selective, but not exclusive, and thus have some COX-1 inhibitory activity. Further studies with large samples are needed to decide whether or not selective coxibs can entirely control GI toxicity.
Niwa et al.36 conducted a prospective, double-blind study of CE using a mucosal protective agent, rebamipide, in healthy subjects. It was found that subjects who received placebo had significantly more mucosal injuries in the small intestine compared to those who received rebamipide. Mucosal protecting agents such as teprenone, rebamipide, and irsogladine showed protective effect against NSAID-induced small intestinal injuries according to animal experiments using rats; more specifically, the increase in iNOS mRNA expression and myeloperoxidase activity due to indomethacin was inhibited by pre-treatment with these mucosal protecting agents.24
Several strategies have been tried to develop new compounds with improved GI tolerability. Several companies are developing drugs that co-release an NSAID (naproxen, aspirin, or ibuprofen) and a PPI (omeprazole, esomeprazole, or lansoprazole); these compounds are already filed with the FDA or in very late stages of development.37 These compounds have been shown to reduce the incidence of NSAID-induced gastropathy but not enteropathy.
Significant part of NSAIDs' GI injury mechanism is related to their topical irritancy. PLx Pharma Inc. (Houston, TX, USA) has taken a strategy to reduce the topical irritant property of NSAIDs and has developed NSAIDs that are associated with phosphatidylcholine.38 Extensive animal works have demonstrated that phosphatidylcholine-NSAIDs are less likely to damage the GI epithelium in rodents, and produce less severe mucosal injury while retaining the anti-inflammatory effects of the parent NSAID. In a randomized, double-blind, endoscopy study, ibuprofen preassociated with phosphatidylcholine showed superior GI toxicity than an equivalent dose of ibuprofen in the subgroup of patients older than 55 years.39
The discovery that nitric oxide (NO) is a potent gastroprotective substance that modulates many aspects of GI mucosal defense led to the development of NO releasing NSAIDs, which are also referred to as COX-inhibiting NO donors.40 The compound has shown improved GI tolerability in some but not all clinical trials. Like NO, hydrogen sulfide (H2S) has been shown to exert protective effects in the GI tract and to accelerate the healing of preexisting ulcers. H2S-releasing NSAIDs, derivatives of naproxen, diclofenac, and indomethacin have been reported.41-43 Phosphatidylcholine associated NSAIDs as well as NO- and H2S-releasing NSAIDs are under extensive preclinical testing for their influence on NSAID-induced enteropathy. Further studies are warranted to develop promising new NSAID with total GI (upper and lower GI tracts) tolerability and without cardiovascular toxicity.
CONCLUSIONS
NSAIDs are one of the most commonly prescribed drugs in the world for analgesic and anti-inflammatory properties. Previous studies have focused primarily on upper GI events; but recently, NSAID-induced enteropathy has gained much attention due to the introduction of new emerging diagnostic modalities, CE and device assisted enteroscopy. Lower GI events accounted for 40% of all serious GI events in patients taking NSAIDs.14 CE studies have demonstrated NSAID use in healthy volunteers raised the incidence (55% to 75%) of intestinal damage.1-6 Unfortunately, however, PPI can't protect NSAID-induced intestinal injuries below Treitz ligament; Selective coxibs improved upper and lower GI safety according to results of clinical trials,23 but coxibs are still capable of triggering GI adverse events and are still concerned to be associated with cardiovascular toxicity issues. Thus, there is still a strong clinical need for effective anti-inflammatory drugs with superior safety profiles than the existing NSAIDs.
Acknowledgments
This work was supported by Dongguk University Research Fund of 2010.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Maiden L, Thjodleifsson B, Seigal A, et al. Long-term effects of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 selective agents on the small bowel: a cross-sectional capsule enteroscopy study. Clin Gastroenterol Hepatol. 2007;5:1040–1045. doi: 10.1016/j.cgh.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 2.Maiden L. Capsule endoscopic diagnosis of nonsteroidal antiinflammatory drug-induced enteropathy. J Gastroenterol. 2009;44(Suppl 19):64–71. doi: 10.1007/s00535-008-2248-8. [DOI] [PubMed] [Google Scholar]
- 3.Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3:55–59. doi: 10.1016/s1542-3565(04)00603-2. [DOI] [PubMed] [Google Scholar]
