Abstract
Background
Hepatic ischemia-reperfusion (I/R) injury is a serious clinical complication that may compromise liver function because of extensive hepatocyte loss. Therefore, the development of novel and effective therapies for hepatic I/R is critical for the improvement of patient outcome. It has been previously shown that administration of milk fat globule-EGF factor VIII (MFG-E8), a membrane-associated secretory glycoprotein, exerts significant beneficial effects under acute inflammatory conditions through multiple physiological processes associated with tissue remodeling.
Methods
To determine whether administration of recombinant human (rh) MFG-E8 attenuates liver injury in an animal model of hepatic I/R. Male adult rats were subjected to 70% hepatic ischemia for 90 min, followed by reperfusion. At the beginning of reperfusion, rats were treated intravenously with normal saline (Vehicle) or rhMFG-E8 (160 μg/kg) over a period of 30 min. MFG-E8 levels and various measurements were assessed 4 h after reperfusion. In addition, survival study was conducted in MFG-E8−/− and rhMFG-E8-treated wild-type (WT) mice using a total hepatic ischemia model.
Results
Liver and plasma MFG-E8 protein levels were significantly decreased after hepatic I/R. Administration of rhMFG-E8 significantly improved liver injury, suppressed apoptosis, attenuated inflammation and oxidative stress, and down-regulated NF-κB pathway. We also noticed that rhMFG-E8 treatment restored the down-regulated PPARγ expression after hepatic I/R. MFG-E8−/− mice showed deterioration on survival and, in contrast, rhMFG-E8-treated WT mice showed a significant improvement of survival compared with vehicle-treated WT mice.
Conclusions
MFG-E8-mediated multiple physiological events may represent an effective therapeutic option in tissue injury following an episode of hepatic I/R.
Keywords: MFG-E8, hepatic ischemia-reperfusion, inflammation, apoptosis, NF-κB, PPARγ
INTRODUCTION
Hepatic ischemia-reperfusion (I/R) damage, which occurs in diverse clinical settings including liver transplantation, trauma, hemorrhagic shock, or liver surgery, is a serious clinical complication that may compromise liver function because of extensive hepatocellular loss. I/R injury represents a complex series of events that result in cellular and tissue damage. It involves the transient deprivation of blood flow and oxygen, and the return of blood flow during reperfusion with concomitant release of reactive oxygen species (ROS), inflammatory mediators, adhesion molecules, adenosine triphosphate (ATP) depletion, and derangements in calcium homeostasis. Finally, these functional changes induce cell death due to apoptosis as well as necrosis [1, 2]. Despite the fact that hepatic injury is a major clinical problem, the precise molecular mechanism of hepatic I/R injury is still not well understood. It has been shown that programmed cell death or apoptosis of liver sinusoidal cells and hepatocytes is a prominent feature of liver I/R injury, in both experimental models and clinical transplantation [1, 3, 4]. Historically, apoptosis has been seen as an ordinary process of cell suicide that, unlike necrosis, does not elicit inflammation [5]. However, recent studies have shown that if the removal process of apoptotic cells fails, apoptotic cells undergo secondary necrosis, which enables to release potentially cytotoxic intracellular contents, and followed by inflammation and impaired tissue repair [6, 7].
Apoptotic cells expose phosphatidylserine (PS) to the external surface of the plasma membrane, which can be recognized by soluble molecules and receptors, thereby enabling their phagocytosis [8]. One of these molecules is the glycoprotein, milk fat globule-EGF factor VIII (MFG-E8), which is mainly secreted by mononuclear cells, and participates in the phagocytosis of apoptotic cells by forming a bridge between PS on apoptotic cells and αvβ3/αvβ5-integrins on phagocytes [9]. Since its discovery, MFG-E8 has been shown to negatively regulate inflammation and autoimmunity by the enhancement of phagocytosis of apoptotic cells [9, 10]. Recently, we have clearly shown that MFG-E8 is a critical molecule for controlling the inflammation caused by inappropriate clearance of apoptotic cells in acute inflammatory disease conditions including sepsis, and gut and renal I/R injury [11–16]. Furthermore, recent vigorous investigations have demonstrated that MFG-E8-mediated therapeutic potential is not only dependent on enhancement of phagocytosis, but also on multiple cellular events associated with tissue remodeling [17–19].
In the present study, to clarify the role of MFG-E8 in hepatic I/R, we evaluated the expression of endogenous MFG-E8 and also examined the effect of exogenous MFG-E8 administration on hepatic I/R injury in an established animal model.
METHODS
Experimental animals and animal model of hepatic I/R
Male Sprague-Dawley rats (275–325 g), purchased from Charles River Laboratories (Wilmington, MA), were used for this study. The animals were housed in a temperature controlled room and on a 12-h light/dark cycle and fed on a standard Purina rat chow diet and allowed water ad libitum. All experiments were performed in accordance with the guidelines for the use of experimental animals by the National Institutes of Health (Bethesda, MD) and were approved by the Institutional Animal Care and Use Committee of The Feinstein Institute for Medical Research (Manhasset, NY). A rat model of partial ischemia was used as previously described [20]. After 90 min of partial hepatic ischemia, the clip was removed to allow reperfusion. The rats were immediately resuscitated with 1 ml normal saline over a period of 30 min via a femoral vein catheter with or without (Vehicle; n=10) recombinant human MFG-E8 (rhMFG-E8; 160 μg/kg in 1 ml normal saline; n=10) intravenously. We have shown that this dose of rhMFG-E8 elicited best survival benefit in dose-dependent study in rat cecal ligation and puncture (CLP) induced-sepsis model [21]. The source of rhMFG-E8 was described previously [21]. Sham animals (n=9) underwent the same surgical procedure without vascular occlusion. Four hours after reperfusion, animals were anesthetized, and blood and liver samples were harvested, frozen immediately in liquid nitrogen, and stored at −80°C until experiments.
Determination of plasma liver enzymes and cytokines
Plasma levels of aspartate aminotransferase (AST), and alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) at 4 h after reperfusion were determined by using commercial assay kits according to the manufacturer’s instructions (Pointe Scientific, Lincoln Park, MI). The plasma TNF-α and IL-6 were determined by using commercially obtained enzyme-linked immunosorbent assay kits (BD Pharmingen, Franklin Lakes, NJ).
Water content determination and histologic examination
Liver edema was estimated by comparing wet-to-dry weight rations as described previously [20]. Morphologic alterations in the liver were examined at 4 h after reperfusion by light microscopy and documented by photographs. The tissue sections were prepared and stained with hematoxylin and eosin as described previously [20].
