Abstract
Isoprenoids constitute an important class of biomolecules that participate in many different cellular processes. Most available detection methods only allow the identification of one or two specific non-sterol isoprenoid intermediates following radioactive or fluorescent labeling. We here report a rapid, non-radioactive and sensitive procedure for the simultaneous detection and quantification of the 8 main non-sterol intermediates of the isoprenoid biosynthesis pathway by means of tandem mass spectrometry. Intermediates were analyzed by HPLC-MS/MS in the multiple reaction monitoring mode using a silica-based C18 HPLC column. For quantification, their stable-isotope-labeled analogues were used as internal standards. HepG2 cells were used to validate the method. Mevalonate, phosphomevalonate and the 6 subsequent isoprenoid-pyrophosphates were readily determined with detection limits ranging from 0.03 to 1.0 μmol/L. The intra- and interassay variations for HepG2 cell homogenates supplemented with isoprenoid intermediates were 3.6–10.9% and 4.4–11.9%, respectively. Under normal culturing conditions, isoprenoid intermediates in HepG2 cells were below detection limits. However, incubation of the cells with pamidronate, an inhibitor of farnesyl pyrophosphate synthase, resulted in increased levels of MVA, IPP/DMAPP and GPP. This method will be suitable to measure profiles of isoprenoid intermediates in cells with compromised isoprenoid biosynthesis, and to determine the specificity of potential inhibitors of the pathway.
Keywords: Isoprenoid biosynthesis, Mevalonate kinase deficiency, Mass spectrometry, Farnesyl pyrophosphate, Geranylgeranyl pyrophosphate
Introduction
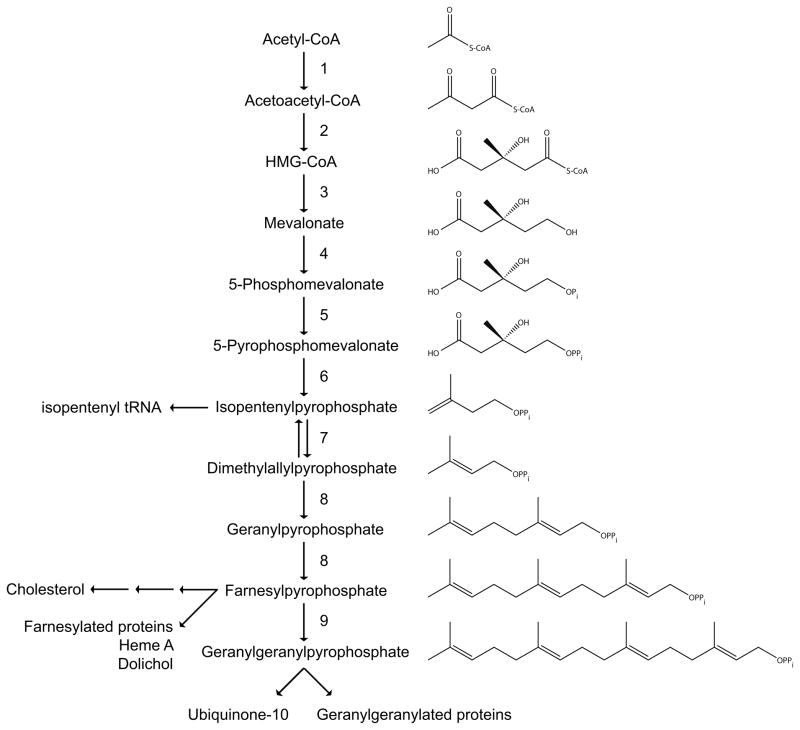
The isoprenoid biosynthesis pathway (Fig. 1) plays an important role in cellular metabolism. It provides the cell with a variety of compounds serving a number of different functions. In addition to sterols, involved in maintaining membrane fluidity, and required for the synthesis of hormones, bile acids and oxysterols, the pathway produces a variety of non-sterol isoprenoids. Examples of these are the side chains of ubiquinone-10 and heme A (which function in the mitochondrial respiratory chain), dolichol (required for protein glycosylation), isopentenyl tRNA (involved in protein translation) and the farnesyl and geranylgeranyl moieties of isoprenylated proteins such as the small GTPases. Although isoprenoids are rather diverse in structure and function, they all are derived from the basic C5 isoprene units, isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). These C5 isoprene units are synthesized in the non-sterol, pre-squalene part of the isoprenoid biosynthesis pathway, also known as the mevalonate pathway [1;2]. The mevalonate pathway starts with three acetyl-CoAs, which are converted into 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) in two consecutive enzyme steps. HMG-CoA is then converted into mevalonate (MVA) by the rate-limiting enzyme of the pathway, HMG-CoA reductase. Subsequently, MVA is phosphorylated twice, which produces 5-pyrophosphomevalonate (MVAPP). Decarboxylation of this latter compound yields IPP. After isomerization of IPP to DMAPP, a head-to-tail condensation of IPP to DMAPP results in the formation of geranyl pyrophosphate (GPP). Addition of another IPP gives farnesyl pyrophosphate (FPP), the branch point metabolite of the pathway, which is the precursor of geranylgeranyl pyrophosphate (GGPP); GGPP is produced by the condensation of one FPP with one IPP molecule.