- 4.Maiden L, Thjodleifsson B, Theodors A, Gonzalez J, Bjarnason I. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128:1172–1178. doi: 10.1053/j.gastro.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein JL, Eisen GM, Lewis B, et al. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3:133–141. doi: 10.1016/s1542-3565(04)00619-6. [DOI] [PubMed] [Google Scholar]
- 6.Fujimori S, Gudis K, Takahashi Y, et al. Distribution of small intestinal mucosal injuries as a result of NSAID administration. Eur J Clin Invest. 2010;40:504–510. doi: 10.1111/j.1365-2362.2010.02290.x. [DOI] [PubMed] [Google Scholar]
- 7.Lanas A, Sopeña F. Nonsteroidal anti-inflammatory drugs and lower gastrointestinal complications. Gastroenterol Clin North Am. 2009;38:333–352. doi: 10.1016/j.gtc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Lanas A, García-Rodríguez LA, Polo-Tomás M, et al. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol. 2009;104:1633–1641. doi: 10.1038/ajg.2009.164. [DOI] [PubMed] [Google Scholar]
- 9.Lanas A, Perez-Aisa MA, Feu F, et al. A nationwide study of mortality associated with hospital admission due to severe gastrointestinal events and those associated with nonsteroidal antiinflammatory drug use. Am J Gastroenterol. 2005;100:1685–1693. doi: 10.1111/j.1572-0241.2005.41833.x. [DOI] [PubMed] [Google Scholar]
- 10.Laine L, Curtis SP, Langman M, et al. Lower gastrointestinal events in a double-blind trial of the cyclo-oxygenase-2 selective inhibitor etoricoxib and the traditional nonsteroidal anti-inflammatory drug diclofenac. Gastroenterology. 2008;135:1517–1525. doi: 10.1053/j.gastro.2008.07.067. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto T, Kudo T, Esaki M, et al. Prevalence of non-steroidal anti-inflammatory drug-induced enteropathy determined by double-balloon endoscopy: a Japanese multicenter study. Scand J Gastroenterol. 2008;43:490–496. doi: 10.1080/00365520701794121. [DOI] [PubMed] [Google Scholar]
- 12.Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet. 2009;374:1449–1461. doi: 10.1016/S0140-6736(09)60938-7. [DOI] [PubMed] [Google Scholar]
- 13.Scarpignato C, Hunt RH. Nonsteroidal antiinflammatory drug-related injury to the gastrointestinal tract: clinical picture, pathogenesis, and prevention. Gastroenterol Clin North Am. 2010;39:433–464. doi: 10.1016/j.gtc.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Reuter BK, Davies NM, Wallace JL. Nonsteroidal anti-inflammatory drug enteropathy in rats: role of permeability, bacteria, and enterohepatic circulation. Gastroenterology. 1997;112:109–117. doi: 10.1016/s0016-5085(97)70225-7. [DOI] [PubMed] [Google Scholar]
- 15.Bjarnason I, Hayllar J, MacPherson AJ, Russell AS. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology. 1993;104:1832–1847. doi: 10.1016/0016-5085(93)90667-2. [DOI] [PubMed] [Google Scholar]
- 16.Wallace JL, Syer S, Denou E, et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011;141:1314–1322. doi: 10.1053/j.gastro.2011.06.075. [DOI] [PubMed] [Google Scholar]
- 17.Konaka A, Kato S, Tanaka A, Kunikata T, Korolkiewicz R, Takeuchi K. Roles of enterobacteria, nitric oxide and neutrophil in pathogenesis of indomethacin-induced small intestinal lesions in rats. Pharmacol Res. 1999;40:517–524. doi: 10.1006/phrs.1999.0550. [DOI] [PubMed] [Google Scholar]
- 18.Uejima M, Kinouchi T, Kataoka K, Hiraoka I, Ohnishi Y. Role of intestinal bacteria in ileal ulcer formation in rats treated with a nonsteroidal antiinflammatory drug. Microbiol Immunol. 1996;40:553–560. doi: 10.1111/j.1348-0421.1996.tb01108.x. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe T, Higuchi K, Kobata A, et al. Non-steroidal anti-inflammatory drug-induced small intestinal damage is Toll-like receptor 4 dependent. Gut. 2008;57:181–187. doi: 10.1136/gut.2007.125963. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y, Dial EJ, Doyen R, Lichtenberger LM. Effect of indomethacin on bile acid-phospholipid interactions: implication for small intestinal injury induced by nonsteroidal anti-inflammatory drugs. Am J Physiol Gastrointest Liver Physiol. 2010;298:G722–G731. doi: 10.1152/ajpgi.00387.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lichtenberger LM, Wang ZM, Romero JJ, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) associate with zwitterionic phospholipids: insight into the mechanism and reversal of NSAID-induced gastrointestinal injury. Nat Med. 1995;1:154–158. doi: 10.1038/nm0295-154. [DOI] [PubMed] [Google Scholar]
- 22.Kent TH, Cardelli RM, Stamler FW. Small intestinal ulcers and intestinal flora in rats given indomethacin. Am J Pathol. 1969;54:237–249. [PMC free article] [PubMed] [Google Scholar]
- 23.Leung WK, Bjarnason I, Wong VW, Sung JJ, Chan FK. Small bowel enteropathy associated with chronic low-dose aspirin therapy. Lancet. 2007;369:614. doi: 10.1016/S0140-6736(07)60282-7. [DOI] [PubMed] [Google Scholar]
