Abstract
Griffithsin (GRFT), Cyanovirin-N (CV-N) and Scytovirin (SVN) are lectins that inhibit HIV-1 infection by binding to multiple mannose-rich glycans on the HIV-1 envelope glycoproteins (Env). Here we show that these lectins neutralize subtype C primary virus isolates in addition to Env-pseudotyped viruses obtained from plasma and cervical vaginal lavages. Among 15 subtype C pseudoviruses, the median IC50 values were 0.4, 1.8 and 20.1 nM for GRFT, CV-N and SVN, respectively, similar to what was found for subtype B and A. Analysis of Env sequences suggested that concomitant lack of glycans at positions 234 and 295 resulted in natural resistance to these compounds, which was confirmed by site-directed mutagenesis. Furthermore, the binding sites for these lectins overlapped that of the 2G12 monoclonal antibody epitope, which is generally absent on subtype C Env. This data support further research on these lectins as potential microbicides in the context of HIV-1 subtype C infection.
Keywords: Griffithsin, Cyanovirin-N, Scytovirin, lectins, HIV-1 subtype C, microbicides, 2G12 monoclonal antibody
INTRODUCTION
Griffithsin (GRFT) is a lectin isolated from the red algae Griffithsia sp. found in the coastal waters off New Zealand (Mori et al., 2005). It is a 121 amino acid dimeric protein with a domain swapped structure (Ziolkowska et al., 2006). This lectin neutralizes HIV-1 by binding to mannose-rich glycans found on the envelope glycoproteins. Both native and recombinant GRFT display potent antiviral activities against laboratory adapted strains and primary isolates of M- and T- tropic HIV-1. This compound was also shown to be active against a broad range of HIV-1 including 4 subtype C viruses (O’Keefe et al., 2009). A second lectin, Cyanovirin-N (CV-N) isolated much earlier from the blue green algae Nostoc ellipsosporum (Boyd et al., 1997) is a 101 amino acid protein that has been shown to exist either as a quasi-symmetric two-domain monomer or as a domain-swapped dimer (Barrientos et al., 2002; Botos and Wlodawer, 2005). Like GRFT, CV-N binds to mannose-rich glycans and both native and recombinant CV-N have shown potent anti-HIV-1 activity in vitro (Bolmstedt et al., 2001; Esser et al., 1999). A third protein, Scytovirin (SVN) is a 95 amino acid lectin isolated from the cyanobacterium Scytonema varium (Bokesch et al., 2003). SVN is expressed as a single amino acid chain with extensive internal sequence duplication. This protein is found exclusively as a monomer and has been shown to neutralize both laboratory adapted strains and primary isolates of HIV-1 by interacting with mannose-rich glycans on the viral envelope (Moulaei et al., 2007; Xiong et al., 2006; Ziolkowska and Wlodawer, 2006).
As a result of their ability to block HIV-1 entry in vitro, GRFT, CV-N and SVN have been proposed as potential microbicides to prevent the sexual transmission of HIV-1. Although these compounds are not HIV-specific, they target high-mannose arrays that are present on all HIV envelope glycoproteins. Since such arrays are uncommon in mammalian cells, these compounds are not likely to be toxic to human cells in vivo even at relatively high concentrations (Balzarini, 2005; Balzarini and Van Damme, 2007). Furthermore, a recombinant GRFT produced in the tobacco-like plant Nicotiana benthamiana was shown to be non-toxic in a rabbit vaginal irritancy model and in human cervical explants (O’Keefe et al., 2009). Although GRFT, CV-N and SVN have not yet been tested in human clinical trials it is noteworthy that CV-N was shown to be effective in protecting pigtailed macaques after vaginal and rectal challenges with high dose of SHIV 86.9P (Tsai et al., 2004; Tsai et al., 2003).
HIV-1 can develop resistance to CV-N by partial deglycosylation, specifically deleting glycans at positions 230, 289, 295, 332, 339, 386, 392 and 448 in HIV-IIIB (Balzarini et al., 2006; Hu, Mahmood, and Shattock, 2007). This suggests that resistance to CV-N may be hard to generate as it will require multiple mutations in the viral genome although additional studies using primary viruses are needed. Some of these glycans also form part of the epitope for the broadly neutralizing monoclonal antibody (mAb) 2G12. This antibody directly binds glycans at positions 332, 339 and 392 (Calarese et al., 2003), while glycans at positions 295, 386 and 448 though not directly involved in this epitope, may influence its conformation (Sanders et al., 2002; Scanlan et al., 2002). Subtype C viruses are commonly resistant to 2G12 neutralization. This has been attributed to their frequent lack of the 295 glycosylation site although re-introduction of this glycan only partially restores sensitivity to this mAb (Binley et al., 2004; Chen et al., 2005; Gray et al., 2007b). Although it is known that GRFT and SVN bind to mannose-rich glycans found on HIV-1 envelope, the specific glycans involved in this binding have not yet been identified.
Despite subtype C viruses being responsible for over 50% of global infections (http://www.unaids.org) many of the antiviral studies with GRFT, CV-N and SVN have been done on subtype B viruses. Significant differences exist in the pattern of glycosylation between subtype B and C envelope glycoproteins (Zhang et al., 2004). For example, about 80% of subtype B envelopes are glycosylated at position 295 while only ~20% of subtype C viruses are glycosylated at the same position. The opposite pattern is observed at position 230 where over 70% of subtype C envelopes are glycosylated compared to only 20% of subtype B. It is known that differences between subtypes can affect their susceptibility to neutralizing agents as for the 2G12 mAb described above (Binley et al., 2004; Gray et al., 2006). Therefore, we aimed to examine the sensitivity of a large collection of subtype C viruses to GRFT, CV-N and SVN and compare these to subtypes B and A strains. Furthermore, we examined the role of the 2G12 epitope in these lectin’s binding sites.