Quantitative real-time PCR
Total RNA was extracted from liver tissue samples after 4 h reperfusion and cDNA was synthesized as described previously [20]. The expressions of target mRNAs were detected by quantitative real-time PCR (7300 Real Time PCR System, Applied Biosystems) with SYBR Green as detection dye. Primer sequences were as follows: TLR4 (NM_019178; forward, 5′-CCT GAG ACC AGG AAG CTT GAA-3′; reverse, 5′-TCT GAT CCA TGC ATT GGT AGG T-3′), ICAM-1 (NM_012967; forward, 5′-CGA GTG GAC ACA ACT GGA AG-3′; reverse, 5′-CGC TCT GGG AAC GAA TAC AC-3′), and GAPDH (AF 106860; forward, ATG ACT CTA CCC ACG GCA AG-3′; reverse, 5′-CTG GAA GAT GGT GAT GGG TT-3′). The relative expression of each mRNA was calculated using ΔΔCt threshold method.
Western blotting
The extracted proteins from the liver (50 μg/sample) and 1 μl plasma were fractionated on a Bis-Tris gel and transferred to a 0.2-μm nitrocellulose membrane. The membranes were blocked and incubated with 1:1,000 goat polyclonal MFG-E8, 1:5,000 rabbit polyclonal nitrotyrosine, 1:1,000 rabbit polyclonal IκBα (Santa Cruz Biotechnology, Santa Cruz, CA), and 1:1,000 rabbit polyclonal PPARγ (Cayman Chemical, Ann Arbor, MI) antibodies. And then membranes were incubated with horseradish peroxidase-labeled secondary antibodies. Bands were detected using Chemiluminescent peroxidase substrate (ECL Plus, Amersham, Little Chalfont, Buckinghamshire, UK).
Apoptosis assay
The presence of apoptotic cells in the liver sections were assessed after 4 hr reperfusion using an immunohistochemical staining of cleaved caspase-3 and TUNEL staining. The sections were stained with 1:80 rabbit polyclonal cleaved caspase-3 (Cell Signaling Technology, Danvers, MA) antibody and stained with terminal deoxynucleotide transferase dUTP nick-end labeling (TUNEL) staining kit (Roche Diagnostics, Indianapolis, IN) according to the manufacture’s instruction. TUNEL-positive cells were counted in 10 fields/section under a fluorescence microscope (× 200), and the number of cells/field are shown.
Survival study
Male C57BL/6J wild-type (WT) mice (20–25 g; Taconic, Albany, NY) with or without rhMFG-E8 treatment (a single dose, 160 μg/kg, intraperitoneal administration at the beginning of reperfusion), and male C57BL/6J MFG-E8−/− mice (20–25 g; a generous gift from Dr. Shigekazu Nagata, Kyoto University, Japan) were subjected to a model of total hepatic ischemia, in which only the ischemic tissue is left in place, as previously described [22]. Briefly, the portal triad for the left and median lobes was occluded for 60 minutes with an atraumatic clip. Immediately after the removal of the clip, the non-ischemic lobes were ligated and resected leaving the ischemic lobes. The mice were resuscitated with 0.5 ml normal saline intraperitoneally. Mice were monitored every 12 h for 10 days.
Statistical analysis
Data are expressed as mean ± standard error (SE) and compared by one-way analysis of variance using Tukey-Kramer’s test. Student t test was used for two-group analysis. The survival study was analyzed using Kaplan-Meier method and compared by log-rank test. Differences in values were considered significant at P < 0.05.
RESULTS
MFG-E8 production is suppressed after hepatic I/R
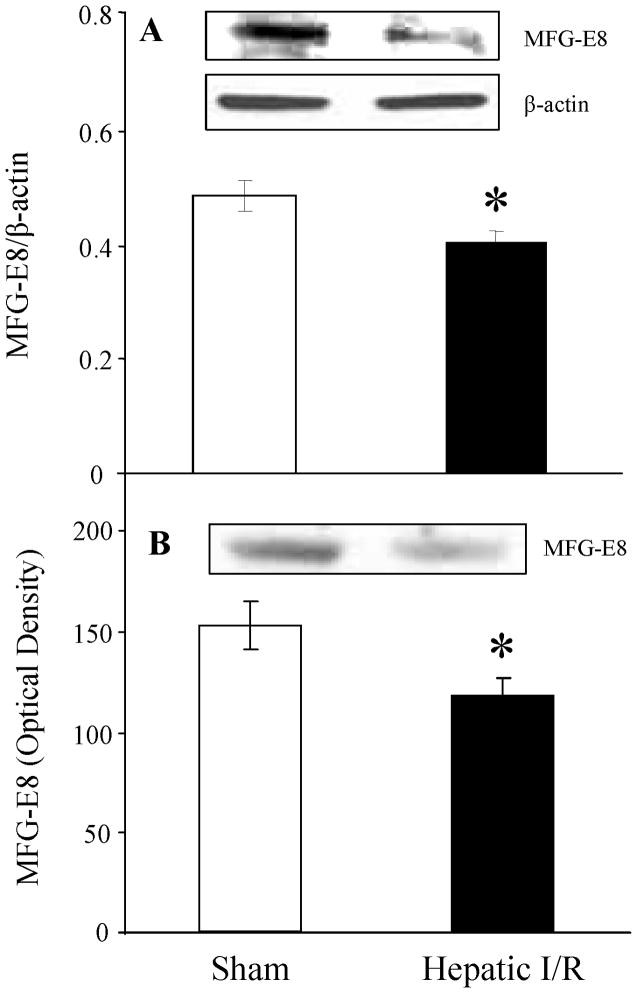
To investigate whether MFG-E8 levels are altered after hepatic I/R, we measured its protein levels at 4 h after reperfusion. MFG-E8 levels in liver tissues showed a significant decrease after hepatic I/R (P = 0.033, Fig. 1A). Similarly, a significant decrease in circulating levels of MFG-E8 was found after hepatic I/R (P = 0.029, Fig. 1B). These results suggest that a decrease in the production of MFG-E8 occurs after hepatic I/R.
FIGURE 1.
Alterations in MFG-E8 protein levels in the liver (A) and plasma (B) at the end of 4-h reperfusion after 90 minutes hepatic ischemia-reperfusion (I/R) or sham operation (Sham). Representative blots are also presented. Data are expressed as means ± SE (n=9–10/group) and compared by Student t test; *P < 0.05 versus Sham group.
