Abstract
Vax1 and Vax2 have been implicated in eye development and the closure of the choroid fissure in mice and zebrafish. We sequenced the coding exons of VAX1 and VAX2 in 70 patients with anophthalmia/microphthalmia. In VAX1, we observed homozygosity for two successive nucleotide substitutions c.453G>A and c.454C>A, predicting p.Arg152Ser, in a proband of Egyptian origin with microphthalmia, small optic nerves, cleft lip/palate and corpus callosum agenesis. This mutation affects an invariant residue in the homeodomain of VAX1 and was absent from 96 Egyptian controls. It is likely that the mutation results in a loss of function, as the mutation results in a phenotype similar to the Vax1 homozygous null mouse. We did not identify any mutations in VAX2. This is the first description of a phenotype associated with a VAX1 mutation in humans and establishes VAX1 as a new causative gene for anophthalmia/microphthalmia.
Keywords: Anophthalmia/microphthalmia, VAX1, VAX2, coloboma
Malformations of the eye such as anophthalmia (absence of the eye) and microphthalmia (reduction in eye size) show a birth prevalence of approximately 1/5000 [Shaw et al. 2005]. Anophthalmia and microphthalmia (A/M) are characterized by absent or rudimentary eye formation despite the presence of adnexal ocular structures [Verma and FitzPatrick, 2007; Ragge et al., 2007]. Mutations in several transcription factors that are expressed during eye development have been implicated in both syndromic and non-syndromic A/M [for reviews, see Verma and FitzPatrick, 2007; Rainger et al., 2008] but it is clear from the frequency of mutations in each causative gene that novel causes of A/M remain to be discovered.
During ocular development, Shh [MIM# 600725] induces ventralization of the optic vesicle and a mutation predicted to interfere with autocatalytic processing of SHH has caused microphthalmia and coloboma [Schimmenti et al., 2003]. Shh induces the expression of two homeobox-containing genes: ventral anterior homeobox 1 [Vax1; MIM# 604294] and ventral anterior homeobox 2 [Vax2; MIM# 604295] [Zhao et al., 2010]. Vax1 and Vax2 share high sequence homology with members of the Emx and Not homeodomain-containing gene families and likely originated from the duplication of a single ancestral gene due to the 100% conservation of the homeodomains [Ohsaki et al., 1999; Schulte et al., 1999]. Vax1 and Vax2 have distinct expression patterns in the developing eye [Ohsaki et al., 1999; Take-uchi et al., 2003]. At embryonic day (E) 8 in the mouse, Vax1 mRNA is present in the anterior neural ridge and adjacent ectoderm and at E10.5, expression is present at site of the putative optic disc and optic stalks, the olfactory placodes and the rostral tip of the neural tube [Bertuzzi et al., 1999; Hallonet et al., 1999]. During mid-embryogenesis, Vax1 is expressed in the diencephalon adjacent to lateral ventricles of the brain and in the septum that is ventral to the corpus callosum [Bertuzzi et al., 1999; Bharti et al., 2011]. At E18.5, Vax1 expression is visible in the ventral optic stalk, the glial cells of the optic nerve, the optic chiasm and in the rostral diencephalon [Hallonet et al., 1999; Bertuzzi et al., 1999]. In contrast, Vax2 expression is present almost exclusively in the ventral part of the developing optic vesicle from E9.0 onwards, although there is weak expression in the optic stalk at E12.5 [Barbieri et al., 1999]. In Danio rerio, Vax1 and Vax2 are both expressed in overlapping domains in the ventral portion of the developing eye [Take-uchi et al., 2003].
Animal models of loss of gene function also implicate the Vax genes in ocular development. Vax1 homozygous null mice had colobomas detectable from the earliest stages of development that were fully penetrant and moderately severe [Hallonet et al., 1998; Bertuzzi et al., 1999]. Colobomas were rare and milder in Vax2 homozygous null mutants, but Vax1 and Vax2 double mutant mice had severe colobomas that were fully penetrant [Barbieri et al., 2002; Mui et al., 2002]. In Danio rerio, injections of antisense morpholinos against Vax1 or Vax2 results in colobomas and reduced retinal pigment at the site of the choroid fissure [Take-uchi et al., 2003]. The severity of the colobomas was increased with loss of both Vax1 and Vax2, thus indicating that the genes act synergistically in fish and mice [Take-uchi et al., 2003].