RESULTS
GRFT, CV-N and SVN neutralize HIV-1 subtype C isolates from blood and cervico-vaginal lavages
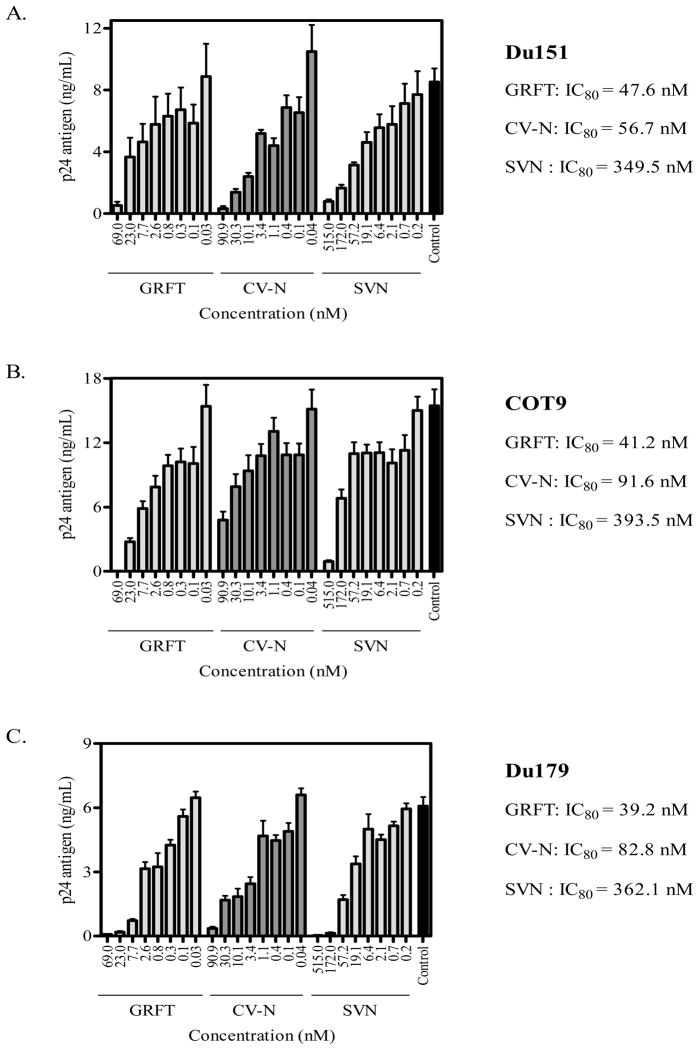
To determine the ability of GRFT, CV-N and SVN to inhibit HIV-1 subtype C infection of peripheral blood mononuclear cells (PBMC), we performed neutralization assays using blood-derived subtype C primary isolates Du151, COT9 and Du179. Viral infection was measured by p24 ELISA after 4 days of culture. The concentration of lectin that inhibited 80% of virus infection (IC80) is reported. All three lectins neutralized these viruses in adose-dependent manner (Figure 1). The IC80 values were similar for all viruses with GRFT and CV-N being more potent than SVN. The mean IC80 values for all 3 viruses were 42.7 ± 4.4, 77.0 ± 18.2 and 368.4 ± 22.7 nM for GRFT, CV-N and SVN, respectively.
Figure 1. GRFT, CV-N, and SVN inhibit HIV-1 subtype C infection of PBMC.
Three primary isolates, Du151 (A), COT9 (B) and Du179 (C) were treated with increasing concentrations of GRFT, CV-N and SVN before infection of PBMC. Data are shown as the average plus standard deviations of three independent experiments. Untreated virus is shown in black (positive control). The IC80 values of the lectins areindicated next to each graph.
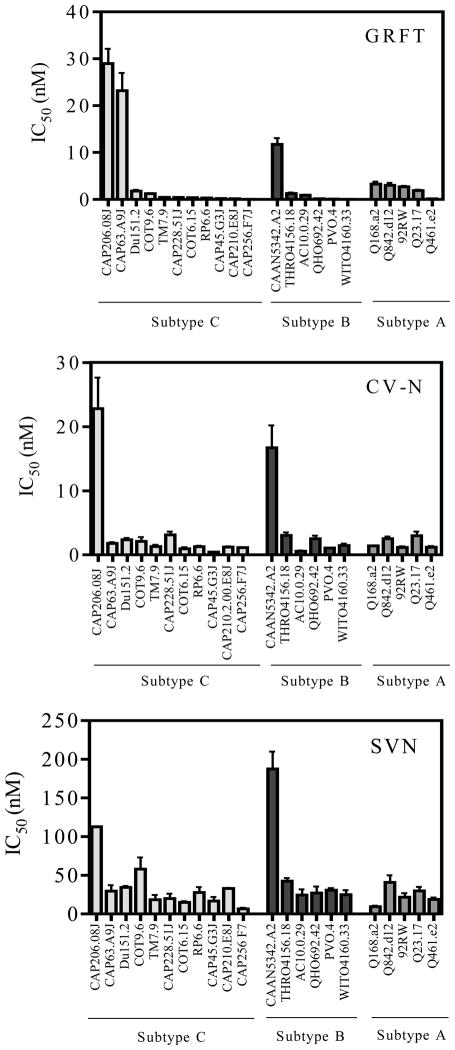
Next we tested the ability of GRFT, CV-N and SVN to inhibit 50% (IC50) HIV-1infection in the TZM-bl cell-line based neutralization assay (Montefiori, 2004). For this we used 11 subtype C Env-pseudotyped viruses cloned from plasma of adult patients with acute or early HIV infection and from pediatric patients during chronic infection (see Methods for details). For comparison we included 6 viruses from the subtype B panel described by Li et al. (Li et al., 2005) and 5 subtype A viruses from acutely infected individuals (Long et al., 2002; Neilson et al., 1999). All viruses used the CCR5 coreceptor. GRFT and CV-N potently neutralized most subtype C viruses with a median IC50 of 0.4 and 1.2 nM, respectively, (Figure 2) which were not significantly different. SVN was the least effective with a median IC50 of 20.1 nM. The single-cycle pseudovirus assay required considerably lower concentrations of lectin to inhibit viral infection compared to the PBMC assay even when comparing IC80 values (not shown). Nevertheless, the trends were the same in both assays as was noted for Du151.2 and COT9.6 pseudoviruses compared to their matched primary viruses in PBMC (Table 1 and Figure 1). There were no significant differences in mean IC50 values between subtypes C, B and A viruses for all 3 lectins (p>0.05) (Table 1). However, 2 subtype C viruses (CAP206.08J and CAP63.A9J) and one subtype B virus (CAAN5342.A2) showed unusually high IC50 values suggesting natural resistance to these compounds (Figure 2). We also found a significant positive correlation (p<0.05) between GRFT and CV-N sensitivity for subtype C, and between CV-N and SVN for both subtype C and B Env-pseudotyped viruses (data not shown).
Figure 2. GRFT, CV-N, and SVN inhibit HIV-1 infection in the TZM-bl assay.
Neutralization of HIV-1 subtype C, B and A pseudoviruses by GRFT (A), CV-N (B), and SVN (C). HIV-1 subtype B and A pseudoviruses were used for comparison to subtype C. Each virus was tested at least three times. Pseudoviruses are ranked by GRFT sensitivity.
Table 1.