Figure 1.
The isoprenoid biosynthesis pathway. The different enzymes involved are numbered as follows: 1. Acetoacetyl-CoA thiolase; 2. 3-Hydroxy-3-methylglutaryl-CoA synthase; 3. 3-Hydroxy-3-methylglutaryl-CoA reductase; 4. Mevalonate kinase; 5. Phosphomevalonate kinase; 6. Mevalonate pyrophosphate decarboxylase; 7. Isopentenyl pyrophosphate isomerase; 8. Farnesyl pyrophosphate synthase; 9. Geranylgeranyl pyrophosphate synthase.
Different methods for the detection of intermediates of the mevalonate pathway have been described in literature. Most of these methods allow the detection of only one specific compound, for example, the detection of MVA in human urine and plasma [3–7], and dog plasma [8], DMAPP in plant leaves, yeast and bacteria [9] or FPP in human and dog plasma [10], and yeast [11]. In addition, methods have been described for the simultaneous determination of FPP and GGPP in rat liver [12] and cultured NIH3T3 cells [13] and detection of IPP and FPP in mice and rat liver [14]. Measuring all the intermediates of the mevalonate pathway in one procedure is a major challenge, because the metabolites differ markedly in structure and physical properties. Indeed, only McCaskill and Croteau [15] reported a procedure for the analysis of all 11 intermediates of the mevalonate pathway from acetyl-CoA through GGPP in plant cells, while Zhang and Poulter [16] described a method to analyze the phosphorylated isoprenoid intermediates. Both procedures require incubation of cells or purified enzymes with radio-labeled precursors after which metabolites are detected by HPLC with radio-detection.
Here we report the development of a sensitive method using high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) that allows the direct detection and quantification of all intermediates of the mevalonate pathway without the use of radioactive or fluorescent compounds. The applicability of our procedure was demonstrated by the analysis of HepG2 cells incubated with pamidronate, an inhibitor of farnesyl pyrophosphate synthase (FPPS), which resulted in the accumulation of MVA, IPP/DMAPP and GPP.
Materials and Methods
Chemicals/materials
The following intermediates of the isoprenoid biosynthesis pathway were purchased from Sigma-Aldrich: mevalonolactone (MVAL), IPP, DMAPP, GPP, FPP and GGPP. MVAL-d7 was purchased from CDN isotopes.
Synthesis of 5-phosphomevalonate and 5-pyrophosphomevalonate
5-Phosphomevalonate (MVAP) and 5-pyrophosphomevalonate (MVAPP) were prepared by enzymatic synthesis. Maltose-binding protein (MBP)-mevalonate kinase (MK) and MBP-phosphomevalonate kinase (PMK) fusion proteins were obtained as described [17] and used to convert mevalonate (MVA) to MVAP and MVAPP. Incubations were performed using the same conditions described for MK or PMK activity measurements [18;19], with a few minor modifications. Instead of 1 M KPi, 1 M NH4HCO3 was used and the incubation time was extended to 1 hour. The reactions were not stopped with 20% formic acid, but samples were immediately deproteinized using Microcon Centrifugal Filter Devices YM-10 (Millipore) according to the manufacturer’s protocol.
Internal standards
All internal standards (IS) were either prepared by enzymatic or chemical synthesis, except MVA-d7. MVA-d7 was prepared by dissolving MVAL-d7 in 0.1 M NaOH followed by incubation at 37°C for 30 min. MVAP-d7 and MVAPP-d7 were synthesized by purified MBP-MK and MBP-PMK using MVA-d7 as substrate and following the same procedure described above for the synthesis of MVAP or MVAPP. IPP-d7 was synthesized by purified MBP-MK, MBP-PMK and MBP-mevalonate pyrophosphate decarboxylase (MPD) using MVA-d7 as substrate. MBP-MPD fusion protein was obtained as described [20] and used to convert MVAPP-d7 to IPP-d7. Incubations were performed using the same conditions described for synthesis of MVAP or MVAPP with one additional step. After the incubation with MBP-MK and MBP-PMK, MPB-MPD was added and incubated for an additional hour. After the enzymatic syntheses, the samples were deproteinized using Microcon Centrifugal Filter Devices YM-10 (Millipore) according to the manufacturer’s protocol. GPP-d3, FPP-d3 and GGPP-d3 were prepared using the vinyl triflate methodology previously developed in the Gibbs laboratory [21;22]. Full details of the synthesis of these compounds will be published elsewhere.
Cell culture
HepG2 cells were cultured in Dulbecco’s Modified Eagles medium (DMEM) containing 10% fetal calf serum (FCS), 1% Penicillin/Streptomycin and 25 mM Hepes in a temperature and humidity controlled incubator (95% air, 5% CO2 as the gas phase) at 37°C. For experiments, cells were grown in T75 cm2 flasks at a density of 3 million cells/flask in DMEM containing 10% lipoprotein (cholesterol)-depleted FCS, 1% Penicillin/Streptomycin and 25 mM Hepes. After 3 days of culturing, 100 μM pamidronate was added and incubated for 6, 12 and 24 hours. Cells were harvested as described under “Sample preparation”.