Treatment with rhMFG-E8 attenuates liver injury after hepatic I/R
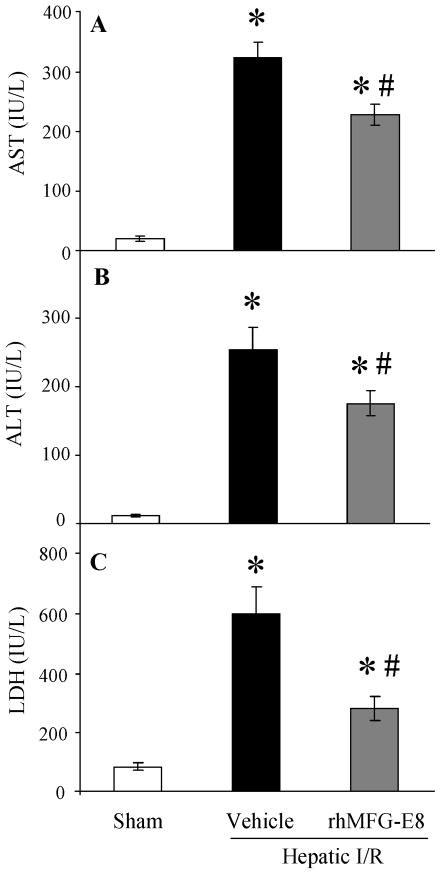
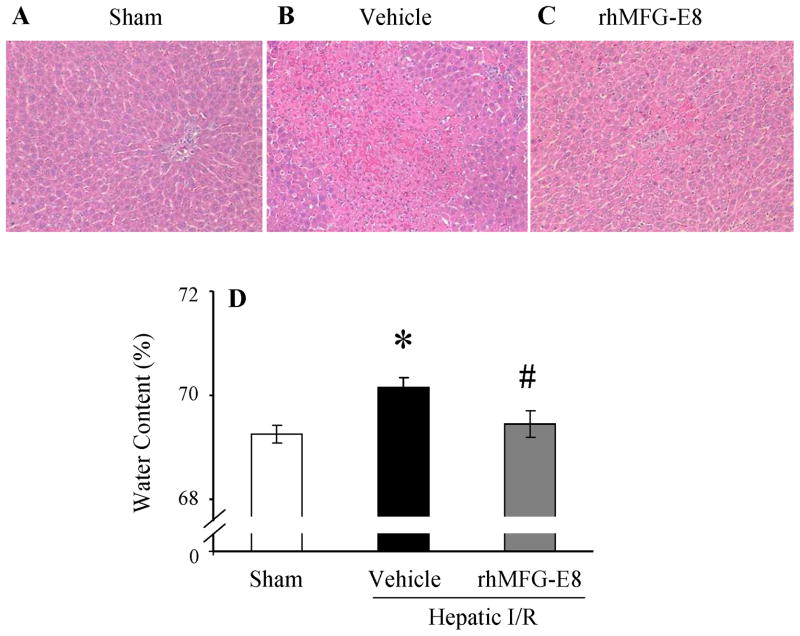
Liver functions were examined by measuring plasma levels of AST, ALT, and LDH at 4 h after reperfusion. These levels increased by 16.6-, 20.8-, and 7.2-fold, respectively, after hepatic I/R (P < 0.0001, 0.0001, and 0.0001, respectively, Figs. 2A–C). In contrast, treatment with rhMFG-E8 at the beginning of reperfusion significantly attenuated I/R-induced liver injury by 29.5%, 30.7%, and 53.3%, respectively (P = 0.0012, 0.0072, and 0.0006, respectively, Figs. 2A–C). These data correlated with the histological alterations in the liver after I/R. Hepatic I/R injury induced hepatocyte necrosis and sinusoidal congestion (Fig. 3B), compared with sham-operated rats which show the normal liver structures were observed (Fig. 3A). In contrast, the architecture of the liver of animals treated with rhMFG-E8 was comparatively preserved and the area of hepatocyte necrosis was relatively small compared with the vehicle-treated liver (Fig. 3C). Additionally, rats subjected to hepatic I/R had a significant increase in water content of the liver at 4 h after reperfusion as compared with sham-operated rats (P = 0.0048, Fig. 3D). When hepatic I/R rats were treated with rhMFG-E8, liver water content was significantly reduced compared with vehicle-treated rats (P = 0.0084, Fig. 3D).
FIGURE 2.
Alterations in plasma liver injury variables (AST (A), ALT (B), LDH (C)) in sham-operated (Sham), hepatic ischemia-reperfusion (I/R) rats treated with normal saline (Vehicle) or recombinant human MFG-E8 (rhMFG-E8) at 4 h after reperfusion. Data are expressed as means ± SE (n=9–10/group) and compared by one-way ANOVA and Tukey-Kramer’s test; *P < 0.05 versus Sham group; #P < 0.05 versus Vehicle group.
FIGURE 3.
Representative liver histology in sham-operated (Sham) (A), hepatic ischemia-reperfusion (I/R) rats treated with normal saline (Vehicle) (B) or recombinant human MFG-E8 (rhMFG-E8) (C) at 4 h after reperfusion (Hematoxylin-eosin staining; Original magnification × 200). Alterations in liver water content in Sham, hepatic I/R animals treated with Vehicle or rhMFG-E8 at 4 h after reperfusion (D). Data are expressed as means ± SE (n=9–10/group) and compared by one-way ANOVA and Tukey-Kramer’s test; *P < 0.05 versus Sham group; #P < 0.05 versus Vehicle group.
Treatment with rhMFG-E8 attenuates inflammatory responses after hepatic I/R
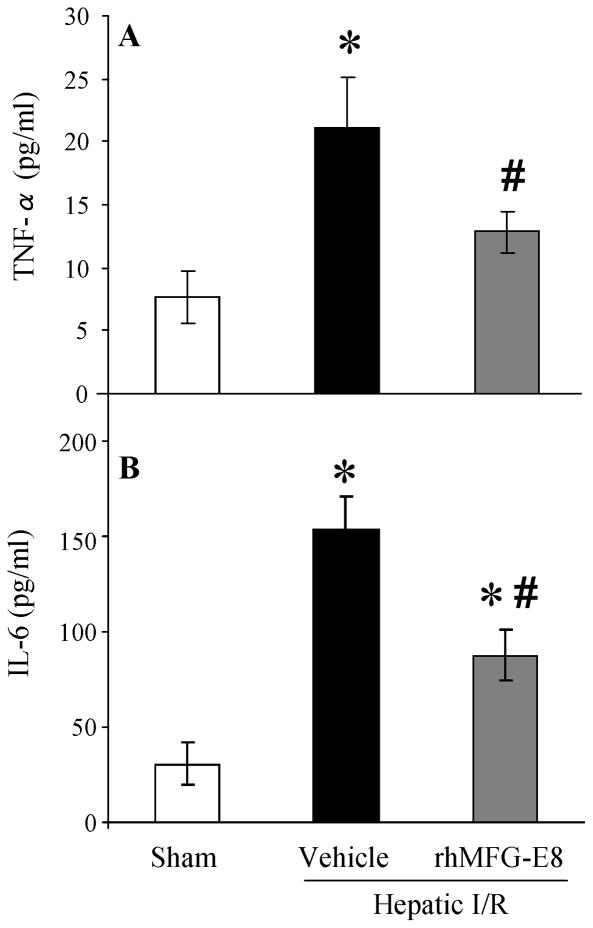
At 4 h after reperfusion, plasma levels of TNF-α was increased by 2.75-fold in vehicle group as compared with those in sham group (P = 0.0017, Fig. 4A). Treatment with rhMFG-E8 significantly decreased plasma TNF-α levels by 39% (P = 0.0137, Fig. 4A). The alterations in plasma IL-6 levels after hepatic I/R were similar to those of TNF-α. Plasma IL-6 levels were significantly increased by 5.06-fold after hepatic I/R (P < 0.0001, Fig. 4B). rhMFG-E8 treatment reduced IL-6 levels by 43% (P = 0.0033, Fig. 4B).