Pattern of predicted mannose-rich glycans on gp120 of HIV-1 and sensitivity to GRFT, CV-N, and SVN
| HIV-1 envelope pseudoviruses | Predicted N-linked mannose-rich glycosylation sitesa | IC50 (nM)b | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 230 | 234 | 241 | 262 | 289 | 295* | 332* | 339* | 386* | 392* | 448* | GRFT | CV-N | SVN | |
| Subtype C (n = 15) | ||||||||||||||
|
| ||||||||||||||
| CAP177.cvl96 | x | x | x | x | x | 55.9±5.0 | 7.5±1.1 | 21.8±5.4 | ||||||
|
| ||||||||||||||
| CAP206.08J | x | x | x | x | x | 30.9±6.2 | 18.0±1.2 | 112.8±1.1 | ||||||
|
| ||||||||||||||
| CAP63.A9J | x | x | 23.2±6.5 | 1.8±0.3 | 29.8±13.3 | |||||||||
|
| ||||||||||||||
| CAP63.cvl6GA | x | x | 25.7±2.6 | 3.1±0.1 | 43.8±8.5 | |||||||||
|
| ||||||||||||||
| CAP261.cvl93 | x | x | 4.2±0.4 | 1.8±0.6 | 6.2±0.9 | |||||||||
|
| ||||||||||||||
| Du151.2 | x | x | x | 1.5±0.8 | 2.0±1.0 | 27.5±9.6 | ||||||||
|
| ||||||||||||||
| COT9.6 | x | 1.2±0.2 | 3.9±2.7 | 57.6±26.9 | ||||||||||
|
| ||||||||||||||
| COT6.15 | x | x | 0.4±0.2 | 0.9±0.5 | 10.8±6.9 | |||||||||
|
| ||||||||||||||
| CAP228.51J | x | x | 0.4±0.1 | 3.1±0.9 | 20.1±11.1 | |||||||||
|
| ||||||||||||||
| CAP270.cvl44 | 0.3±0.1 | 0.8±0.07 | 11.7±4.8 | |||||||||||
|
| ||||||||||||||
| RP6.6 | x | x | 0.2±0.1 | 1.1±0.1 | 11.3±3.3 | |||||||||
|
| ||||||||||||||
| TM7.9 | x | x | 0.2±0.1 | 1.1±0.4 | 12.1±1.6 | |||||||||
|
| ||||||||||||||
| CAP45.G3J | x | x | x | x | 0.2±0.1 | 0.4±0.1 | 16.9±8.8 | |||||||
|
| ||||||||||||||
| CAP210.E8J | x | 0.2±0.0 | 1.2±0.2 | 33.1±1.1 | ||||||||||
|
| ||||||||||||||
| CAP256.F7J | x | x | 0.1±0.0 | 1.1±0.1 | 6.7±2.3 | |||||||||
|
| ||||||||||||||
| Median | 0.4 | 1.8 | 20.1 | |||||||||||
|
| ||||||||||||||
| Subtype B (n = 6) | ||||||||||||||
|
| ||||||||||||||
| CAAN5342.A2 | x | x | x | 11.7±2.3 | 16.7±6.0 | 187.6±38.6 | ||||||||
|
| ||||||||||||||
| THRO4156.18 | x | x | 1.2±0.3 | 3.0±0.8 | 42.6±6.9 | |||||||||
|
| ||||||||||||||
| AC10.0.29 | x | x | x | 0.9±0.2 | 0.3±0.1 | 24.5±12.9 | ||||||||
|
| ||||||||||||||
| QHO692.42 | x | x | 0.2±0.1 | 2.5±0.8 | 27.3±14.1 | |||||||||
|
| ||||||||||||||
| PVO.4 | x | x | 0.1±0.0 | 1.0±0.1 | 25.2±14.0 | |||||||||
|
| ||||||||||||||
| WITO4160.33 | x | x | 0.05±0.0 | 1.5±0.5 | 24.8±10.1 | |||||||||
|
| ||||||||||||||
| Median | 0.6 | 2.0 | 26.2 | |||||||||||
|
| ||||||||||||||
| Subtype A (n = 5) | ||||||||||||||
|
| ||||||||||||||
| Q168.a2 | x | x | x | x | x | 3.3±0.9 | 1.4±0.1 | 9.4±2.3 | ||||||
|
| ||||||||||||||
| Q842.d12 | x | x | x | x | 3.0±0.9 | 2.5±0.6 | 40.9±15.8 | |||||||
|
| ||||||||||||||
| Q23.17 | x | x | x | 1.9±0.3 | 3.0±1.1 | 29.8±9.0 | ||||||||
|
| ||||||||||||||
| 92RW009 | x | x | x | x | 1.8±0.4 | 1.2±0.3 | 21.8±8.9 | |||||||
|
| ||||||||||||||
| Q461.e2 | x | x | x | x | x | 0.12±0.06 | 1.2±0.3 | 18.9±4.2 | ||||||
|
| ||||||||||||||
| Median | 1.9 | 1.4 | 21.8 | |||||||||||
Mannose-rich glycosylations were identified from the amino acid sequence of each envelope clone (Kwong et al., 1998; Leonard et al., 1990). Missing mannose-rich glycosylation sites are marked by X. Viruses are ranked according to GRFT sensitivity.
The IC50 is the concentration of GRFT, CV-N, and SVN that reduced HIV-1 infection by 50%. Mean ± SD of 3 independent is shown.
Glycans involved in binding the monoclonal antibody 2G12
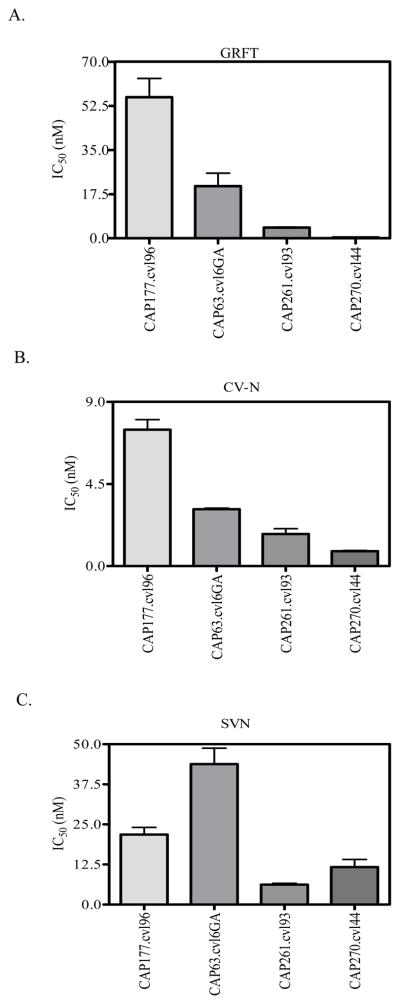
We also tested the sensitivity of four subtype C Env isolated from cervical-vaginal lavages (CVL) of HIV-1 positive women during acute infection (Bronwen Lambson, unpublished data). These viruses also showed varying sensitivities to the lectins with one (CAP177.cvl96) showing a high level of resistance to both GRFT and CV-N (Figure 3). For CAP63.cvl6GA a matched blood sample was also available and the CVL and plasma derived viruses showed similar sensitivities to the three lectins (Table 1). In general, viruses from CVL were inhibited by GRFT, CV-N and SVN with IC50 in the nanomolar range similar to those derived from plasma.
Figure 3. GRFT, CV-N, and SVN inhibit HIV-1 isolates from CVL.
Sensitivity of 4 HIV-1 subtype C pseudoviruses containing functional envelope genes amplified from cervico-vaginal lavages against GRFT (A), CV-N (B) and SVN (C) are shown.
Correlation between HIV-1 mannose-rich glycosylation patterns and sensitivity to GRFT, CV-N and SVN
Given that these lectins bind mannose-rich glycans, we analyzed the predicted glycosylation patterns of all the viruses used in our neutralization assays to determine if there was any correlation with their sensitivity to GRFT, CV-N and SVN. This analysis included all 11 predicted mannose-rich sites on HIV-1 gp120 some of which are involved in mAb 2G12 binding (labeled with asterisks in Table 1). Significant differences were noted between subtypes as previously reported (Zhang et al., 2004). In this study, 13 of the 15 subtype C viruses lacked the 295 glycan compared to more than half of the subtype B and A viruses where this glycan was intact. On average, viruses from subtypes C and B lacked 2 of the 11 predicted glycans although there was a range with some viruses lacking as many as 5 sites. The subtype A viruses had a larger number of missing sites and they all lacked the 230 and 289 glycans. Only one virus was predicted to have all 11 glycans, a subtype C virus (CAP270.cvl44) isolated from CVL.