In humans, VAX1 has two isoforms, the first containing 3 exons and encoding a 334 amino acid protein and the second containing 4 exons and encoding a 186 amino acid protein. VAX2 has 3 exons and encodes a 290 amino acid protein. VAX1 and VAX2 have been little studied in human patients with eye disease. Homozygosity mapping in a consanguineous family excluded VAX1 in two affected individuals diagnosed with Temtamy syndrome who manifested optic colobomas, agenesis of the corpus callosum, intractable seizures, craniofacial dysmorphism and skeletal anomalies [Li et al., 2007].
In view of the paucity of human data, we chose to sequence both genes in patients with A/M. Two patient groups were studied. In the first group, DNA samples from 70 patients with A/M were sequenced for VAX1 and VAX2 sequence alterations. From this first group, 35 samples were collected under an Institutional Review Board (IRB)-approved protocol for the Anophthalmia/Microphthalmia Registry and Gene-screening project (Albert Einstein Medical Center, Philadelphia, PA) and 35 samples were collected under an IRB-approved protocol from the Institut de Recherche en Ophtalmologie (Ecole Polytechnique Fédérale de Lausanne and Université de Lausanne, Sion, Switzerland). A second group of 10 A/M patients was then selected with at least one additional finding of cleft palate and/or agenesis of the corpus callosum. These patients were identified from the population-based California Birth Defects Monitoring Program with IRB approvals from the California Committee for the Protection of Human Subjects and the University of California, San Francisco (UCSF) Committee for Human Research. Finally, control chromosomes were obtained using a protocol from the UCSF Committee for Human Research. We sequenced the coding exons and intron-exon boundaries of VAX1 [NM_001112704.1 (long isoform) and NM_199131.2 (short isoform); http://www.ncbi.nlm.nih.gov/gene)] and VAX2 [NM_012476.2] as previously described [Slavotinek et al., 2006]. Primers are provided in Supp. Table S1.
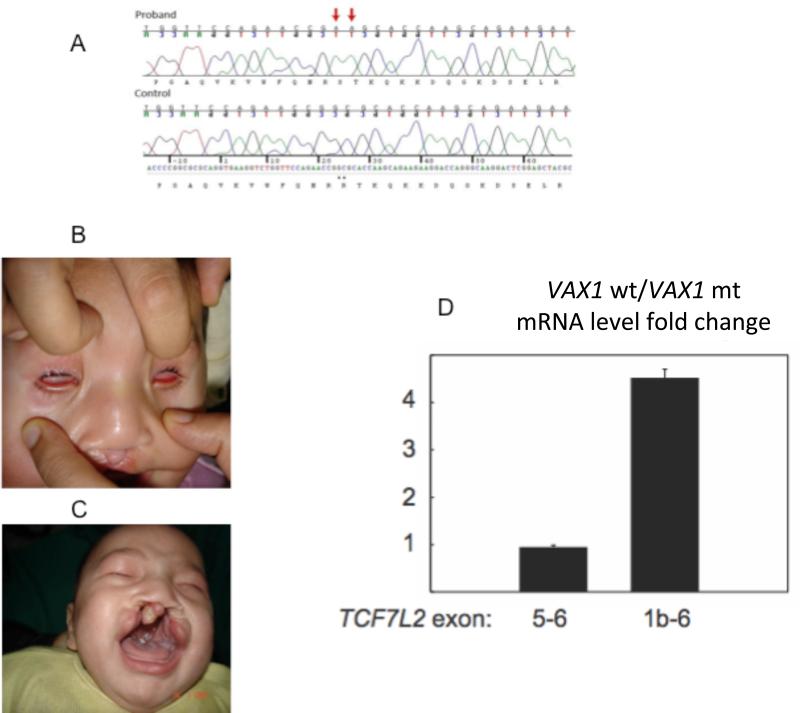
Sequencing of VAX1 in the 70 unselected patients with A/M revealed one mutation, with homozygosity for two adjacent nucleotide substitutions, c.453G>A and c.454C>A, that predicted p.Arg152Ser in the long isoform of VAX1 (Fig. 1A) in a single patient (Table 1). This patient was an Egyptian male with bilateral microphthalmia and small optic nerves, cleft lip and palate, corpus callosum agenesis, hippocampal malformations and absence of the pineal gland. Birth weight was 3750 g, but no head circumference measurement from the time of birth was available. Bilateral severe microphthalmia (Fig. 1B) and bilateral cleft lip and palate (Fig. 1C) were present. A TORCH screen at birth was negative and chromosome analysis showed an apparently normal male karyotype, 46,XY. At 4 months of age, his development was delayed and he had no head control. His weight was 5.5 kg (3rd percentile), height 57 cm (3rd percentile) and head circumference was 41 cm (25th percentile). His anterior fontanelle was open and measured 3cm x 3cm. Examinations of the chest, heart, abdomen, genitalia, skeletal system, skin and muscle tone were described as unremarkable. His hearing was also reported to be normal.