FIGURE 4.
Alterations in plasma cytokine levels (TNF-α (A), IL-6 (B)) in sham-operated (Sham), hepatic ischemia-reperfusion (I/R) rats treated with normal saline (Vehicle) or recombinant human MFG-E8 (rhMFG-E8) at 4 h after reperfusion. Data are expressed as means ± SE (n=7–9/group) and compared by one-way ANOVA and Tukey-Kramer’s test; *P < 0.05 versus Sham group; #P < 0.05 versus Vehicle group.
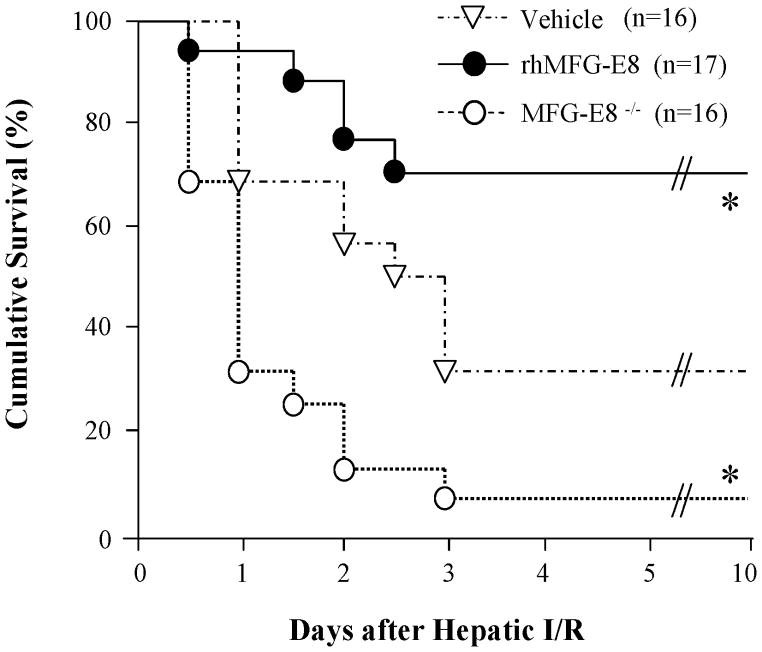
MFG-E8 has a critical role in survival after hepatic I/R
To address whether MFG-E8 has a beneficial effect in hepatic I/R, we conducted a survival study using C57BL/6J MFG-E8−/− and WT mice. It is reported that the model of partial hepatic ischemia, which is used in our study, is nonlethal and could not be used for survival analysis. Therefore, we used total hepatic I/R model as previously described [22]. Indeed, MFG-E8−/− mice were more susceptible to total hepatic I/R-mediated mortality than WT mice (Vehicle) (survival rate; 6.3% and 31.3%, respectively; P = 0.0054, Fig. 5). Thus, this finding demonstrates that lack of MFG-E8 sensitizes the liver to I/R. Furthermore, exogenous administration of rhMFG-E8 in WT mice (rhMFG-E8) improved the survival rate to 70.6% (P = 0.0384 vs. Vehicle group, Fig. 5). Our result of survival rate of WT mice is in line with previous report by Llacuna, et al [22].
FIGURE 5.
Alterations in the survival rate after hepatic ischemia-reperfusion (I/R) in MFG-E8−/− mice and wild-type mice treated with normal saline (Vehicle) or recombinant human MFG-E8 (rhMFG-E8). Each group of mice received normal saline or a single dose (160 μg/kg) rhMFG-E8 intraperitoneally at the beginning of reperfusion and observed for 10 days. The survival rate was estimated by the Kaplan-Meier method and compared by using the log-rank test (n=16–17/group); *P < 0.05 versus Vehicle group.
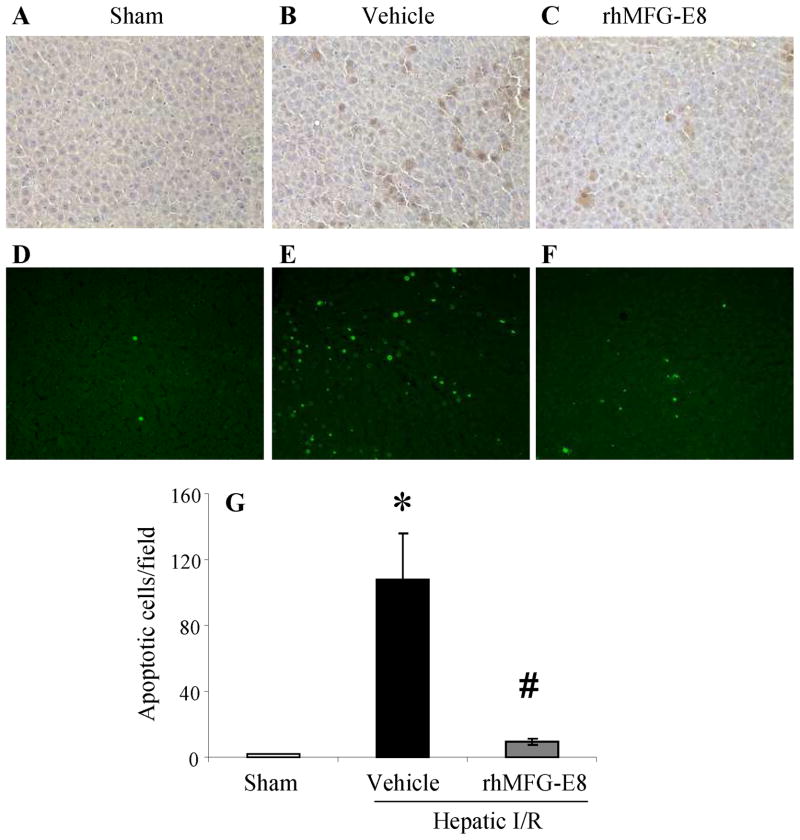
Treatment with rhMFG-E8 decreases apoptosis after hepatic I/R
To investigate the beneficial effects that rhMFG-E8 decreases apoptotic cell death, immunohistochemistry of cleaved caspase-3 was used as a measure of apoptosis in the liver. As shown in Figs. 6A–C, minimal immunostaining of cleaved caspase-3 was detected in the liver of sham-operated animals. In the vehicle-treated animals, the expression of cleaved caspase-3 in the hepatocytes was markedly increased at 4 h after hepatic I/R and rhMFG-E8 treatment decreased the immunostaining. Additionally, apoptosis was detected in situ using the TUNEL assay. As shown in Figs. 6D–G, TUNEL-positive cells were markedly increased in the liver of vehicle-treated animals compared with sham-operated animals (P = 0.0003, Fig. 6G). Treatment with rhMFG-E8, however, significantly decreased the number of apoptotic cells in the liver (P = 0.0006, Fig. 6G).