The five viruses (four subtype C, including two from CVL, and one subtype B) that were the least sensitive to GRFT lacked both the 234 and 295 glycosylation sites. Two of these five viruses (CAP206.08J and CAAN5342.A2) were also the least sensitive to CV-N and SVN and a third (CAP177.cvl96) also required above average levels of CV-N for inhibition. We concluded that the absence of both the 234 and 295 glycosylation sites was responsible for this decreased sensitivity to these lectins. However, the lack of either of these two glycans in isolation did not affect sensitivity. Thus WITO4160.33 and Q842.d12 which lacked the 234 glycan but had the 295 glycan showed similar sensitivity to viruses with both glycans. Similarly viruses that lacked the 295 glycan but retained the glycan at position 234 (most subtype C viruses) were not unusually resistant. Overall there was no correlation between the number of missing glycans and resistance to these lectins. The fewer mannose-rich glycosylation sites on subtype A Envs did not reduce their sensitivity.
The 234 and 295 glycosylation sites are involved in GRFT, CV-N and SVN neutralization of HIV-1
In order to examine the contribution of the glycan at position 295 to lectin neutralization, we introduced this glycosylation site in 3 viruses, Du151.2, COT9.6 and COT6.15, that already had the 234 glycan (see Table 1). For all three viruses the introduction of the 295 glycosylation site further increased their sensitivity to GRFT by 7 to 50 fold (Table 2). However, the addition of the 295 glycan had no effect on their sensitivity to CV-N and SVN suggesting that this glycan is essential for GRFT binding only. As GRFT is more promiscuous in its binding to high-mannose oligosaccharides than either SVN or CV-N (which only bind to oligomannose-8 or -9) this could also indicate that the glycan at position 295 is a smaller oligosaccharide such as oligomannose-5 or -7.
Table 2.
Effect of the 295 glycan on sensitivity to GRFT, CV-N and SVN
| Envelope | GRFT | CV-N | SVN | |
|---|---|---|---|---|
|
| ||||
| IC50 (nM)* | ||||
| Du151.2 | Wild-type | 1.5±0.8 | 2.0±1.0 | 27.5±9.6 |
| V295N | 0.03±0.02 | 1.2±0.4 | 32.5±4.9 | |
| COT9.6 | Wild-type | 1.2±0.2 | 3.9±2.7 | 57.6±26.9 |
| V295N | 0.06±0.04 | 4.4±3.9 | 63.0±35.9 | |
| COT6.15 | Wild-type | 0.4±0.2 | 0.9±0.5 | 10.8±6.8 |
| V295N | 0.06±0.0 | 1.1±0.6 | 16.1±9.2 | |
IC50 is the concentration of GRFT, CV-N, and SVN that reduces viral infection by 50%.
Cases were the mutant IC50 was more than 5-fold lower than the wild-type virus IC50 are bolded.
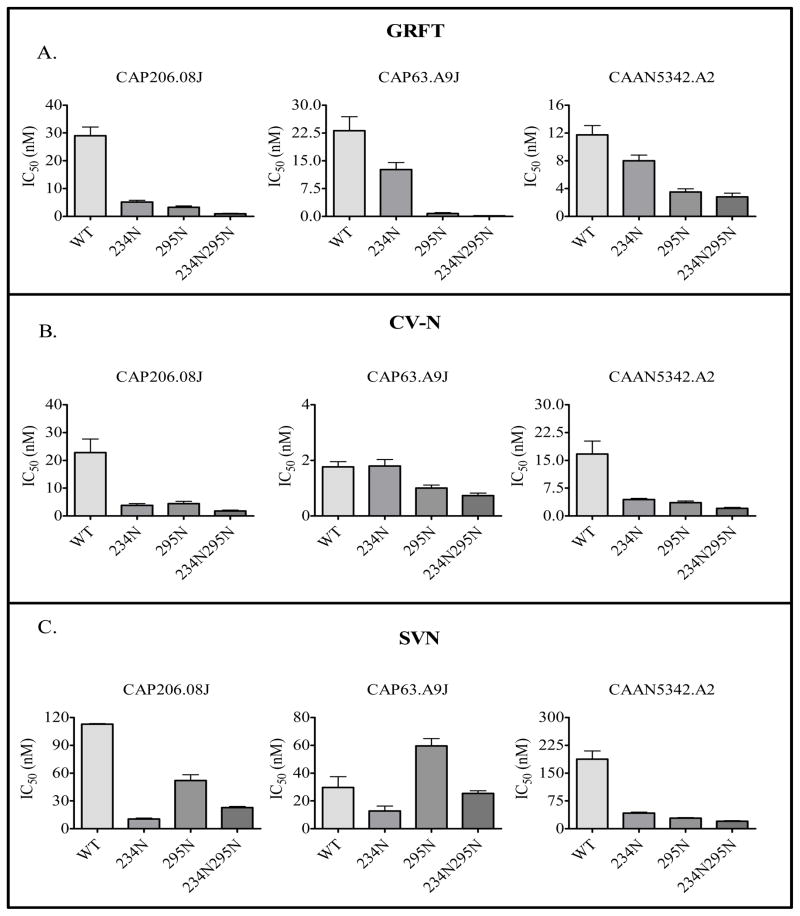
We also introduced both the 234 and 295 glycosylation sites into 3 viruses, CAP206.08J, CAP63.A9J and CAAN5342.A2 that lacked both of these sites and were the most resistant to the lectins, to determine if this would increase their sensitivity. Reconstruction of the 234 glycosylation site in both CAP206.08J and CAP63.A9J reduced virus infectivity of TZMbl-cells by ~2-fold. We were unable to determine whether this was due to a decrease in the production of viral particles in 293T cells or a defect in the virus’s capacity to infect cells. However, the introduction of 295 and to a lesser extent 234, increased the sensitivity of all three viruses to GRFT (Figure 4A). Furthermore, when these two glycans were introduced simultaneously there was an additional increase in sensitivity. The introduction of the 234 and 295 glycosylation sites also had a similar effect on CV-N sensitivity, with the exception of CAP63.A9J where addition of the 234 glycosylation site had no effect (Figure 4B). For SVN, the 234 glycosylation site had a more significant impact on sensitivity than the 295 although for CAP63.A9J addition of a glycan at position 295 actually increased sensitivity above the wild-type (Figure 4C). Collectively, these data suggested that the 234 and 295 glycosylation sites are involved in lectin binding to the HIV-1 envelope glycoproteins and subsequent virus neutralization, although for CV-N and SVN their involvement may be strain-dependent.
Figure 4. Glycans at positions 234 and 295 increase HIV-1 sensitivity to GRFT, CV-N and SVN.
Glycosylation sites at 234 and/or 295 were introduced in CAP206.08J, CAP63.A9J and CAAN5342.A2 by site-directed mutagenesis. Mutant viruses were tested in neutralization assays against GRFT (A), CV-N (B) and SVN (C) in JC53bl-13 cells. Each virus was tested at least three times.