Figure 1.
A. Chromatogram from the proband and a control, showing homozygosity for c.453G>A and c.454C>A (c.453_454delinsAA) in the proband (marked by arrows). B. Frontal view of the proband, showing bilateral severe microphthalmia. C. Frontal view of the proband, showing bilateral cleft lip. D. Mutated VAX1 cannot activate endogenous dnTCF7L2. As determined by Q-RTPCR, the expression of full-length TCF7L2 mRNA (exons 5-6) is not affected by overexpression of the human wild-type VAX1 in HEK293 cells, while the expression of dnTCF7L2 (exons 1b-6) is induced. This induction is not seen with mutated VAX1. Fold change is the ratio between expression normalized to GAPDH in cells transfected with the wild-type VAX1 expression construct versus cells transfected with the mutated VAX1 expression construct. Error bars are mean ± standard deviation (n = 3).
Table 1.
Sequence Alterations in VAX1 and VAX2 in 70 Anophthalmia/Microphthalmia Patients
| Gene/Location | Nucleotide Alteration | Amino Acid Alteration | Allele Frequency (n = 140 chromosomes) | dbSNPa | Allele Frequency from dbSNP | Polyphen-2 Scoreb |
|---|---|---|---|---|---|---|
| VAX1 | ||||||
| Exon 3 | c.453_454delinsAA | p.Arg152Ser | GC = 0.993 AA = 0.007 |
- | - | 1.0; probably damaging |
| VAX2 | ||||||
| Exon 1 | c.43C>A | p.Arg15Arg | C = 0.864 A = 0.136 |
rs2234495 | C = 0.875 (120)c A = 0.125 |
- |
| Exon 1 | c.70C>G | p.Arg24Gly | C = 0.993 G = 0.007 |
rs2234496 | C = 0.924 (118)d G = 0.076 |
0.444; possibly damaging |
| Exon 3 | c.761C>G | p.Pro254Arg | C = 0.907 G = 0.093 |
rs2234500 | C = 0.933 (120)e G = 0.067 |
0.995; probably damaging |
| Exon 3 | c.825A>G | p.Leu275Leu | A = 0.993 G = 0.007 |
rs2234501 | A = 0.983 (120)f G = 0.017 |
- |
Nucleotide numbering reflects cDNA numbering with +1 corresponding to the A of the ATG translation initiation codon in the reference sequence, according to journal guidelines (www.hgvs.org/mutnomen) for both genes. The initiation codon is codon 1.
The VAX1 exon 3 sequence alteration is in isoform 1 of VAX1.
dbSNP = Database of Single Nucleotide Polymorphisms; http://www.ncbi.nlm.nih.gov/proiects/SNP/
Polyphen-2 Score = http://genetics.bwh.harvard.edu/pph2/
=CEU Allele frequency = ss231220873_pilot_1_CEU_low_coverage_panel; number of chromosomes in brackets
= YRI Allele frequency = ss219317729_piot_1_YRI_low_coverage_panel; number of chromosomes in brackets
= CEU Allele frequency = ss231220980_pilot_1_CEU_low_coverage_panel; number of chromosomes in brackets
= CEU Allele frequency = ss231220981_pilot_1_CEU_low_coverage_panel; number of chromosomes in brackets
The child was reviewed at 3.5 years of age. His weight, height and head circumference were at the 3rd percentile. He had global developmental delays and was able to sit and to transfer objects from one hand to the other. His only words were ‘dada’ and ‘mama’ and his developmental level was assessed as that of a 6-month child. An echocardiogram and renal ultrasound scan were normal as were skeletal radiographs. A magnetic resonance imaging (MRI) scan of the brain was reported to show absence of the normal ocular globes bilaterally, with the right orbit showing a fluid-filled cyst. The corpus callosum was absent. There was a small nodule of soft tissue in the anterior hemispheric fissure of unknown origin. The pineal gland was absent and the hippocampus had a vertical orientation. No hormonal analyses have been done to test pituitary function. The parents have had no obvious eye malformations.