Sample preparation
Cells in culture flasks were washed 2 times with 100 mM NH4HCO3, pH 7.8. One or two ml of 2-propanol:100 mM NH4HCO3, pH 7.8 (1:1 v/v) was added to a T75 cm2 or T162 cm2 flask, respectively, and cells were scraped from the bottom. Cells were collected in a test tube, sonicated on ice (twice, 40 J at 8 W output) and 250 μl of the resulting cell homogenate was used for further preparation. To each cell homogenate (with or without supplemented isoprenoid intermediates), 500 μl 2-propanol:100 mM NH4HCO3 pH 7.8 (1:1 v/v) and IS (1 nmol MVA-d7, MVAP-d7 and MVAPP-d7, 0.2 nmol IPP-d7, GPP-d3, FPP-d3 and GGPP-d3) were added and samples were vortexed. Subsequently, 750 μl acetonitrile was added for deproteinization and samples were kept on ice for 10 min. The samples were then centrifuged for 10 min at 14000g at 4°C. After centrifugation, supernatants were transferred to glass tubes and dried under a stream of nitrogen at 40°C. The residues were then dissolved in 120 μl milliQ water and 10 μl of this solution was injected into the HPLC-MS/MS. Protein concentration of each cell suspension was determined using bicinchoninic acid [23].
Intra- and interassay determination
HepG2 cells were grown for three days in culture flasks at a density of 55000 cells/cm2 in regular DMEM as described under “Cell culture”. Cells were harvested as described under “Sample preparation”. The intraassay variation of the method was established with HepG2 cell homogenates without additions and with HepG2 cell homogenates supplemented with one of three different mixtures of isoprenoid intermediates. Low: 1 nmol MVA, MVAP and MVAPP, 0.2 nmol IPP, 0.1 nmol GPP, 0.11 nmol FPP and 0.12 nmol GGPP. Medium: 1.5 nmol of each calibrator. High: 3 nmol of each calibrator. The interassay variation was established with HepG2 cell homogenates without additions and with HepG2 cell homogenates supplemented with the same mixtures of isoprenoids used for the intraassay, during a period of 12 weeks. The recovery was determined using the intra- and interassay samples enriched with relevant intermediates.
Calibration curves
Calibration mixtures containing different concentrations of intermediates were used to construct calibrations curves. MVA: 10, 20, 30, 40 and 50 μmol/L. MVAP and MVAPP: 5, 10, 20, 30 and 40 μmol/L. IPP: 1.5, 13.7, 25.8, 37.9 and 50 μmol/L. GPP, FPP and GGPP: 0.5, 12.9, 25.3, 37.7 and 50 μmol/L. Constant amounts of IS were added to each calibration mixture (1 nmol MVA-d7, MVAP-d7 and MVAPP-d7, 0.2 nmol IPP-d7, GPP-d3, FPP-d3 and GGPP-d3). Calibration curves were used to determine linearity and the concentration of each compound in prepared samples.
HPLC-MS/MS
The HPLC system consisted of a Surveyor quaternary gradient pump, a vacuum degasser, a column temperature controller and an autosampler (Thermo Finnigan Corporation). The column temperature was maintained at 20°C. The samples were injected onto a 4.6 × 50 mm Luna C18 (2) column, 3 μm particle diameter (Phenomenex). The intermediates of the isoprenoid biosynthesis pathway were separated by a linear gradient between solution A (20 mM NH4HCO3, 0.1% triethylamine) and solution B (acetonitrile/H2O, 4:1, 0.1% triethylamine). The gradient was as follows: 0–2 min, 100% A to 80% A; 2–6 min, 80% A to 0% A; 6–7 min, 0% A; 7–7.1 min, 0% A to 100% A; 7.1–12 min, equilibration with 100% A. The flow rate was set to 1 ml/min and was split after the HPLC column in a ratio of 1/20, producing an inlet flow into the tandem mass spectrometer of 50 μl/min. For each analysis, 10 μl of sample was injected onto the column, and the total analysis time, including the equilibration, was 12 min.
A TSQ Quantum AM (Thermo Finnigan Corporation) was used in the negative electrospray ionization mode. Nitrogen was used as nebulizing gas, and argon was used as the collision gas at a pressure of 0.5 mTorr. The ion spray voltage was set at 3000 V, and the capillary temperature was 350°C. The collision cell energy was optimized for each particular intermediate of the isoprenoid biosynthesis pathway. The intermediates were detected with the mass spectrometer in multiple reaction monitoring (MRM) mode.
Results
Chromatography and mass spectra
We first optimized the mass spectrometer for each intermediate of the mevalonate pathway. Calibration mixtures containing 12.5 μmol/L MVA, MVAP, MVAPP, IPP, DMAPP, GPP, FPP or GGPP were used to determine MS/MS fragmentation patterns and HPLC retention behavior for each compound. The isoforms IPP and DMAPP elute as one peak and with this procedure can not be measured separately. To allow reliable quantification and exclude misinterpretation due to different physical behaviour of the various intermediates, we used for each intermediate its own IS, i.e. MVA-d7, MVAP-d7, MVAPP-d7, IPP-d7, GPP-d3, FPP-d3 and GGPP-d3. Because we observed interference of IPP-d7 detection by MVAP and MVAPP, we separated the preparation and detection of MVAP and MVAPP from the other compounds. The specific transitions obtained for each metabolite are listed in Table 1. All the isoprenoid intermediates containing a phosphate or pyrophosphate moiety produced a collision induced fragment ion of m/z 79.