GRFT, CV-N and SVN binding sites overlap the 2G12 epitope
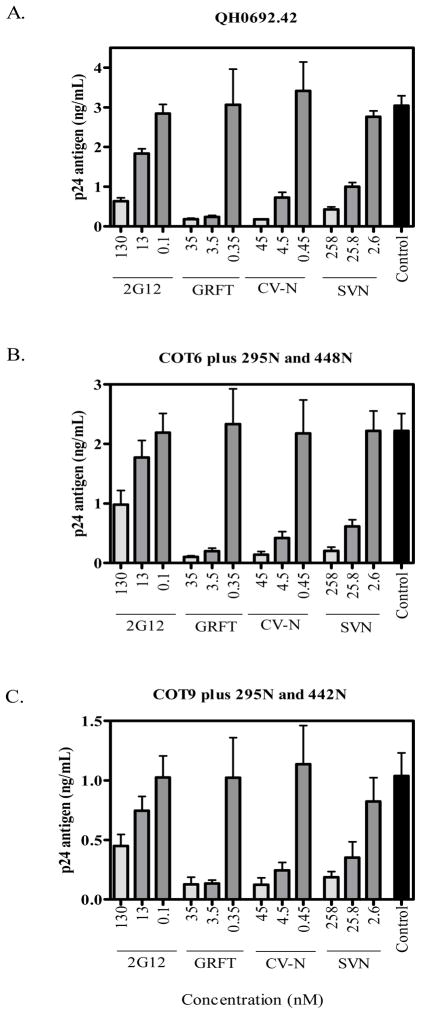
Since five of the 11 mannose-rich sites on gp120 form the 2G12 mAb epitope, we investigated whether lectin binding interfered with access to this epitope. We used a competition ELISA to measure the amount of p24 antigen captured onto 2G12-coated plates after pre-incubation of the virus with GRFT, CV-N or SVN. The 2G12 mAb was competed against itself as a positive control. We tested the subtype B virus QH0692.42which is sensitive to 2G12 with an IC50 of 2.8 μg/ml (Li et al., 2005). Subtype C viruses are generally insensitive to 2G12 and so we used modified subtype C gp160 where the 2G12 epitope had been partially reconstituted (Gray et al., 2007b). Thus, for COT6.15 the addition of glycans at positions 295 and 448 (COT6-V295N/S448N) increased sensitivity from IC50 >100 μg/ml to 64.5 μg/ml. There was also increased binding of the 2G12 mAb to this mutant (Gray et al., 2007b). For COT9.6 the addition of glycans at positions 295 and 442 (COT9-V295N/K442N) increased the sensitivity to 2G12 from an IC50 of 68.7 μg/ml to 33.9 μg/ml.
Pre-incubation of the 3 viruses with each lectin significantly reduced the amount of virus captured onto 2G12-coated plates suggesting competition between these compounds (Figure 5). Similar to what was seen in the neutralization assays; GRFT and CV-N were more potent than SVN at inhibiting virus capture. The effect was dose-dependent although higher amounts of lectin were required to inhibit virus capture compared to neutralization. However, for all 3 viruses, virus capture by 2G12 was inhibited at equivalent lectin concentrations despite the 10–20 fold difference in sensitivities to 2G12 neutralization. Together with the mutagenesis results (Table 2) these data suggested an overlap between the 2G12 epitope and GRFT, CV-N and SVN binding sites.
Figure 5. GRFT, CV-N, and SVN compete with the 2G12 mAb for binding to HIV-1.
Pseudoviruses QH0692.42 (A), COT6.15-V295N/S448N (B) and COT9.6-V295N/K442N (C), the latter two with a reconstituted 2G12 epitope, were incubated with GRFT, CV-N and SVN prior to capture with the 2G12 mAb. 2G12 was competed against itself as the experimental control. Experiments were done at least 3 times.
DISCUSSION
In this study we have shown that HIV-1 subtype C viruses, including those from the vaginal tract are sensitive to the mannose-binding lectins, GRFT, CV-N and SVN. Despite subtype-specific differences in mannosylation patterns, their sensitivity was similar to what has been previously reported for subtype B viruses (Bokesch et al., 2003; Boyd et al., 1997; Mori et al., 2005). GRFT and CV-N were more potent than SVN which required higher concentrations to prevent HIV-1 entry. We identified glycans at positions 234 and 295, in addition to the 2G12 mAb epitope, as playing an important role in the binding of these lectins. Our data support the continued development of GRFT, CV-N and SVN as potential microbicides since these compounds are likely to be effective against HIV-1 subtype C mucosal transmissions.
Inhibition of HIV-1 subtype C infection by GRFT, CV-N and SVN in both primary cells and a cell line were similar although higher concentrations were required for PBMC neutralization. The increased sensitivity of TZM-bl cells compared to PBMC is consistent with studies of antibody neutralization, where single-cycle pseudovirus assays are generally more sensitive than PBMC infectivity assays (Fenyo et al., 2009). The longer duration of culture and multiple replication cycles in the PBMC assay probably account for this. Unlike Huskens and colleagues, we did not find any enhanced infectivity of HIV-1 in PBMC treated with CV-N, indicating that CV-N was not mitogenic in our hands (Huskens et al., 2008). The greater neutralization potency of GRFT and CV-N compared to SVN may be due to the fact that a GRFT dimer has six mannose binding sites and a CV-N dimer has four binding sites. In contrast, the SVN monomer has only two mannose residue binding sites (Moulaei et al., 2007; Ziolkowskaet al., 2006; Ziolkowska and Wlodawer, 2006). The correlation between IC50 values of the compounds within certain subtypes suggested that these lectins share some binding sites, a finding which we confirmed for a few loci. Although, we have shown efficacy of these compounds against subtype C viruses in primary PBMC, it will be important to test the anti-HIV activity of GRFT, CV-N and SVN using dendritic cells and macrophages as these cells play an important role during the sexual transmission of HIV (Lederman, Offord, and Hartley, 2006; Pohlmann, Baribaud, and Doms, 2001).
Subtype C viruses isolated from both adult and pediatric HIV infections as well as those from acute and chronic infection were sensitive to these lectins. Our data are in line with previously reported inhibitory concentrations for subtype B viruses and confirm that these compounds are active against multiple subtypes (Bokesch et al., 2003; Boyd et al., 1997; Mori et al., 2005; O’Keefe et al., 2009). Given that CV-N and GRFT are being proposed as microbicides, we also considered it important to test them against viruses found in the vaginal tract. It has been reported that virus compartmentalization can occur during HIV infection raising the possibility of differential sensitivity of viruses from CVL compared to blood (Kemal et al., 2003). However, our data showed that CAP63.A9J from plasma and CAP63.cvl6GA from CVL of the same HIV-positive woman had similar sensitivity to all three lectins. We attributed this to the fact that these two viruses have minimal genetic differences and the same mannose-rich glycosylation patterns (Table 1). Also these viruses are from early infection and, therefore, it may be too early for compartmentalization to develop. Nevertheless, further studies with more pairs of plasma and CVL viruses, from the same individual with a demonstrated virus compartmentalization, are needed to determine if viruses from CVL are as sensitive as those present in blood.
Despite differences in mannose-rich glycosylation patterns, subtype C, B and A viruses (Zhang et al., 2004) showed similar sensitivities to GRFT, CV-N, and SVN. This result was unexpected given that these compounds bind high-mannose residues. Analysis of the glycosylation patterns suggested that absence of glycans at positions 234 and 295 was associated with natural resistance to GRFT and in some cases to CV-N and SVN. Since subtype C viruses generally lack 295N it was surprising that these viruses were as sensitive to the lectins as subtype B and A viruses that generally retained this site. However, a concomitant lack of the 234 glycosylation site was also required to reduce sensitivity and since this glycan is present on ~80% of subtype C viruses, this may account for the comparable sensitivity with subtype B. The important role of these glycans in conferring sensitivity to these lectins was confirmed by site-directed mutagenesis. Interestingly, when the 295 or 234 glycosylation sites were individually reconstituted they had a significant impact in some viruses. This suggests that in natural Env there may be considerable redundancy or overlap in the glycans that are bound by these compounds. We did not find a correlation between the number of predicted mannose-rich glycans and sensitivity to the lectins, unlike an earlier study on CV-N in subtype B viruses (Balzarini et al., 2006). For example, CAP270.cvl44 which had all predicted 11 mannose-rich glycans was less sensitive to GRFT than viruses that had fewer predicted glycans. In fact our data suggested that the location of the glycan was more important than the number of glycans. Thus, in addition to mannose-rich glycans there may be other factors in the viral envelope that determine the sensitivity to GRFT, CV-N and SVN. These factors may include the Env structure, that can result in glycans that occupy the same position being exposed differently, and the fact that not all potential sites are glycosylated (Go et al., 2009).