The p.Arg152Ser mutation affects an invariant residue in the third helix of the homeodomain (Supp. Table S2) and was not present in 96 Egyptian controls; the phenotypically normal, but consanguineous parents were heterozygotes and an unaffected sibling was wildtype for these two nucleotides (data not shown). Polyphen-2 (Prediction of Functional Effects of Human Non-synonymous Single Nucleotide Polymorphisms) predicted that the mutation was ‘probably damaging’, with a score of 1.0 (sensitivity 0.0 and specificity 1.0) and it was not present in either the Database of Single Nucleotide Polymorphisms (dbSNP; http://www.ncbi.nlm.nih.gov/projects/SNP/; date of search 9.21.11) or the 1000 Genomes Project website (http://www.1000genomes.org/; date of search 9.21.11).
It has recently been shown that the Vax proteins function as activators of a potent dominant-negative isoform of the canonical Wnt signaling mediator Tcf7l2, designated dnTcf7l2 [Vacik et al., 2011]. They achieve this by binding to and activating an internal promoter located in the fifth intron of the Tcf7l2 gene. DnTcf7l2 lacks the activating beta-catenin domain for Tcf7l2 and therefore functions as a strong repressor of canonical Wnt target genes. This dominant-negative antagonist is expressed throughout the developing forebrain and its morpholino-mediated loss in Xenopus leads to embryos lacking the anterior head region [Vacik et al., 2011]. To assess the effect of the VAX1 mutation on its ability to activate dnTCF7L2, we used quantitative RT-PCR (Q-RT-PCR) to measure expression of the most abundant dnTCF7L2 mRNA, the exon 1b isoform [Vacik et al., 2011], in HEK293 cells following transfection with either wild-type or mutated VAX1 expression constructs. Wild-type VAX1 and mutated VAX1/pArg152Ser expression constructs were purchased from Origene (Rockville, MD). 1 μg of the expression construct was transfected into HEK293 cells in a 24-well plate using Fugene6 (Roche, Indianapolis, IN). Total RNA was isolated 40 hours after transfection using TRIZOL (Invitrogen, Carlsbad, CA) and reverse transcription was performed using Superscript III (Invitrogen, Carlsbad, CA). Quantitative RT-PCR reactions were performed in triplicate using SYBRGreen PCR master mix (Applied Biosystems, Foster City, CA) and normalized against expression of GAPDH. The Q-RT-PCR primers have been provided in Supp. Table S1.
As expected, neither wild-type nor mutant VAX1/p.Arg152Ser induced the expression of full-length TCF7L2 mRNAs (Fig. 1D). However, we found that wild-type VAX1 stimulates the expression of dnTCF7L2 mRNA 1b, while the mutant form does not (Fig. 1D). These results suggest that the VAX1 mutation prevents this homeodomain transcription factor from activating dnTCF7L2, and that this in turn leads to de-repression of TCF7L2 target genes and hyperactivation of Wnt signaling. It is possible that this mechanism explains at least part of the observed phenotype found with the VAX1 p.Arg152Ser mutation.
Similar to the phenotype that was observed in the patient with p.Arg152Ser, Vax1 homozygous null mice had clefting of the secondary palate with 100% penetrance and agenesis of the corpus callosum [Bertuzzi et al., 1999]. We therefore sequenced VAX1 in 10 A/M patients selected to have additional phenotypic findings of orofacial clefting and/or agenesis of the corpus callosum (Supp. Table S3). In a patient with bilateral anophthalmia and bilateral cleft lip and palate, we detected one heterozygous sequence alteration, c.945C>T, in exon 3 (Supp. Table S3; data not shown). This alteration is not listed in dbSNP or 1000 genomes and was absent in the DNA from 160 control chromosomes obtained from patients with diaphragmatic hernia; the parents were unavailable for testing. However, c.945C>T is a silent substitution of unclear significance as it does not alter an amino acid residue (p.Ala315Ala) and was not predicted to alter splicing [Automated Splice Site Analysis; Rogan et al., 1998; Nalla and Rogan, 2005]. Furthermore, we did not find a second alteration in this patient that would have been consistent with the autosomal recessive inheritance pattern found in the patient with p.Arg152Ser.