Table 1.
MRM transitions for each isoprenoid intermediate and internal standards.
| Compound | Parent ion (m/z) | Product ion (m/z) | Collision energy (eV) |
|---|---|---|---|
| MVA | 147.10 | 59.10 | 14 |
| MVAP | 227.10 | 79.00 | 23 |
| MVAPP | 306.90 | 79.00 | 23 |
| IPP/DMAPP | 245.00 | 79.00 | 23 |
| GPP | 313.10 | 79.00 | 21 |
| FPP | 381.10 | 79.00 | 40 |
| GGPP | 449.15 | 79.00 | 46 |
| MVA-d7 | 154.10 | 59.10 | 14 |
| MVAP-d7 | 234.00 | 79.00 | 23 |
| MVAPP-d7 | 313.90 | 79.00 | 23 |
| IPP-d7 | 252.00 | 79.00 | 23 |
| GPP-d3 | 316.10 | 79.00 | 21 |
| FPP-d3 | 384.10 | 79.00 | 40 |
| GGPP-d3 | 452.15 | 79.00 | 46 |
Limits of quantification (LOQ) and detection (LOD)
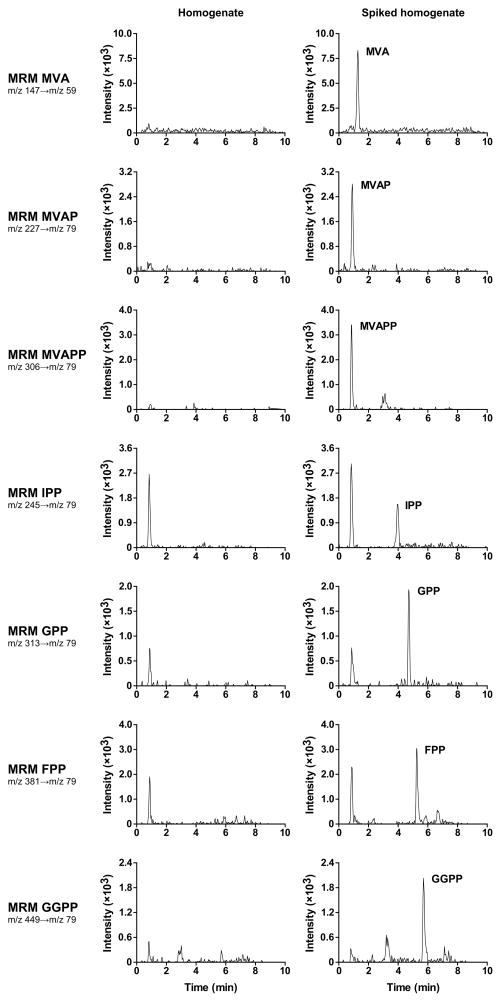
HepG2 cell homogenates were supplemented with decreasing concentrations of calibration mixture containing MVA, IPP, GPP, FPP and GGPP or MVAP and MVAPP thereby decreasing the concentration of the isoprenoid intermediates to undetectable levels. Samples were subsequently prepared for HPLC-MS/MS as described in “materials and methods”. The LOQ and the LOD were defined as the lowest concentration that gave a signal-to-noise ratio of 10 and 3, respectively. The LOQ and LOD for the isoprenoid intermediates were 0.1–4.2 μmol/L and 0.03–1.0 μmol/L, respectively (Table 2). Figure 2 shows the MRM chromatograms of HepG2 cell homogenate spiked with LOQ levels of each compound.
Table 2.
LOQ and LOD of all isoprenoid intermediates.
| Compound | LOQ (μmol/L) | LOD (μmol/L) |
|---|---|---|
| MVA | 4.17 | 1.04 |
| MVAP | 4.17 | 1.04 |
| MVAPP | 4.17 | 1.04 |
| IPP | 0.42 | 0.10 |
| GPP | 0.10 | 0.03 |
| FPP | 0.11 | 0.03 |
| GGPP | 0.13 | 0.06 |
Figure 2.
MRM chromatograms of HepG2 cell homogenate and HepG2 cell homogenate spiked with LOQ levels of each isoprenoid intermediate.
Linearity
Calibration curves were constructed for each isoprenoid intermediate as described in “materials and methods”. The calibration curves were linear up to at least 50 μmol/L (r2 >0.990). Because the HPLC column was overloaded for IPP, GPP, FPP and GGPP when using calibration mixtures of 100 μmol/L, higher concentrations than 50 μmol/L could not be measured accurately.