Three studies examining CV-N escape pathways using laboratory-adapted HIV-1 subtype B viruses selected in vitro showed that deletion of glycans caused resistance to CV-N (Balzarini et al., 2006; Hu, Mahmood, and Shattock, 2007; Witvrouw et al., 2005). The large number of glycans involved suggested that these compounds have a high genetic barrier to resistance. In this study, we showed that for subtype C primary viruses that lack the 295 glycan, the absence of a single glycan at position 234 could in some cases confer natural resistance to these lectins. However, it is important to bear in mind that generating resistant variants in vitro is likely to be different to identifying loci associated with natural resistance as we have done here. Furthermore, naturally-occurring resistant viruses may have compensatory mutations that are isolate-specific or not commonly present. Indeed, glycan 234 may play an important functional role given its relative conservation in subtype C, and this is first study to report its involvement in CV-N resistance in both subtypes C and B. Further studies are needed to clearly elucidate the position and number of glycans involved in CV-N resistance in primary viruses. It will also be important to investigate the ability of HIV-1 to develop resistance to GRFT and SVN, which may help to identify other GRFT and SVN binding sites on the virus.
The ability of GRFT, CV-N and SVN to compete against the 2G12 MAb for binding to HIV-1 implicates the 2G12 epitope in the binding of these compounds to HIV-1. These data are in agreement with previous reports that glycans in the 2G12 epitope are involved in CV-N and GRFT binding to HIV-1 gp120 (Esser et al., 1999; Mori et al., 2005). However, our study is the first to demonstrate the involvement of the 2G12 epitope in SVN binding to HIV-1. Nevertheless, the involvement of this epitope in the activity of these lectins to subtype C viruses is unclear as these viruses are generally insensitive to the 2G12 mAb (Chen et al., 2005; Gray et al., 2007b; Sanders et al., 2002). The sensitivity of subtype C viruses to GRFT, CV-N and SVN is likely due to the fact that these lectins bind many more sites than those involved in the 2G12 epitope.
GRFT, CV-N and SVN are being actively pursued as microbicides (Bokesch et al., 2003; O’Keefe et al., 2009; Shattock and Moore, 2003; Xiong et al., 2006). The search for HIV microbicides is driven by the challenges encountered in developing an effective HIV vaccine (Johnston and Fauci, 2007) and the fact that the majority of HIV infections around the world are sexually transmitted (Stein, 2003). Potential microbicides include non-specific inhibitors such as buffering agents that inactivate viruses by maintaining the acidic pH in the vaginal tract and moderately specific inhibitors such as anionic polymers that neutralize the virus by binding to its positively charged envelope glycoproteins (Cutler and Justman, 2008). Anti-retroviral agents that target the reverse transcriptase enzyme are being actively researched and herald a new phase in prevention strategies. GRFT, CV-N and SVN, however, hold an advantage over the above-mentioned compounds by selectively targeting the virus to inhibit its entry into susceptible cells. These compounds are small proteins that can be readily and cheaply produced. Furthermore, commensal lactobacilli can be engineered to produce these compounds which could be used to colonize vaginal mucosa and create an environment hostile to HIV-1 (Pusch et al., 2005). Since these compounds are not being developed as therapeutics, their exclusive use as prevention agents would avert the complications that may arise with the use of ARV as microbicides. In conclusion, our data support the continued development of GRFT, CV-N and SVN as microbicides against HIV-1 subtype C mucosal transmissions.
Materials and Methods
Viruses, gp160 envelope (Env) clones, MAbs, cell lines and lectins
HIV-1 subtype C isolates Du151, and Du179 were isolated in South Africa from individuals infected with HIV-1 subtype C (van Harmelen et al., 2001). COT9 is a South African pediatric isolate (Choge et al., 2006). HIV-1 subtype C Env clones Du151.2, CAP45.G3J, CAP210.E8J, CAP206.08J, CAP63.A9J, CAP228.51J and CAP256.F7J were amplified from South African individuals at the acute or early stage of HIV infection (Gray et al., 2007a; Li et al., 2006). HIV-1 subtype B Env, CAAN5342.A2, WITO4160.33, THRO4156.18, AC10.0.29, QH0692.42 and PVO.4 were amplified from acutely infected individuals from the U.S.A, Trinidad and Tobago and Italy (Li et al., 2005). Subtype C Env COT9.6, COT6.15, RP6.6 and TM7.9 were derived from South African pediatric HIV-1 isolates. CAP63.cvl6GA, CAP177.cvl96, CAP261.cvl93 and CAP270.cvl44 were amplified from cervico-vaginal lavages (CVL) obtained during the acute phase of infection from HIV positive women in the CAPRISA cohort in South Africa. The pSG3Δenv plasmid was obtained from Beatrice Hahn. The mAb 2G12 was obtained from the NIH Reference and Reagent Program and the IAVI Neutralizing Antibody Consortium. The TZM-bl cell line was obtained from the NIH Reference and Reagent Program (Cat No 8129) and the 293T cell line was obtained from the American Type Culture Collection. Both cell lines were cultured in DMEM containing 10% fetal bovine serum (FBS). Cell monolayers were disrupted at confluence by treatment with 0.25% trypsin in 1 mM EDTA. Recombinant GRFT, CV-N, and SVN purified from E.coli were provided by Dr. Barry O’Keefe from NCI.
HIV-1 neutralization assay in peripheral blood mononuclear cells
The neutralization assay in PBMC was carried out as described by Bures et al. (Bures et al., 2000). Briefly, a three-fold dilution series of GRFT, CV-N, and SVN in 40 μL of RPMI 1640 containing 20% FBS and interleukin-2 (growth medium) was prepared in triplicate in a U-bottom 96-well plate. Five hundred TCID50 of HIV-1 primary isolate in 15 μL of growth medium was added to each well and the plate was incubated at 37°C for 1 hour. Then 100 μL of 5 × 106 cells/mL phytohemagglutin/interleukin-2 stimulated PBMC (PHA-PBMCs) was added to each well. The following day cells were washed 3 times with RPMI 1640 with 20% FBS and resuspended in 155 μL of fresh growth medium. The culture supernatant was collected twice daily and replaced with an equal amount of fresh growth medium. For each harvest the p24 antigen concentration in the virus control wells was measured by ELISA using the Vironostika HIV-1 Antigen Microelisa System (Biomerieux, Boseind, the Netherlands), according to the manufacturer’s instructions. The inhibitory activity of the lectins were measured at the time-point that corresponded to the early part of the linear growth period of the virus control (Zhou and Montefiori, 1997). The IC80 were calculated by plotting the lectin concentration vs. the percentage inhibition in a linear regression using GraphPad Prism 4.0.