We have described a new homozygous sequence alteration in VAX1, p.Arg152Ser, in a male with a phenotype of severe microphthalmia, agenesis of the corpus callosum and cleft palate that is highly reminiscent of the mouse model for loss of function of this gene. We consider that this mutation is pathogenic on the basis of the homozygosity observed in the patient and the segregation of the mutation with the disease phenotype in the family, the in-silico predictions of likely pathogenicity and the absence of the sequence alteration in control individuals of the same ethnicity. The mutation is likely to cause microphthalmia because of loss of function. Our functional studies suggest that one mechanism whereby the mutation exerts it phenotypic effects is through failure to produce dnTCF7L2 and consequent hyperactivation of Wnt signaling. However, the involvement of other pathways has not been excluded. In the chick, cVAX has been shown to interact with Paired box gene 6 [PAX6; MIM# 607108] and this interaction may be important in delineating the boundary between the dorsal and ventral retina [Leconte et al., 2004]. At the stage of optic cup development, PAX6 and cVAX are expressed in a gradient from the dorsal retina (primarily PAX6 expression) to the ventral retina (primarily cVAX expression) [Leconte et al., 2004]. cVAX can bind to both the paired domain and the homeodomain of PAX6, although it is unknown if the cVAX/PAX6 interaction occurs via the paired domain, homeodomain, or both [Leconte et al., 2004]. During normal eye development, Pax6 represses VAX1; in turn, cVAX can repress the activity of the intronic, PAX6 α-enhancer in the retina, thus repressing PAX6 transactivation and contributing to PAX6 downregulation [Leconte et al., 2004]. As the p.Arg152Ser amino acid substitution occurs in the VAX1 homeodomain, we reasoned that it could alter the ability of VAX1 to repress PAX6. In addition, mutations causing loss of function in PAX6 have also been associated with agenesis of the corpus callosum [Abouzeid et al., 2009].
It is also still possible that the mutation in VAX1 has altered the function or regulation of another gene or genes besides Tcf7l2 and Pax6. In a double-knockout mouse for Chicken Ovalbumin Upstream Promoter Transcription Factor 1 [COUP-TFI; MIM# 132890] and Chicken Ovalbumin Upstream Promoter Transcription Factor 2 [COUP-TFII; MIM# 107773] targeted specifically to the eye, COUP-TFI and COUP-TFII mutants had greatly reduced Vax1 and Vax2 expression and manifested microphthalmia and colobomas [Tang et al., 2010] and a possible interaction between VAX1 and a COUP-TF gene was not investigated.
The finding of a corpus callosum defect and other brain malformations in the patient with the p.Arg152Ser mutation was strongly suggestive for a role for VAX1 in human brain development. This has been supported by animal models of loss of Vax1 function, as Vax1 homozygous null mutant mice have previously been reported to have agenesis of the corpus callosum [Bertuzzi et al., 1999]. More recently, Vax1 mutant mice have been found to have a second, rostrally located Rathke's pouch that has the potential to develop into a pituitary gland with hormone secreting capabilities [Bharti et al., 2011]. The pituitary duplication was attributed to loss of Vax1, with failure to repress Fibroblast growth factor 10 [Fgf10; MIM# 602115] in the neuroectoderm rostral to the infundibulum of the pituitary gland [Bharti et al., 2011].