MS/MS variation, intra- and interassay variations, and recovery
The variation of the MS/MS was determined by 10 consecutive analyses of 10 μl of a single sample, i.e. processed HepG2 cell homogenates supplemented with 1.5 nmol of each calibrator. The MS/MS variation was 3.7–9.3% (Table 3). The intra- and interassay variations were established by measurement of HepG2 cell homogenates and HepG2 cell homogenates supplemented with isoprenoid intermediates at 3 different concentrations (Table 4 and 5). The lowest concentrations of added calibrators used (1 nmol MVA, MVAP and MVAPP; 0.2 nmol IPP; 0.1 nmol GPP, 0.11 nmol FPP and 0.12 nmol GGPP), were based on the observed difference in MS/MS sensitivity. The medium and high concentrations of calibrators were 1.5 and 3 nmol, respectively. Intracellular levels of isoprenoid intermediates in HepG2 cells were below detection limits. The intra- and interassay variations determined with the homogenates supplemented with the intermediates were 3.6–10.9% and 4.4–11.9%, respectively. Recoveries of the added calibrators were in the range of 91–124%.
Table 3.
| Compound | Mean (nmol) | CV (%) |
|---|---|---|
| MVA | 1.53 | 9.3 |
| MVA-P | 1.41 | 7.8 |
| MVA-PP | 1.56 | 8.7 |
| IPP | 1.37 | 7.9 |
| GPP | 1.51 | 4.4 |
| FPP | 1.51 | 5.1 |
| GGPP | 1.63 | 3.7 |
n = 10 for each compound.
HepG2 cell homogenates were supplemented with 1.5 nmol of each intermediate.
Every sample contains 775 μg of protein.
Table 4.
| Compound | Input (nmol) | Mean (nmol) | CV (%) | Recoveryd (%) |
|---|---|---|---|---|
| MVA | 1.00 | 0.98 | 9.8 | 98 |
| MVA-P | 1.00 | 1.14 | 9.9 | 114 |
| MVA-PP | 1.00 | 1.06 | 10.3 | 106 |
| IPP | 0.20 | 0.22 | 10.3 | 109 |
| GPP | 0.10 | 0.12 | 4.3 | 124 |
| FPP | 0.11 | 0.13 | 6.0 | 116 |
| GGPP | 0.12 | 0.13 | 5.8 | 106 |
| Compound | Input (nmol) | Mean (nmol) | CV (%) | Recoveryd (%) |
| MVA | 1.50 | 1.48 | 9.6 | 99 |
| MVA-P | 1.50 | 1.63 | 7.1 | 108 |
| MVA-PP | 1.50 | 1.60 | 9.6 | 106 |
| IPP | 1.50 | 1.48 | 8.4 | 98 |
| GPP | 1.50 | 1.44 | 4.7 | 96 |
| FPP | 1.50 | 1.61 | 5.6 | 108 |
| GGPP | 1.50 | 1.62 | 4.1 | 108 |
| Compound | Input (nmol) | Mean (nmol) | CV (%) | Recoveryd (%) |
| MVA | 3.00 | 2.96 | 10.9 | 99 |
| MVA-P | 3.00 | 2.94 | 7.8 | 98 |
| MVA-PP | 3.00 | 2.90 | 8.7 | 97 |
| IPP | 3.00 | 2.74 | 8.8 | 91 |
| GPP | 3.00 | 2.90 | 3.7 | 97 |
| FPP | 3.00 | 3.11 | 4.3 | 104 |
| GGPP | 3.00 | 3.08 | 3.6 | 103 |
n = 10 for each compound concentration.
No intermediates were detected in HepG2 cell homogenates without supplementation.
Every sample contains 775 μg of protein.
Recoveries were determined using cell homogenates supplemented with the indicated intermediates.
Table 5.
| Compound | Input (nmol) | Mean (nmol) | CV (%) | Recoveryd (%) |
|---|---|---|---|---|
| MVA | 1.00 | 1.02 | 9.5 | 102 |
| MVA-P | 1.00 | 0.99 | 10.2 | 99 |
| MVA-PP | 1.00 | 1.06 | 11.9 | 106 |
| IPP | 0.20 | 0.22 | 11.2 | 109 |
| GPP | 0.10 | 0.12 | 8.5 | 123 |
| FPP | 0.11 | 0.12 | 11.1 | 107 |
| GGPP | 0.12 | 0.11 | 11.1 | 94 |
| Compound | Input (nmol) | Mean (nmol) | CV (%) | Recoveryd (%) |
| MVA | 1.50 | 1.62 | 10.6 | 108 |
| MVA-P | 1.50 | 1.52 | 9.5 | 101 |
| MVA-PP | 1.50 | 1.47 | 11.5 | 98 |
| IPP | 1.50 | 1.54 | 10.6 | 103 |
| GPP | 1.50 | 1.61 | 9.6 | 108 |
| FPP | 1.50 | 1.69 | 9.3 | 113 |
| GGPP | 1.50 | 1.61 | 4.6 | 107 |
| Compound | Input (nmol) | Mean (nmol) | CV (%) | Recoveryd (%) |
| MVA | 3.00 | 3.12 | 8.5 | 104 |
| MVA-P | 3.00 | 2.88 | 9.8 | 96 |
| MVA-PP | 3.00 | 2.81 | 10.5 | 94 |
| IPP | 3.00 | 2.82 | 9.9 | 94 |
| GPP | 3.00 | 3.05 | 4.4 | 102 |
| FPP | 3.00 | 3.14 | 6.7 | 105 |
| GGPP | 3.00 | 3.09 | 7.7 | 103 |
n = 10 for each compound concentration.
No intermediates were detected in HepG2 cell homogenates without supplementation.
Every sample contains 775 μg of protein.