Generation of Env-pseudotyped virus stock
HIV-1 pseudoviruses were generated by co-transfection of the Env and pSG3Δenv plasmids (Wei et al., 2003) into 293T cells using the Fugene transfection reagent (Roche Applied Science, Indianapolis, IN). The TCID50 of each virus stock was quantified by infecting TZM-bl cells with serial 5-fold dilutions of the supernatant in quadruplicate in the presence of DEAE dextran (37.5 μg/mL) (Sigma-Aldrich, St. Louis, MO). The Bright Glo™ Reagent (Promega, Madison, WI) was used to measure infection after 48 hours of tissue culture, according to the manufacturer’s instructions. Luminescence was measured in a Wallac 1420 Victor Multilabel Counter (Perkin-Elmer, Norwalk, CT). The TCID50 was calculated as described elsewhere (Johnson and Byington, 1990).
Single cycle neutralization assay (TZM-bl assay)
The pseudovirus neutralization assay was performed as described elsewhere (Montefiori, 2004). Briefly, three-fold dilution series of each lectin in 100 μL of DMEM with 10% FBS (growth medium) were prepared in a 96-well plate in duplicate. Two hundred TCID50 of pseudovirus in 50 μL of growth medium was added and the mixture was incubated for 1 hour at 37°C. Then 100 μL of TZM-bl cells at a concentration of 1×105 cells/mL containing 37.5 μg/mL of DEAE dextran was added to each well and cultured at 37°C for 48 hours. Infection was evaluated by measuring the activity of the firefly luciferase. Titers were calculated as the inhibitory concentration that causes 50% reduction (IC50) of relative light unit (RLU) compared to the virus control (wells with no inhibitor) after the subtraction of the background (wells without both the virus and the inhibitor).
HIV-1 virion capture assay
High binding 96-well plates (Corning Incorporated Corning, New York, U.S.A) were coated overnight with 1 μg/well of the mAb 2G12 in NaHCO3 (pH 8.5). The coated plates were washed three times with phosphate buffered saline (PBS) and blocked with 3% bovine serum albumin (BSA) in PBS at 37°C for 2 hours. Pseudoviruses were pre-incubated for an hour with different concentrations of 2G12, GRFT, CV-N, or SVN. After discarding the blocking solution the virus was added to the plate and left at 37°C for 2 hours. The plate was then washed three times with PBS and captured viruses were lysed with 150 μL of 0.5% Triton-X 100. The amount of p24 was measured using the Vironostika HIV-1 Antigen Microelisa System (Biomerieux, Boseind, the Netherlands), according to the manufacturer’s instructions.
Site-directed mutagenesis
Putative glycosylation sites were introduced in HIV-1 gp120 using the QuikChange Site Directed Mutagenesis Kit (Stratagene, LaJolla, CA). The presence of the mutation was confirmed by sequencing using the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystem, Foster City, CA) and resolved on the ABI 3100 automated genetic analyzer.
Statistical analysis
The Mann-Whitney t-test was used to compare the median IC50 of each lectin for the neutralization of subtype C, B and A viruses and the Wilcoxon matched pairs test was used to compare the median IC50 of GRFT and CV-N for the neutralization of subtype C. GraphPad Prism 4.0 was used for the Spearman’s nonparametric rank test. p-values of ≤0.05 were considered statistically significant.
Acknowledgments
We thank Natasha Taylor-Meyer for her help with the single cycle neutralization assay. This work was funded by a Biofisa grant from NEPAD, the South African AIDS Vaccine Initiative (SAAVI) and by CAPRISA. CAPRISA is supported by the National Institute of Allergy and infectious Disease (NIAID), National Institutes of Health (NIH), the National Research Foundation, the Columbia University-Southern African Fogarty AIDS International Training and Research Programme (AITRP) funded by the Fogarty International Center, NIH and a training grant from LifeLab, a biotechnology centre of the South African Government Department of Science and Technology. This research was also supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (B. O. & J. M.).
References
- Balzarini J. Targeting the glycans of gp120: a novel approach aimed at the Achilles heel of HIV. Lancet Infect Dis. 2005;5(11):726–31. doi: 10.1016/S1473-3099(05)70271-1. [DOI] [PubMed] [Google Scholar]
- Balzarini J, Van Damme L. Microbicide drug candidates to prevent HIV infection. Lancet. 2007;369:787–97. doi: 10.1016/S0140-6736(07)60202-5. [DOI] [PubMed] [Google Scholar]
- Balzarini J, Van Laethem K, Peumans WJ, Van Damme EJ, Bolmstedt A, Gago F, Schols D. Mutational pathways, resistance profile, and side effects of cyanovirin relative to human immunodeficiency virus type 1 strains with N-glycan deletions in their gp120 envelopes. J Virol. 2006;80(17):8411–21. doi: 10.1128/JVI.00369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos LG, Louis JM, Botos I, Mori T, Han Z, O’Keefe BR, Boyd MR, Wlodawer A, Gronenborn AM. The domain-swapped dimer of cyanovirin-N is in a metastable folded state: reconciliation of X-ray and NMR structures. Structure. 2002;10(5):673–86. doi: 10.1016/s0969-2126(02)00758-x. [DOI] [PubMed] [Google Scholar]
- Binley JM, Wrin T, Korber B, Zwick MB, Wang M, Chappey C, Stiegler G, Kunert R, Zolla-Pazner S, Katinger H, Petropoulos CJ, Burton DR. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol. 2004;78(23):13232–52. doi: 10.1128/JVI.78.23.13232-13252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokesch HR, O’Keefe BR, McKee TC, Pannell LK, Patterson GM, Gardella RS, Sowder RC, 2nd, Turpin J, Watson K, Buckheit RW, Jr, Boyd MR. A potent novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Biochemistry. 2003;42(9):2578–84. doi: 10.1021/bi0205698. [DOI] [PubMed] [Google Scholar]
- Bolmstedt AJ, O’Keefe BR, Shenoy SR, McMahon JB, Boyd MR. Cyanovirin-N defines a new class of antiviral agent targeting N-linked, high-mannose glycans in an oligosaccharide-specific manner. Mol Pharmacol. 2001;59(5):949–54. doi: 10.1124/mol.59.5.949. [DOI] [PubMed] [Google Scholar]
- Botos I, Wlodawer A. Proteins that bind high-mannose sugars of the HIV envelope. Prog Biophys Mol Biol. 2005;88(2):233–82. doi: 10.1016/j.pbiomolbio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Boyd MR, Gustafson KR, McMahon JB, Shoemaker RH, O’Keefe BR, Mori T, Gulakowski RJ, Wu L, Rivera MI, Laurencot CM, Currens MJ, Cardellina JH, 2nd, Buckheit RW, Jr, Nara PL, Pannell LK, Sowder RC, 2nd, Henderson LE. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother. 1997;41(7):1521–30. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bures R, Gaitan A, Zhu T, Graziosi C, McGrath KM, Tartaglia J, Caudrelier P, El Habib R, Klein M, Lazzarin A, Stablein DM, Deers M, Corey L, Greenberg ML, Schwartz DH, Montefiori DC. Immunization with recombinant canarypox vectors expressing membrane-anchored glycoprotein 120 followed by glycoprotein 160 boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDSRes Hum Retroviruses. 2000;16(18):2019–35. doi: 10.1089/088922200750054756. [DOI] [PubMed] [Google Scholar]