The corpus callosum abnormalities associated with loss of Vax1 function in the mouse may also be due to altered Pax6 signaling. Vax1 is expressed in the ventral telencephalon [Ellison-Wright et al., 2004] and Pax6 in the dorsal telencephalon [Haubst et al., 2004], suggesting that Pax6 and Vax1 share adjacent expression domains in the developing cortex, similar to the situation in the developing eye. Mice homozygous for mutations or null alleles of the Pax6 gene also harbor a wide variety of neurodevelopmental abnormalities including absence of the pineal gland and corpus callosum [Schmahl et al., 1993; Estivill-Torrus et al., 2001]. Brain imaging studies in patients with PAX6 loss of function mutations have also revealed a wide range of malformations, including absence of the pineal gland, hypogenesis of the corpus callosum, significantly smaller corpus callosum volumes and Probst bundles [Ellison-Wright et al., 2004; Bamiou et al., 2007; Abouzeid et al., 2009]. However, there are other known Vax1 target genes that have a role in axon guidance, such as Netrin-1 and the receptor tyrosine kinase EphB3 [Mui et al., 2005]. In contrast to its involvement in brain development, little is known about the role of Vax1 in palate development. The cleft palate observed in Vax1 homozygous null mice may be associated with the transient expression of Vax1 in the first branchial arch [Hallonet et al. 1999]. It is interesting to note that several SNPs in PAX6 showed significant evidence of linkage disequilibrium in cleft lip and/or cleft palate case-parent trios from four populations [Sull et al., 2009].
In VAX2, we identified four coding SNPs in the 70 A/M patients: c.43C>A, predicting p.Pro15Pro; c.70C>G, predicting p.Arg24Gly; c.761C>G, predicting p.Pro254Arg and c.825A>G, predicting p.Leu275Leu (Table 1). Two of these SNPs were non-synonymous - p.Pro254Arg, which was predicted to be ‘probably damaging’ by Polyphen-2 with a score of 0.995 (sensitivity 0.67 and specificity 0.97) and p.Arg24Gly, which was predicted to be ‘possibly damaging’, with a score of 0.444 (sensitivity 0.89 and specificity: 0.90). However, the allele frequencies for these SNPs in the 70 A/M patients were not significantly different from allele frequencies reported for control chromosomes in dbSNP (Table 1). We therefore did not find evidence of a direct involvement of VAX2 in the pathogenesis of human A/M in the small cohort sequenced.
In conclusion, we present the first description of a homozygous VAX1 mutation, c.453G>A and c.454C>A, that predicted p.Arg152Ser, in a male with severe bilateral microphthalmia and small optic nerves, cleft lip and palate, corpus callosum agenesis, hippocampal malformations and absence of the pineal gland. A similar phenotype to the patient of microphthalmia, agenesis of the corpus callosum and cleft palate has also been observed in mice with loss of Vax1 function, making this the most likely mechanism. The mutation may act by preventing the formation of dnTCF7L2, resulting in hyperactivation of the Wnt pathway. This is the first description of a patient with a VAX1 mutation and establishes VAX1 as a new causative gene for A/M in humans.
Supplementary Material
Acknowledgments
Anne Slavotinek was funded by grant K08 grant K08HD053476 from The Eunice Kennedy Shriver National Institute of Child Health and Development at the National Institutes of Health. This publication was supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131. We thank the California Department of Public Health Maternal Child and Adolescent Health Division for providing data for these analyses. We thank James Huang for technical assistance. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the California Department of Public Health or the NIH.
Grant support: This work was supported by grant K08 HD053476 from The Eunice Kennedy Shriver National Institute of Child Health and Development at the National Institutes of Health to AS (to G.L. R01 EY017478). This work was also supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131.