FIGURE 6.
Representative immunohistochemical staining for cleaved caspase-3 and TUNEL assay (green fluorescent) of liver sections from sham-operated (Sham) (A, D), hepatic ischemia-reperfusion (I/R) rats treated with normal saline (Vehicle) (B, E) or recombinant human MFG-E8 (rhMFG-E8) (C, F) at 4 h after reperfusion. Original magnification × 200. Alterations in the number of apoptotic cells by TUNEL assay in Sham, hepatic I/R rats treated with Vehicle or rhMFG-E8 at 4 h after reperfusion (G). Data are expressed as means ± SE (n=6/group) and compared by one-way ANOVA and Tukey-Kramer’s test; *P < 0.05 versus Sham group; #P < 0.05 versus Vehicle group.
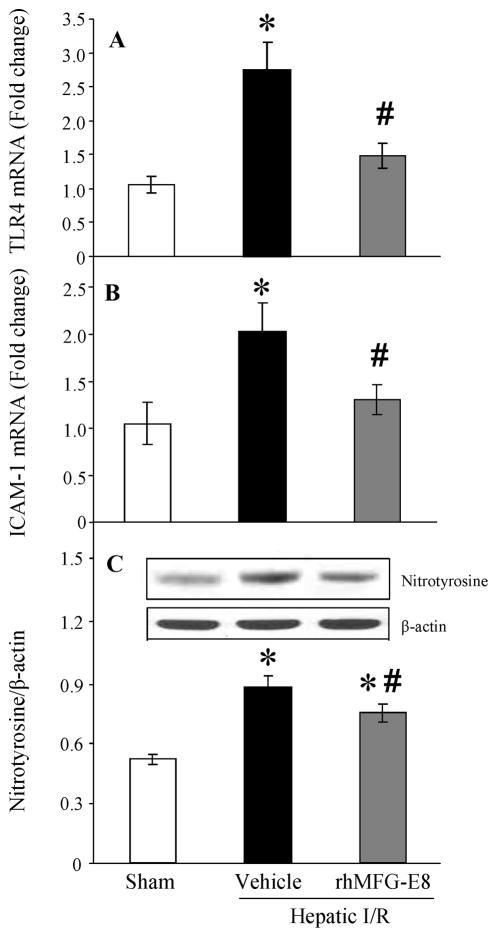
Treatment with rhMFG-E8 attenuates TLR4, ICAM-1 expressions and oxidative stress after hepatic I/R
The innate immune responses via TLR4 signaling pathway and endothelial activation after hepatic I/R are already known as major contributors in liver injury after I/R [1, 23]. Both TLR4 and intercellular adhesion molecule (ICAM)-1 mRNA expressions were significantly increased after I/R (P = 0.0003 and 0.0033, respectively, Figs. 7A, B). When animals were treated with rhMFG-E8, these mRNA levels were significantly decreased (P = 0.0029 and 0.0093, respectively) and there were no statistical differences between sham and rhMFG-E8-treated animals (Figs. 7A, B). Excessive nitric oxide (NO) produced in the liver reacts with superoxide and forms the potent oxidant peroxynitrite (ONOO−), which can be a major cause of hepatocellular injury. Nitrotyrosine is recognized as a marker of peroxynitrite formation [24]. Although hepatic I/R induced higher levels of nitrotyrosine in the liver (P < 0.0001, Fig. 7C), treatment with rhMFG-E8 showed a significant reduction of nitrotyrosine levels compared with that in vehicle-treated animals (P = 0.0131, Fig. 7C).
FIGURE 7.
Alterations in liver TLR4 (A), ICAM-1 (B) mRNA expressions, and nitrotyrosine (C) protein expression in sham-operated (Sham), hepatic ischemia-reperfusion (I/R) rats treated with normal saline (Vehicle) or recombinant human MFG-E8 (rhMFG-E8) at 4 h after reperfusion. Data are expressed as means ± SE (n=7–9/group) and compared by one-way ANOVA and Tukey-Kramer’s test; *P < 0.05 versus Sham group; #P < 0.05 versus Vehicle group.
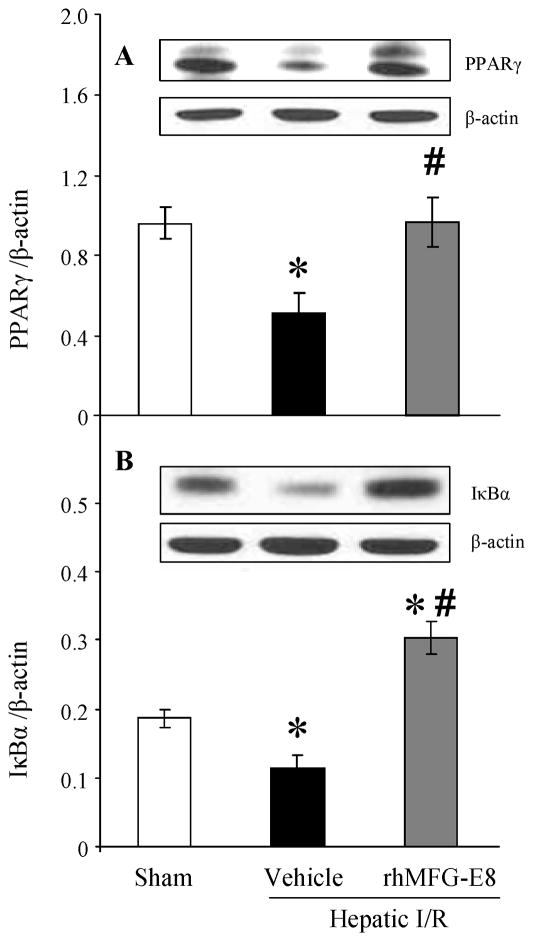
Treatment with rhMFG-E8 modulates PPARγ/NF-κB pathway after hepatic I/R
Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear receptor superfamily of ligand-dependent transcription factors and three different PPARs (α, δ, γ) have been identified. PPARγ affects various cellular processes at the level of gene expression regulation [25, 26]. PPARγ activation has been shown to have anti-inflammatory effects by repressing the expression of inflammatory response genes in activated macrophages; therefore, it is a key regulator of macrophage-mediated immune responses [27, 28]. At 4 h after reperfusion, PPARγ protein levels in the liver were significantly decreased in the vehicle group as compared with those of sham group (P = 0.0180, Fig. 8A). Treatment with rhMFG-E8 significantly increased PPARγ levels compared with those of vehicle group (P = 0.0087, Fig. 8A). Consequently, IκBα protein expression was significantly decreased in the liver of vehicle-treated animals at 4 h after reperfusion compared with sham-operated animals (P = 0.0025, Fig. 8B), however, treatment with rhMFG-E8 significantly increased IκBα expression after hepatic I/R (P < 0.0001, Fig. 8B).