Recoveries were determined using cell homogenates supplemented with the indicated intermediates.
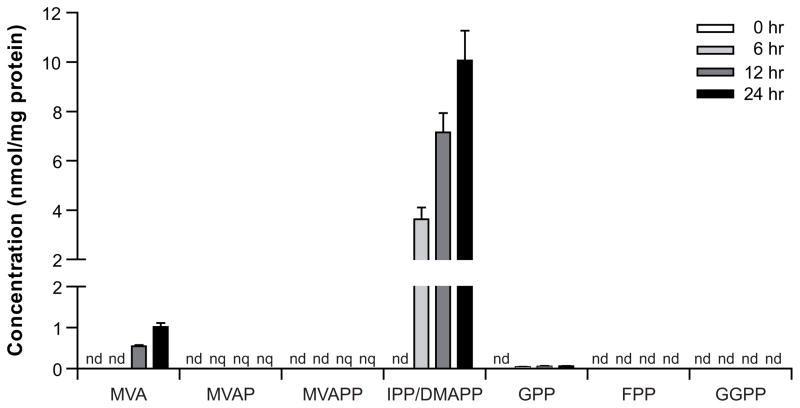
Intracellular accumulation of intermediates of the mevalonate pathway
Application of the method to determine intracellular levels of intermediates was demonstrated by blocking the isoprenoid biosynthesis pathway with pamidronate, which inhibits FPPS (Fig. 1). HepG2 cells were cultured in medium supplemented with lipoprotein-depleted FCS to induce isoprenoid biosynthesis [24;25], and incubated with 100 μM pamidronate for 6, 12 and 24 hours (Fig. 3). Accumulation of MVA and IPP/DMAPP was observed in a time-dependent manner. Furthermore, small amounts of MVAP, MVAPP and GPP were detected, although levels of MVAP and MVAPP were below quantification limits. When HepG2 cells were cultured in medium with regular FCS and incubated with pamidronate no accumulation of intermediates was observed (data not shown).
Figure 3.
Inhibition of the isoprenoid biosynthesis pathway with pamidronate. HepG2 cells treated with 100 μM pamidronate for 6, 12 and 24 hours. n = 4, mean±sd. nd, not detected. nq, not quantified.
Discussion
We developed a sensitive and specific method for the detection and quantification of nearly all isoprenoid intermediates of the mevalonate pathway using HPLC-MS/MS. Our method covers the measurement of MVA up to GGPP and is most sensitive for the phosphorylated compounds. Previously, Seker et al. [26] described a method to analyze the first three metabolites of the pathway, i.e., acetyl-CoA, acetoacetyl-CoA and HMG-CoA, using reversed-phase ion-pair HPLC, which can be used as a complementary method to allow detection of all isoprenoid intermediates. To assure that our method would be suitable for studies in cells and tissue, we determined the detection and quantification parameters of the various intermediates after supplying these to homogenates of the hepatoma cell line HepG2 rather than using the intermediates dissolved in buffer. Due to the wide diversity in structure, the various isoprenoid intermediates behaved quite differently in our extraction procedure, which makes the use of stable-isotope-labeled compounds of each metabolite as internal standard important for accurate quantification. Moreover, MVA, MVAP and MVAPP have a somewhat higher limit of detection (1.0 μmol/L) than the other intermediates (0.03–0.1 μmol/L).
Currently, only one genetic disorder is known which is due to an enzyme defect in the mevalonate pathway, namely mevalonate kinase deficiency (MKD). MKD is autosomal recessively inherited and characterized by periodic episodes of fever and inflammation. Due to the deficient activity of mevalonate kinase the patients have elevated levels of mevalonic acid in plasma and urine [27]. A deficiency of one of the other enzymes of the mevalonate pathway is predicted to result in the accumulation of a phosphorylated isoprenoid intermediate. In contrast to mevalonic acid, however, compounds containing a phosphate moiety are expected not to cross the cell membrane easily and thus this accumulation would predominantly occur intracellularly. Indeed, in the experiments in which we incubated HepG2 cells with pamidronate we also analyzed the culture medium. Despite the marked accumulation of IPP/DMAPP in the cells (Fig. 3), these phosphorylated metabolites were not detected in the culture medium, while the non-phosphorylated intermediate MVA could be readily detected in the medium (data not shown). This implies that patients with a deficiency in an enzyme of the mevalonate pathway other than MK, may not be detected by plasma and/or urine analysis, although some accumulation of mevalonic acid may provide a first clue. Analysis of (cultured) cells, PBMCs or tissue by our HPLC-MS/MS method therefore may be helpful to identify these potential patients.
Our method can also be useful to asses the effect of manipulation of the isoprenoid biosynthesis pathway with specific inhibitors directed against enzymes of this pathway. For example, isoprenylation of proteins is an important therapeutic target in cancer research. This posttranslational modification promotes membrane association and contributes to protein-protein interactions. Ras, a member of the small G protein superfamily, is one of many proteins that is prenylated by farnesyl transferase. Because of the high frequency of Ras mutations in cancer, farnesyl transferase inhibitors have been widely developed and are being tested for potential use in cancer therapy [28–30]. There has also been renewed interest recently in the development of squalene synthase inhibitors as potential agents for the treatment of hypercholesterolemia, and such inhibitors could also lead to enhanced cellular levels of isoprenoid intermediates [31]. With our method the specificity of these two classes of inhibitors can be studied by determining their effect on overall isoprenoid biosynthesis.