References
- Abouzeid H, Youssef MA, ElShakankiri N, Hauser P, Munier FL, Schorderet DF. PAX6 aniridia and interhemispheric brain anomalies. Mol Vis. 2009;15:2074–2083. [PMC free article] [PubMed] [Google Scholar]
- Bamiou DE, Free SL, Sisodiya SM, Chong WK, Musiek F, Williamson KA, van Heyningen V, Moore AT, Gadian D, Luxon LM. Auditory interhemispheric transfer deficits, hearing difficulties, and brain magnetic resonance imaging abnormalities in children with congenital aniridia due to PAX6 mutations. Arch Pediatr Adolesc Med. 2007;161:463–469. doi: 10.1001/archpedi.161.5.463. [DOI] [PubMed] [Google Scholar]
- Barbieri AM, Lupo G, Bulfone A, Andreazzoli M, Mariani M, Fougerousse F, Consalez GG, Borsani G, Beckmann JS, Barsacchi G, Ballabio A, Banfi S. A homeobox gene, vax2, controls the patterning of the eye dorsoventral axis. Proc Natl Acad Sci USA. 1999;96:10729–10734. doi: 10.1073/pnas.96.19.10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri AM, Broccoli V, Bovolenta P, Alfano G, Marchitiello A, Mocchetti C, Crippa L, Bulfone A, Marigo V, Ballabio A, Banfi S. Vax2 inactivation in mouse determines alteration of the eye dorsal-ventral axis, misrouting of the optic fibres and eye coloboma. Development. 2002;129:805–813. doi: 10.1242/dev.129.3.805. [DOI] [PubMed] [Google Scholar]
- Bertuzzi S, Hindges R, Mui SH, O'Leary DD, Lemke G. The homeodomain protein vax1 is required for axon guidance and major tract formation in the developing forebrain. Genes Dev. 1999;13:3092–3105. doi: 10.1101/gad.13.23.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti K, Gasper M, Bertuzzi S, Arnheiter H. Lack of the ventral anterior homeodomain transcription factor VAX1 leads to induction of a second pituitary. Development. 2011;138:873–878. doi: 10.1242/dev.056465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison-Wright Z, Heyman I, Frampton I, Rubia K, Chitnis X, Ellison-Wright I, Williams SC, Suckling J, Simmons A, Bullmore E. Heterozygous PAX6 mutation, adult brain structure and fronto-striato-thalamic function in a human family. Eur J Neurosci. 2004;19:1505–1512. doi: 10.1111/j.1460-9568.2004.03236.x. [DOI] [PubMed] [Google Scholar]
- Estivill-Torrus G, Vitalis T, Fernandez-Llebrez P, Price DJ. The transcription factor Pax6 is required for development of the diencephalic dorsal midline secretory radial glia that form the subcommissural organ. Mech Dev. 2001;109:215–224. doi: 10.1016/s0925-4773(01)00527-5. [DOI] [PubMed] [Google Scholar]
- Hallonet M, Hollemann T, Pieler T, Gruss P. Vax1, a novel homeobox-containing gene, directs development of the basal forebrain and visual system. Genes Dev. 1999;13:3106–3114. doi: 10.1101/gad.13.23.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubst N, Berger J, Radjendirane V, Graw J, Favor J, Saunders GF, Stoykova A, Götz M. Molecular dissection of Pax6 function: the specific roles of the paired domain and homeodomain in brain development. Development. 2004;131:6131–6140. doi: 10.1242/dev.01524. [DOI] [PubMed] [Google Scholar]
- Kammandel B, Chowdhury K, Stoykova A, Aparicio S, Brenner S, Gruss P. Distinct cis-essential modules direct the time-space pattern of the Pax6 gene activity. Dev Biol. 1999;205:79–97. doi: 10.1006/dbio.1998.9128. [DOI] [PubMed] [Google Scholar]
- Leconte L, Lecoin L, Martin P, Saule S. Pax6 interacts with cVax and Tbx5 to establish the dorsoventral boundary of the developing eye. J Biol Chem. 2004;279:47272–47277. doi: 10.1074/jbc.M406624200. [DOI] [PubMed] [Google Scholar]
- Li J, Shivakumar S, Wakahiro M, Mukherjee P, Barkovich AJ, Slavotinek A, Sherr EH. Agenesis of the corpus callosum, optic coloboma, intractable seizures, craniofacial and skeletal dysmorphisms: an autosomal recessive disorder similar to Temtamy syndrome. Am J Med Genet A. 2007;143A:1900–1905. doi: 10.1002/ajmg.a.31855. [DOI] [PubMed] [Google Scholar]
- Mui SH, Hindges R, O'Leary DD, Lemke G, Bertuzzi S. The homeodomain protein Vax2 patterns the dorsoventral and nasotemporal axes of the eye. Development. 2002;129:797–804. doi: 10.1242/dev.129.3.797. [DOI] [PubMed] [Google Scholar]