- 24.Higuchi K, Umegaki E, Watanabe T, et al. Present status and strategy of NSAIDs-induced small bowel injury. J Gastroenterol. 2009;44:879–888. doi: 10.1007/s00535-009-0102-2. [DOI] [PubMed] [Google Scholar]
- 25.Bjarnason I, Price AB, Zanelli G, et al. Clinicopathological features of nonsteroidal antiinflammatory drug-induced small intestinal strictures. Gastroenterology. 1988;94:1070–1074. doi: 10.1016/0016-5085(88)90568-9. [DOI] [PubMed] [Google Scholar]
- 26.Lang J, Price AB, Levi AJ, Burke M, Gumpel JM, Bjarnason I. Diaphragm disease: pathology of disease of the small intestine induced by non-steroidal anti-inflammatory drugs. J Clin Pathol. 1988;41:516–526. doi: 10.1136/jcp.41.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sunada K, Yamamoto H. Double-balloon endoscopy: past, present, and future. J Gastroenterol. 2009;44:1–12. doi: 10.1007/s00535-008-2292-4. [DOI] [PubMed] [Google Scholar]
- 28.Rahme E, Barkun A, Nedjar H, Gaugris S, Watson D. Hospitalizations for upper and lower GI events associated with traditional NSAIDs and acetaminophen among the elderly in Quebec, Canada. Am J Gastroenterol. 2008;103:872–882. doi: 10.1111/j.1572-0241.2008.01811.x. [DOI] [PubMed] [Google Scholar]
- 29.Zuccaro G. Epidemiology of lower gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2008;22:225–232. doi: 10.1016/j.bpg.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Aabakken L, Osnes M. 51Cr-ethylenediaminetetraacetic acid absorption test: effects of naproxen, a non-steroidal, antiinflammatory drug. Scand J Gastroenterol. 1990;25:917–924. doi: 10.3109/00365529008997613. [DOI] [PubMed] [Google Scholar]
- 31.Smale S, Tibble J, Sigthorsson G, Bjarnason I. Epidemiology and differential diagnosis of NSAID-induced injury to the mucosa of the small intestine. Best Pract Res Clin Gastroenterol. 2001;15:723–738. doi: 10.1053/bega.2001.0231. [DOI] [PubMed] [Google Scholar]
- 32.Tibble JA, Sigthorsson G, Foster R, et al. High prevalence of NSAID enteropathy as shown by a simple faecal test. Gut. 1999;45:362–366. doi: 10.1136/gut.45.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Damms A, Bischoff SC. Validation and clinical significance of a new calprotectin rapid test for the diagnosis of gastrointestinal diseases. Int J Colorectal Dis. 2008;23:985–992. doi: 10.1007/s00384-008-0506-0. [DOI] [PubMed] [Google Scholar]
- 34.Fujimori S, Seo T, Gudis K, et al. Prevention of nonsteroidal anti-inflammatory drug-induced small-intestinal injury by prostaglandin: a pilot randomized controlled trial evaluated by capsule endoscopy. Gastrointest Endosc. 2009;69:1339–1346. doi: 10.1016/j.gie.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe T, Sugimori S, Kameda N, et al. Small bowel injury by low-dose enteric-coated aspirin and treatment with misoprostol: a pilot study. Clin Gastroenterol Hepatol. 2008;6:1279–1282. doi: 10.1016/j.cgh.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 36.Niwa Y, Nakamura M, Ohmiya N, et al. Efficacy of rebamipide for diclofenac-induced small-intestinal mucosal injuries in healthy subjects: a prospective, randomized, double-blinded, placebo-controlled, cross-over study. J Gastroenterol. 2008;43:270–276. doi: 10.1007/s00535-007-2155-4. [DOI] [PubMed] [Google Scholar]
- 37.Wallace JL, Ferraz JG. New pharmacologic therapies in gastrointestinal disease. Gastroenterol Clin North Am. 2010;39:709–720. doi: 10.1016/j.gtc.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 38.Lichtenberger LM, Barron M, Marathi U. Association of phosphatidylcholine and NSAIDs as a novel strategy to reduce gastrointestinal toxicity. Drugs Today (Barc) 2009;45:877–890. doi: 10.1358/dot.2009.45.12.1441075. [DOI] [PubMed] [Google Scholar]
- 39.Lanza FL, Marathi UK, Anand BS, Lichtenberger LM. Clinical trial: comparison of ibuprofen-phosphatidylcholine and ibuprofen on the gastrointestinal safety and analgesic efficacy in osteoarthritic patients. Aliment Pharmacol Ther. 2008;28:431–442. doi: 10.1111/j.1365-2036.2008.03765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallace JL, Del Soldato P. The therapeutic potential of NO-NSAIDs. Fundam Clin Pharmacol. 2003;17:11–20. doi: 10.1046/j.1472-8206.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- 41.Fiorucci S, Antonelli E, Distrutti E, et al. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology. 2005;129:1210–1224. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 42.Lim YJ, Lee JS, Ku YS, Hahm KB. Rescue strategies against non-steroidal anti-inflammatory drug-induced gastroduodenal damage. J Gastroenterol Hepatol. 2009;24:1169–1178. doi: 10.1111/j.1440-1746.2009.05929.x. [DOI] [PubMed] [Google Scholar]
- 43.Wallace JL. Hydrogen sulfide-releasing anti-inflammatory drugs. Trends Pharmacol Sci. 2007;28:501–505. doi: 10.1016/j.tips.2007.09.003. [DOI] [PubMed] [Google Scholar]