Acknowledgments
This research was supported by grant 912-03-024 of ZonMW. The synthetic work at Purdue was supported by NIH grant R01 CA78819. We thank Novartis for kindly providing pamidronate. We thank Drs. Sander M. Houten and Ronald J.A. Wanders for their valuable input and critical discussions throughout this study.
Abbreviations
- MVA
Mevalonate
- MVAP
5-Phosphomevalonate
- MVAPP
5-Pyrophosphomevalonate
- IPP
Isopentenyl pyrophosphate
- DMAPP
Dimethylallyl pyrophosphate
- GPP
Geranyl pyrophosphate
- FPP
Farnesyl pyrophosphate
- GGPP
Geranylgeranyl pyrophosphate
- HMG-CoA
3-Hydroxy-3-methylglutaryl-CoA
- HPLC-MS/MS
High performance liquid chromatography-tandem mass spectrometry
- FPPS
Farnesyl pyrophosphate synthase
- MVAL
Mevalonolactone
- MBP
Maltose-binding protein
- MK
Mevalonate kinase
- PMK
Phosphomevalonate kinase
- IS
Internal standard
- MPD
Mevalonate pyrophosphate decarboxylase
- MRM
Multiple reaction monitoring
- LOQ
Limit of quantification
- LOD
Limit of detection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Casey PJ, Seabra MC. Protein prenyltransferases. J Biol Chem. 1996;271:5289–5292. doi: 10.1074/jbc.271.10.5289. [DOI] [PubMed] [Google Scholar]
- 2.McTaggart SJ. Isoprenylated proteins. Cell Mol Life Sci. 2006;63:255–267. doi: 10.1007/s00018-005-5298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal M, Schuster A, Whigan DB. Liquid chromatography/tandem mass spectrometry methods for quantitation of mevalonic acid in human plasma, urine: method validation, demonstration of using a surrogate analyte, and demonstration of unacceptable matrix effect in spite of use of a stable isotope analog internal standard. Rapid Commun Mass Spectrom. 2003;17:1723–1734. doi: 10.1002/rcm.1112. [DOI] [PubMed] [Google Scholar]
- 4.Saini GS, Wani TA, Gautam A, Varshney B, Ahmed T, Rajan KS, Pillai KK, Paliwal JK. Validation of the LC-MS/MS method for the quantification of mevalonic acid in human plasma and determination of the matrix effect. J Lipid Res. 2006;47:2340–2345. doi: 10.1194/jlr.D600018-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Scoppola A, Maher VM, Thompson GR, Rendell NB, Taylor GW. Quantitation of plasma mevalonic acid using gas chromatography-electron capture mass spectrometry. J Lipid Res. 1991;32:1057–1060. [PubMed] [Google Scholar]
- 6.Siavoshian S, Simoneau C, Maugeais P, Marks L, Rodary L, Gardette J, Krempf M. Measurement of mevalonic acid in human urine by bench top gas chromatography-mass spectrometry. Clin Chim Acta. 1995;243:129–136. doi: 10.1016/0009-8981(95)06162-2. [DOI] [PubMed] [Google Scholar]
- 7.Woollen BH, Holme PC, Northway WJ, Martin PD. Determination of mevalonic acid in human urine as mevalonic acid lactone by gas chromatography-mass spectrometry. J Chromatogr B Biomed Sci Appl. 2001;760:179–184. doi: 10.1016/s0378-4347(01)00247-x. [DOI] [PubMed] [Google Scholar]
- 8.Saisho Y, Kuroda T, Umeda T. A sensitive and selective method for the determination of mevalonic acid in dog plasma by gas chromatography/negative ion chemical ionization-mass spectrometry. J Pharm Biomed Anal. 1997;15:1223–1230. doi: 10.1016/s0731-7085(96)02012-2. [DOI] [PubMed] [Google Scholar]
- 9.Fisher AJ, Rosenstiel TN, Shirk MC, Fall R. Nonradioactive assay for cellular dimethylallyl diphosphate. Anal Biochem. 2001;292:272–279. doi: 10.1006/abio.2001.5079. [DOI] [PubMed] [Google Scholar]
- 10.Saisho Y, Morimoto A, Umeda T. Determination of farnesyl pyrophosphate in dog and human plasma by high-performance liquid chromatography with fluorescence detection. Anal Biochem. 1997;252:89–95. doi: 10.1006/abio.1997.2314. [DOI] [PubMed] [Google Scholar]
- 11.Song L. Detection of farnesyl diphosphate accumulation in yeast ERG9 mutants. Anal Biochem. 2003;317:180–185. doi: 10.1016/s0003-2697(03)00138-6. [DOI] [PubMed] [Google Scholar]
- 12.Keller RK. Squalene synthase inhibition alters metabolism of nonsterols in rat liver. Biochim Biophys Acta. 1996;1303:169–179. doi: 10.1016/0005-2760(96)00081-1. [DOI] [PubMed] [Google Scholar]
- 13.Tong H, Holstein SA, Hohl RJ. Simultaneous determination of farnesyl and geranylgeranyl pyrophosphate levels in cultured cells. Anal Biochem. 2005;336:51–59. doi: 10.1016/j.ab.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 14.Bruenger E, Rilling HC. Determination of isopentenyl diphosphate and farnesyl diphosphate in tissue samples with a comment on secondary regulation of polyisoprenoid biosynthesis. Anal Biochem. 1988;173:321–327. doi: 10.1016/0003-2697(88)90196-0. [DOI] [PubMed] [Google Scholar]