- Mui SH, Kim JW, Lemke G, Bertuzzi S. Vax genes ventralize the embryonic eye. Genes Dev. 2005;19:1249–1259. doi: 10.1101/gad.1276605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalla Rogan. Automated splicing mutation analysis by information theory. Hum Mutat. 2005;25:334–342. doi: 10.1002/humu.20151. [DOI] [PubMed] [Google Scholar]
- Ohsaki K, Morimitsu T, Ishida Y, Kominami R, Takahashi N. Expression of the Vax family homeobox genes suggests multiple roles in eye development. Genes Cells. 1999;4:267–276. doi: 10.1046/j.1365-2443.1999.00257.x. [DOI] [PubMed] [Google Scholar]
- Ragge NK, Subak-Sharpe ID, Collin JR. A practical guide to the management of anophthalmia and microphthalmia. Eye. 2007;21:1290–1300. doi: 10.1038/sj.eye.6702858. [DOI] [PubMed] [Google Scholar]
- Rainger J, Van Heyningen V, FitzPatrick DR. Development of the Eye. In: Epstein CJ, Erickson RP, Wynshaw-Boris A, editors. Inborn Errors of Development. 2e. Oxford University Press; Oxford: 2008. pp. 94–106. [Google Scholar]
- Rogan PK, Faux B, Schneider TD. Information analysis of human splice site mutations. Hum Mutat. 1998;12:153–171. doi: 10.1002/(SICI)1098-1004(1998)12:3<153::AID-HUMU3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Schimmenti LA, de la Cruz J, Lewis RA, Karkera JD, Manligas GS, Roessler E, Muenke M. Novel mutation in sonic hedgehog in non-syndromic colobomatous microphthalmia. Am J Med Genet A. 2003;116A:215–22. doi: 10.1002/ajmg.a.10884. [DOI] [PubMed] [Google Scholar]
- Schmahl W, Knoedlseder M, Favor J, Davidson D. Defects of neuronal migration and the pathogenesis of cortical malformations are associated with Small eye (Sey) in the mouse, a point mutation at the Pax-6-locus. Acta Neuropathol. 1993;86:126–135. doi: 10.1007/BF00334879. [DOI] [PubMed] [Google Scholar]
- Schulte D, Furukawa T, Peters MA, Kozak CA, Cepko CL. Misexpression of the Emx-related homeobox genes cVax and mVax2 ventralizes the retina and perturbs the retinotectal map. Neuron. 1999;24:541–553. doi: 10.1016/s0896-6273(00)81111-3. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Carmichael SL, Yang W, Harris JA, Finnell RH, Lammer EJ. Epidemiologic characteristics of anophthalmia and bilateral microphthalmia among 2.5 million births in California, 1989-1997. Am J Med Genet A. 2005;137:36–40. doi: 10.1002/ajmg.a.30840. [DOI] [PubMed] [Google Scholar]
- Slavotinek AM, Moshrefi A, Davis R, Leeth E, Schaeffer GB, Burchard GE, Shaw GM, James B, Ptacek L, Pennacchio LA. Array comparative genomic hybridization in patients with congenital diaphragmatic hernia: mapping of four CDH-critical regions and sequencing of candidate genes at 15q26.1-15q26.2. Eur J Hum Genet. 2006;614:999–1008. doi: 10.1038/sj.ejhg.5201652. [DOI] [PubMed] [Google Scholar]
- Sull JW, Liang KY, Hetmanski JB, Fallin MD, Ingersoll RG, Park J, Wu-Chou YH, Chen PK, Chong SS, Cheah F, Yeow V, Park BY, Jee SH, Jabs EW, Redett R, Scott AF, Beaty TH. Maternal transmission effects of the PAX genes among cleft case-parent trios from four populations. Eur J Hum Genet. 2009;17:831–839. doi: 10.1038/ejhg.2008.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Take-uchi M, Clarke JD, Wilson SW. Hedgehog signalling maintains the optic stalk-retinal interface through the regulation of Vax gene activity. Development. 2003;130:955–968. doi: 10.1242/dev.00305. [DOI] [PubMed] [Google Scholar]
- Vacik T, Stubbs JL, Lemke G. A novel mechanism for the transcriptional regulation of Wnt signaling in development. Genes Dev. 2011;25:1783–1795. doi: 10.1101/gad.17227011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma AS, Fitzpatrick DR. Anophthalmia and microphthalmia. Orphanet J Rare Dis. 2007;2:47. doi: 10.1186/1750-1172-2-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Saitsu H, Sun X, Shiota K, Ishibashi M. Sonic hedgehog is involved in formation of the ventral optic cup by limiting Bmp4 expression to the dorsal domain. Mech Dev. 2010;127:62–72. doi: 10.1016/j.mod.2009.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.