FIGURE 8.
Alterations in liver PPARγ (A), and IκBα (B) expression in sham-operated (Sham), hepatic ischemia-reperfusion (I/R) rats treated with normal saline (Vehicle) or recombinant human MFG-E8 (rhMFG-E8) at 4 h after reperfusion. Data are expressed as means ± SE (n=5–8/group) and compared by one-way ANOVA and Tukey-Kramer’s test; *P < 0.05 versus Sham group; #P < 0.05 versus Vehicle group.
DISCUSSION
Using a well-established rat model of liver ischemia-reperfusion injury, we found that rhMFG-E8-mediated protective mechanisms included (i) amelioration of the hepatocellular damage; (ii) suppression of pro-inflammatory responses and oxidative stress; (iii) reduction of apoptotic cell death; and (iv) modulation of PPARγ/NF-κB pathway. Furthermore, we have shown that in the survival study using MFG-E8−/− mice, disruption of MFG-E8 expression renders animals more susceptible to hepatic I/R, providing further evidence for the crucial role of MFG-E8 in hepatic I/R. To our knowledge, this is the first study to demonstrate the beneficial effects of MFG-E8 against I/R injury in the liver.
Since its initial description as a milk fat protein, the functional roles of MFG-E8–associated tissue remodeling have been investigated extensively, they include promoting removal of apoptotic lymphocytes by macrophages [9, 10], clearance of mammary epithelial cells in involution [29], mediating sperm-egg bindings [30], maintenance of intestinal epithelium [18, 31], and facilitating VEGF-dependent neovascularization [32]. Among these, much attention on subsequent research has been paid on its role for the clearance of apoptotic cells. We have previously shown that CLP induced-sepsis, and intestinal and renal I/R, suppressed MFG-E8 production in vital organs. This suppression of MFG-E8 production contributes to the impairment of phagocytic activity against apoptotic cells, which leads to the accumulation of apoptotic cells and subsequent exaggerated inflammatory responses induced by secondary necrosis [11–16]. Herein, we demonstrated that liver and plasma levels of MFG-E8 were decreased after hepatic I/R. Although we have shown that sepsis-induced down-regulation of MFG-E8 production is mainly lipopolysaccharide (LPS)-CD14 pathway dependent [33], the underlying mechanism of MFG-E8 reduction in I/R is unknown. During hepatic I/R, portal vein occlusion results in congestion of the intestinal wall, leading to the release of gut-derived molecules, including LPS, into the portal vein and following systemic circulation. Therefore, we speculate that gut-derived LPS could be a possible candidate for the decreased liver and systemic MFG-E8 production in this hepatic I/R model.
One of the remarkable findings of the current study is that hepatic I/R induces significant accumulation of apoptotic cells in the liver and that rhMFG-E8 treatment dramatically attenuated the number of the apoptotic cells. It has been shown that apoptosis of liver cells, which is not only hepatocytes but sinusoidal epithelial cells, is a prominent feature of liver injury in the setting of I/R. The severity of apoptosis correlates with the degree of hepatic injury and the inhibition of certain elements of the apoptotic pathway seemed to ameliorate the injury and enhance the survival after hepatic I/R [1, 34]. Based on previous reports [9–11, 13, 35], our data strongly suggest that the reduction of MFG-E8 production in this model is associated with impaired phagocytosis of apoptotic hepatocytes, and that rhMFG-E8-mediated suppression of apoptotic cells is partially due to its restoration. On the other hand, the direct anti-apoptotic effect of MFG-E8 is under investigation. We have previously demonstrated that addition of MFG-E8-containing exosomes did not inhibit apoptosis of apoptotic thymocytes induced by dexamethasone treatment [15]. However, Jinushi, et al [36]. have shown that MFG-E8 increases the resistance of B16 melanoma cells to apoptosis which is induced by etoposide and Fas ligand treatment via αVβ3 integrin signaling pathway. Therefore, further studies are warranted to examine the direct anti-apoptotic effect of MFG-E8 in specific disease condition. It is also well known that TNF-α, one of the central mediators in liver response to I/R, initiate the apoptotic cascade through the death receptor/caspases pathway [37, 38]. Consistent with present results, we have reported that MFG-E8 can suppress TNF-α production in vivo and in vitro from immune cells not only through the indirect pathway resulted from the enhancement of phagocytosis of apoptotic cells, but also via direct mechanisms [11, 13, 15, 16]. Hence, the suppressive effect of TNF-α production is one of the plausible mechanisms for MFG-E8-mediated attenuation of apoptotic cell accumulations. In the preliminary study, we also assessed necrotic and apoptotic hepatocellular damage in the liver after 24 hours after I/R. It was found that much necrotic damage and less apoptotic cells are shown 24 hour after I/R than 4 hours. This result implied us that the process of apoptotic cell death occurs in relatively early time course and apoptotic cells have already shifted to secondary necrosis in 24 hours after I/R. Therefore, we chose 4 hour time point for the current experiments to show the clear picture which exogenous MFG-E8 treatment attenuates hepatic I/R injury through the reduction of apoptotic cells.
The pathophysiology of hepatic I/R is complex [1, 23]. During the initial stages of reperfusion, Kupffer cells are activated, producing a large amount of pro-inflammatory cytokines such as TNF-α and IL-6 via various signaling pathways including TLR4/NF-κB and mitogen-activated protein kinases (MAPK) [39, 40]. These cytokines have a direct cytotoxic effect on hepatocytes and endothelial cells, but they also induce the expression of ICAM on endothelial cells. Thereafter, activated neutrophils accumulate in the liver and adhere to endothelial cells through the interaction between integrins and ICAM-1 [1, 41]. They release ROS and diverse proteases, which are responsible for the induced oxidative stress during late stages of reperfusion. Thus, hepatic I/R involves various contributory hallmarks, making it difficult to attain effective protection by targeting only an individual mechanism. In this regard, our results suggest that MFG-E8 can be a potential supportive drug for hepatic I/R due to multiple protective functions against liver injury including inhibition of pro-inflammatory cytokine production, oxidative stress, and endothelial activation.