- 15.McCaskill D, Croteau R. Procedures for the isolation and quantification of the intermediates of the mevalonic acid pathway. Anal Biochem. 1993;215:142–149. doi: 10.1006/abio.1993.1566. [DOI] [PubMed] [Google Scholar]
- 16.Zhang D, Poulter CD. Analysis and purification of phosphorylated isoprenoids by reversed-phase HPLC. Anal Biochem. 1993;213:356–361. doi: 10.1006/abio.1993.1432. [DOI] [PubMed] [Google Scholar]
- 17.Hogenboom S, Romeijn GJ, Houten SM, Baes M, Wanders RJ, Waterham HR. Absence of functional peroxisomes does not lead to deficiency of enzymes involved in cholesterol biosynthesis. J Lipid Res. 2002;43:90–98. [PubMed] [Google Scholar]
- 18.Hoffmann GF, Brendel SU, Scharfschwerdt SR, Shin YS, Speidel IM, Gibson KM. Mevalonate kinase assay using DEAE-cellulose column chromatography for first-trimester prenatal diagnosis and complementation analysis in mevalonic aciduria. J Inherit Metab Dis. 1992;15:738–746. doi: 10.1007/BF01800016. [DOI] [PubMed] [Google Scholar]
- 19.Wanders RJA, Romeijn GJ. Differential Deficiency of Mevalonate Kinase and Phosphomevalonate Kinase in Patients with Distinct Defects in Peroxisome Biogenesis: Evidence for a Major Role of Peroxisomes in Cholesterol Biosynthesis. Biochemical and Biophysical Research Communications. 1998;247:663–667. doi: 10.1006/bbrc.1998.8836. [DOI] [PubMed] [Google Scholar]
- 20.Hogenboom S, Romeijn GJ, Houten SM, Baes M, Wanders RJ, Waterham HR. Absence of functional peroxisomes does not lead to deficiency of enzymes involved in cholesterol biosynthesis. J Lipid Res. 2002;43:90–98. [PubMed] [Google Scholar]
- 21.Gibbs RA, Krishnan U, Dolence JM, Poulter CD. A Stereoselective Palladium/Copper-Catalyzed Route to Isoprenoids: Synthesis and Biological Evaluation of 13-Methylidenefarnesyl Diphosphate. J Org Chem. 1995;60:7821–7829. [Google Scholar]
- 22.Zahn TJ, Eilers M, Guo Z, Ksebati MB, Simon M, Scholten JD, Smith SO, Gibbs RA. Evaluation of Isoprenoid Conformation in Solution and in the Active Site of Protein-Farnesyl Transferase Using Carbon-13 Labeling in Conjunction with Solution-and Solid-State NMR. J Am Chem Soc. 2000;122:7153–7164. [Google Scholar]
- 23.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 24.Gibson KM, Hoffmann G, Schwall A, Broock RL, Aramaki S, Sweetman L, Nyhan WL, Brandt IK, Wappner RS, Lehnert W. 3-Hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured fibroblasts from patients with mevalonate kinase deficiency: differential response to lipid supplied by fetal bovine serum in tissue culture medium. J Lipid Res. 1990;31:515–521. [PubMed] [Google Scholar]
- 25.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 26.Seker T, Moller K, Nielsen J. Analysis of acyl CoA ester intermediates of the mevalonate pathway in Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2005;67:119–124. doi: 10.1007/s00253-004-1697-0. [DOI] [PubMed] [Google Scholar]
- 27.Houten SM, Frenkel J, Waterham HR. Isoprenoid biosynthesis in hereditary periodic fever syndromes and inflammation. Cell Mol Life Sci. 2003;60:1118–1134. doi: 10.1007/s00018-003-2296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basso AD, Kirschmeier P, Bishop WR. Lipid posttranslational modifications. Farnesyl transferase inhibitors. J Lipid Res. 2006;47:15–31. doi: 10.1194/jlr.R500012-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Caraglia M, Budillon A, Tagliaferri P, Marra M, Abbruzzese A, Caponigro F. Isoprenylation of intracellular proteins as a new target for the therapy of human neoplasms: preclinical and clinical implications. Curr Drug Targets. 2005;6:301–323. doi: 10.2174/1389450053765833. [DOI] [PubMed] [Google Scholar]
- 30.McTaggart SJ. Isoprenylated proteins. Cell Mol Life Sci. 2006;63:255–267. doi: 10.1007/s00018-005-5298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charlton-Menys V, Durrington PN. Squalene synthase inhibitors: clinical pharmacology and cholesterol-lowering potential. Drugs. 2007;67:11–16. doi: 10.2165/00003495-200767010-00002. [DOI] [PubMed] [Google Scholar]