Several investigators have reported that PPARγ is an important regulator of postischemic liver injury and that activation of PPARγ by synthetic ligands ameliorates liver injury through the anti-inflammatory properties [42–44]. Furthermore, it has been shown that PPARγ inhibits the protein kinases IκB kinase complex (IKK), which negatively regulate IκBα by degradation, and suppress NF-κB translocation to the nucleus where it regulates the expression of a variety of genes involved in the inflammatory process. In sepsis and hemorrhagic shock, the anti-inflammatory effect of PPARγ ligands is shown to be mediated via inhibition of IKK/NF-κB pathway [45, 46]. Our results indicated that hepatic I/R induces the down-regulation of PPARγ expression and IκBα degradation and rhMFG-E8 treatment recovered the PPARγ expression and inhibited the IκBα degradation. As a consequence, this rhMFG-E8-mediated inhibition of IκBα degradation may lead to inactivation of NF-κB and subsequent signaling events such as suppression of pro-inflammatory cytokines, ICAM-1 expression, and ROS production. The underlying mechanisms for the MFG-E8-mediated up-regulation of PPARγ expression are unknown. However, recent reports have shown the close interaction between PPARs and MFG-E8. Interestingly, PPARγ and PPARδ play roles as regulators for the phagocytosis of apoptotic cells in macrophages. Genetic deletion of these PPARs decreases the expression of MFG-E8 and induces subsequent elimination of phagocytic activity of macrophages [47, 48]. Taken together, it is suggested that the modulation of PPARγ/NF-κB pathway is one of the plausible mechanisms of the anti-inflammatory property of MFG-E8 and MFG-E8-mediated activation of PPARγ, in collaboration with MFG-E8, may facilitate apoptotic cell engulfment in our model.
In conclusion, the results of this study clearly demonstrate the protective effect of the administration of rhMFG-E8, as a compensation for the deficit of endogenous MFG-E8 production, after hepatic I/R. Although the most effective dose, timing, and duration of rhMFGF-E8 treatment for clinical use in liver injury remain to be determined, MFG-E8 treatment appears to be a novel and promising therapeutic approach for liver injury after hepatic I/R.
Acknowledgments
This work is supported by the National Institutes of Health (NIH) grants, R01 GM057468 and R01 GM053008 (P.W.). One of the authors (P Wang) is an inventor of the pending PCT application #WO/2006/122327: “Milk fat globule epidermal growth factor-factor VIII and sepsis” and PCT application #WO/2009/064448: “Prevention and treatment of inflammation and organ injury after ischemia/reperfusion using MFG-E8”. These patent applications cover the fundamental concept of using MFG-E8 for the treatment of sepsis and ischemia/reperfusion injury.
Footnotes
Some of the results of this study were previously presented on 34th Annual Conference on Shock, Norfolk, Virginia, June 11 to June 14, 2011.
Other authors report no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Montalvo-Jave EE, Escalante-Tattersfield T, Ortega-Salgado JA, et al. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J Surg Res. 2008;147:153. doi: 10.1016/j.jss.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181:160. doi: 10.1016/s0002-9610(00)00573-0. [DOI] [PubMed] [Google Scholar]
- 3.Borghi-Scoazec G, Scoazec JY, Durand F, et al. Apoptosis after ischemia-reperfusion in human liver allografts. Liver Transpl Surg. 1997;3:407. doi: 10.1002/lt.500030408. [DOI] [PubMed] [Google Scholar]
- 4.Contreras JL, Vilatoba M, Eckstein C, et al. Caspase-8 and caspase-3 small interfering RNA decreases ischemia/reperfusion injury to the liver in mice. Surgery. 2004;136:390. doi: 10.1016/j.surg.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell CW, Jiang W, Reich CF, 3rd, et al. The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol. 2006;291:C1318. doi: 10.1152/ajpcell.00616.2005. [DOI] [PubMed] [Google Scholar]
- 7.Silva MT, do Vale A, dos Santos NM. Secondary necrosis in multicellular animals: an outcome of apoptosis with pathogenic implications. Apoptosis. 2008;13:463. doi: 10.1007/s10495-008-0187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Y, Tibrewal N, Birge RB. Phosphatidylserine recognition by phagocytes: a view to a kill. Trends Cell Biol. 2006;16:189. doi: 10.1016/j.tcb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Hanayama R, Tanaka M, Miwa K, et al. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 10.Hanayama R, Tanaka M, Miyasaka K, et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 11.Aziz M, Jacob A, Matsuda A, et al. Review: milk fat globule-EGF factor 8 expression, function and plausible signal transduction in resolving inflammation. Apoptosis. 2011;16:1077. doi: 10.1007/s10495-011-0630-0. [DOI] [PubMed] [Google Scholar]
- 12.Cui T, Miksa M, Wu R, et al. Milk fat globule epidermal growth factor 8 attenuates acute lung injury in mice after intestinal ischemia and reperfusion. Am J Respir Crit Care Med. 2010;181:238. doi: 10.1164/rccm.200804-625OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuda A, Jacob A, Wu R, et al. Milk fat globule-EGF factor VIII in sepsis and ischemia-reperfusion injury. Mol Med. 2011;17:126. doi: 10.2119/molmed.2010.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuda A, Wu R, Jacob A, et al. Protective effect of milk fat globule-epidermal growth factor-factor VIII after renal ischemia-reperfusion injury in mice. Crit Care Med. 2011;39:2039. doi: 10.1097/CCM.0b013e3182227a3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miksa M, Wu R, Dong W, et al. Dendritic cell-derived exosomes containing milk fat globule epidermal growth factor-factor VIII attenuate proinflammatory responses in sepsis. Shock. 2006;25:586. doi: 10.1097/01.shk.0000209533.22941.d0. [DOI] [PubMed] [Google Scholar]
- 16.Miksa M, Wu R, Dong W, et al. Immature dendritic cell-derived exosomes rescue septic animals via milk fat globule epidermal growth factor-factor VIII [corrected] J Immunol. 2009;183:5983. doi: 10.4049/jimmunol.0802994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atabai K, Jame S, Azhar N, et al. Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages. J Clin Invest. 2009;119:3713. doi: 10.1172/JCI40053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bu HF, Zuo XL, Wang X, et al. Milk fat globule-EGF factor 8/lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J Clin Invest. 2007;117:3673. doi: 10.1172/JCI31841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raymond A, Ensslin MA, Shur BD. SED1/MFG-E8: a bi-motif protein that orchestrates diverse cellular interactions. J Cell Biochem. 2009;106:957. doi: 10.1002/jcb.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J, Wu R, Qiang X, et al. Human adrenomedullin and its binding protein attenuate organ injury and reduce mortality after hepatic ischemia-reperfusion. Ann Surg. 2009;249:310. doi: 10.1097/SLA.0b013e3181961d43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah KG, Wu R, Jacob A, et al. Recombinant human milk fat globule-EGF factor 8 produces dose-dependent benefits in sepsis. Intensive Care Med. 2012;38:128. doi: 10.1007/s00134-011-2353-7. [DOI] [PubMed] [Google Scholar]
- 22.Llacuna L, Mari M, Garcia-Ruiz C, et al. Critical role of acidic sphingomyelinase in murine hepatic ischemia-reperfusion injury. Hepatology. 2006;44:561. doi: 10.1002/hep.21285. [DOI] [PubMed] [Google Scholar]
- 23.Vardanian AJ, Busuttil RW, Kupiec-Weglinski JW. Molecular mediators of liver ischemia and reperfusion injury: a brief review. Mol Med. 2008;14:337. doi: 10.2119/2007-00134.Vardanian. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kliewer SA, Xu HE, Lambert MH, et al. Peroxisome proliferator-activated receptors: from genes to physiology. Recent Prog Horm Res. 2001;56:239. doi: 10.1210/rp.56.1.239. [DOI] [PubMed] [Google Scholar]
- 26.Zingarelli B, Cook JA. Peroxisome proliferator-activated receptor-gamma is a new therapeutic target in sepsis and inflammation. Shock. 2005;23:393. doi: 10.1097/01.shk.0000160521.91363.88. [DOI] [PubMed] [Google Scholar]
- 27.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 28.Ricote M, Li AC, Willson TM, et al. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 29.Hanayama R, Nagata S. Impaired involution of mammary glands in the absence of milk fat globule EGF factor 8. Proc Natl Acad Sci U S A. 2005;102:16886. doi: 10.1073/pnas.0508599102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ensslin MA, Shur BD. Identification of mouse sperm SED1, a bimotif EGF repeat and discoidin-domain protein involved in sperm-egg binding. Cell. 2003;114:405. doi: 10.1016/s0092-8674(03)00643-3. [DOI] [PubMed] [Google Scholar]
- 31.Aziz MM, Ishihara S, Mishima Y, et al. MFG-E8 attenuates intestinal inflammation in murine experimental colitis by modulating osteopontin-dependent alphavbeta3 integrin signaling. J Immunol. 2009;182:7222. doi: 10.4049/jimmunol.0803711. [DOI] [PubMed] [Google Scholar]
- 32.Silvestre JS, Thery C, Hamard G, et al. Lactadherin promotes VEGF-dependent neovascularization. Nat Med. 2005;11:499. doi: 10.1038/nm1233. [DOI] [PubMed] [Google Scholar]
- 33.Komura H, Miksa M, Wu R, et al. Milk fat globule epidermal growth factor-factor VIII is down-regulated in sepsis via the lipopolysaccharide-CD14 pathway. J Immunol. 2009;182:581. doi: 10.4049/jimmunol.182.1.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudiger HA, Graf R, Clavien PA. Liver ischemia: apoptosis as a central mechanism of injury. J Invest Surg. 2003;16:149. [PubMed] [Google Scholar]
- 35.Thery C, Regnault A, Garin J, et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jinushi M, Nakazaki Y, Carrasco DR, et al. Milk fat globule EGF-8 promotes melanoma progression through coordinated Akt and twist signaling in the tumor microenvironment. Cancer Res. 2008;68:8889. doi: 10.1158/0008-5472.CAN-08-2147. [DOI] [PubMed] [Google Scholar]
- 37.Lentsch AB, Kato A, Yoshidome H, et al. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169. doi: 10.1053/jhep.2000.9323. [DOI] [PubMed] [Google Scholar]
- 38.Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King LA, Toledo AH, Rivera-Chavez FA, et al. Role of p38 and JNK in liver ischemia and reperfusion. J Hepatobiliary Pancreat Surg. 2009;16:763. doi: 10.1007/s00534-009-0155-x. [DOI] [PubMed] [Google Scholar]
- 40.Tsung A, Hoffman RA, Izuishi K, et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol. 2005;175:7661. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 41.Farhood A, McGuire GM, Manning AM, et al. Intercellular adhesion molecule 1 (ICAM-1) expression and its role in neutrophil-induced ischemia-reperfusion injury in rat liver. J Leukoc Biol. 1995;57:368. [PubMed] [Google Scholar]
- 42.Akahori T, Sho M, Hamada K, et al. Importance of peroxisome proliferator-activated receptor-gamma in hepatic ischemia/reperfusion injury in mice. J Hepatol. 2007;47:784. doi: 10.1016/j.jhep.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 43.Kuboki S, Shin T, Huber N, et al. Peroxisome proliferator-activated receptor-gamma protects against hepatic ischemia/reperfusion injury in mice. Hepatology. 2008;47:215. doi: 10.1002/hep.21963. [DOI] [PubMed] [Google Scholar]
- 44.Shin T, Kuboki S, Huber N, et al. Activation of peroxisome proliferator-activated receptor-gamma during hepatic ischemia is age-dependent. J Surg Res. 2008;147:200. doi: 10.1016/j.jss.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chima RS, Hake PW, Piraino G, et al. Ciglitazone ameliorates lung inflammation by modulating the inhibitor kappaB protein kinase/nuclear factor-kappaB pathway after hemorrhagic shock. Crit Care Med. 2008;36:2849. doi: 10.1097/ccm.0b013e318187810e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zingarelli B, Sheehan M, Hake PW, et al. Peroxisome proliferator activator receptor-gamma ligands, 15-deoxy-Delta(12,14)-prostaglandin J2 and ciglitazone, reduce systemic inflammation in polymicrobial sepsis by modulation of signal transduction pathways. J Immunol. 2003;171:6827. doi: 10.4049/jimmunol.171.12.6827. [DOI] [PubMed] [Google Scholar]
- 47.Mukundan L, Odegaard JI, Morel CR, et al. PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat Med. 2009;15:1266. doi: 10.1038/nm.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roszer T, Menendez-Gutierrez MP, Lefterova MI, et al. Autoimmune kidney disease and impaired engulfment of apoptotic cells in mice with macrophage peroxisome proliferator-activated receptor gamma or retinoid X receptor alpha deficiency. J Immunol. 2011;186:621. doi: 10.4049/jimmunol.1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]