- Calarese DA, Scanlan CN, Zwick MB, Deechongkit S, Mimura Y, Kunert R, Zhu P, Wormald MR, Stanfield RL, Roux KH, Kelly JW, Rudd PM, Dwek RA, Katinger H, Burton DR, Wilson IA. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300(5628):2065–71. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- Chen H, Xu X, Bishop A, Jones IM. Reintroduction of the 2G12 epitope in an HIV-1 clade C gp120. Aids. 2005;19(8):833–5. doi: 10.1097/01.aids.0000168980.74713.9e. [DOI] [PubMed] [Google Scholar]
- Choge I, Cilliers T, Walker P, Taylor N, Phoswa M, Meyers T, Viljoen J, Violari A, Gray G, Moore PL, Papathanosopoulos M, Morris L. Genotypic and phenotypic characterization of viral isolates from HIV-1 subtype C-infected children with slow and rapid disease progression. AIDS Res Hum Retroviruses. 2006;22(5):458–65. doi: 10.1089/aid.2006.22.458. [DOI] [PubMed] [Google Scholar]
- Cutler B, Justman J. Vaginal microbicides and the prevention of HIV transmission. Lancet Infect Dis. 2008;8(11):685–97. doi: 10.1016/S1473-3099(08)70254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser MT, Mori T, Mondor I, Sattentau QJ, Dey B, Berger EA, Boyd MR, Lifson JD. Cyanovirin-N binds to gp120 to interfere with CD4-dependent human immunodeficiency virus type 1 virion binding, fusion, and infectivity but does not affect the CD4 binding site on gp120 or soluble CD4-induced conformational changes in gp120. J Virol. 1999;73(5):4360–71. doi: 10.1128/jvi.73.5.4360-4371.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenyo EM, Heath A, Dispinseri S, Holmes H, Lusso P, Zolla-Pazner S, Donners H, Heyndrickx L, Alcami J, Bongertz V, Jassoy C, Malnati M, Montefiori D, Moog C, Morris L, Osmanov S, Polonis V, Sattentau Q, Schuitemaker H, Sutthent R, Wrin T, Scarlatti G. International network for comparison of HIV neutralization assays: the NeutNet report. PLoS One. 2009;4(2):e4505. doi: 10.1371/journal.pone.0004505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go EP, Chang Q, Liao HX, Sutherland LL, Alam SM, Haynes BF, Desaire H. Glycosylation site-specific analysis of clade C HIV-1 envelope proteins. J Proteome Res. 2009;8(9):4231–42. doi: 10.1021/pr9002728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray ES, Meyers T, Gray G, Montefiori DC, Morris L. Insensitivity of paediatric HIV-1 subtype C viruses to broadly neutralising monoclonal antibodies raised against subtype B. PLoS Med. 2006;3(7):e255. doi: 10.1371/journal.pmed.0030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray ES, Moore PL, Choge IA, Decker JM, Bibollet-Ruche F, Li H, Leseka N, Treurnicht F, Mlisana K, Shaw GM, Karim SS, Williamson C, Morris L. Neutralizing antibody responses in acute human immunodeficiency virus type 1 subtype C infection. J Virol. 2007a;81(12):6187–96. doi: 10.1128/JVI.00239-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray ES, Moore PL, Pantophlet RA, Morris L. N-linked glycan modifications in gp120 of human immunodeficiency virus type 1 subtype C render partial sensitivity to 2G12 antibody neutralization. J Virol. 2007b;81(19):10769–76. doi: 10.1128/JVI.01106-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Mahmood N, Shattock RJ. High-mannose-specific deglycosylation of HIV-1 gp120 induced by resistance to cyanovirin-N and the impact on antibody neutralization. Virology. 2007;368(1):145–54. doi: 10.1016/j.virol.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskens D, Vermeire K, Vandemeulebroucke E, Balzarini J, Schols D. Safety concerns for the potential use of cyanovirin-N as a microbicidal anti-HIV agent. Int J Biochem Cell Biol. 2008;40(12):2802–14. doi: 10.1016/j.biocel.2008.05.023. [DOI] [PubMed] [Google Scholar]
- Johnson VA, Byington RE. Quantitative assays for virus infectivity. In: Aldovini A, Walker BD, editors. Techniques in HIV Research. Stockton Press; New York: 1990. pp. 71–76. [Google Scholar]
- Johnston MI, Fauci AS. An HIV vaccine--evolving concepts. N Engl JMed. 2007;356(20):2073–81. doi: 10.1056/NEJMra066267. [DOI] [PubMed] [Google Scholar]
- Kemal KS, Foley B, Burger H, Anastos K, Minkoff H, Kitchen C, Philpott SM, Gao W, Robison E, Holman S, Dehner C, Beck S, Meyer WA, 3rd, Landay A, Kovacs A, Bremer J, Weiser B. HIV-1 in genital tract and plasma of women: compartmentalization of viral sequences, coreceptor usage, and glycosylation. Proc Natl Acad Sci U S A. 2003;100(22):12972–7. doi: 10.1073/pnas.2134064100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman MM, Offord RE, Hartley O. Microbicides and other topical strategies to prevent vaginal transmission of HIV. Nat Rev Immunol. 2006;6(5):371–82. doi: 10.1038/nri1848. [DOI] [PubMed] [Google Scholar]
- Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79(16):10108–25. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Salazar-Gonzalez JF, Derdeyn CA, Morris L, Williamson C, Robinson JE, Decker JM, Li Y, Salazar MG, Polonis VR, Mlisana K, Karim SA, Hong K, Greene KM, Bilska M, Zhou J, Allen S, Chomba E, Mulenga J, Vwalika C, Gao F, Zhang M, Korber BT, Hunter E, Hahn BH, Montefiori DC. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J Virol. 2006;80(23):11776–90. doi: 10.1128/JVI.01730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long EM, Rainwater SM, Lavreys L, Mandaliya K, Overbaugh J. HIV type 1 variants transmitted to women in Kenya require the CCR5 coreceptor for entry, regardless of the genetic complexity of the infecting virus. AIDS Res Hum Retroviruses. 2002;18(8):567–76. doi: 10.1089/088922202753747914. [DOI] [PubMed] [Google Scholar]
- Montefiori DC. Evaluating neutralizing antibodies againts HIV, SIV and SHIV in luciferase reporter gene assays. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, Coico R, editors. Current Protocols in Immunology. John Wiley & Sons; 2004. pp. 12.11.1–12.11.15. [DOI] [PubMed] [Google Scholar]
- Mori T, O’Keefe BR, Sowder RC, 2nd, Bringans S, Gardella R, Berg S, Cochran P, Turpin JA, Buckheit RW, Jr, McMahon JB, Boyd MR. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J Biol Chem. 2005;280(10):9345–53. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- Moulaei T, Botos I, Ziolkowska NE, Bokesch HR, Krumpe LR, McKee TC, O’Keefe BR, Dauter Z, Wlodawer A. Atomic-resolution crystal structure of the antiviral lectin scytovirin. Protein Sci. 2007;16(12):2756–60. doi: 10.1110/ps.073157507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson JR, John GC, Carr JK, Lewis P, Kreiss JK, Jackson S, Nduati RW, Mbori-Ngacha D, Panteleeff DD, Bodrug S, Giachetti C, Bott MA, Richardson BA, Bwayo J, Ndinya-Achola J, Overbaugh J. Subtypes of human immunodeficiency virus type 1 and disease stage among women in Nairobi, Kenya. J Virol. 1999;73(5):4393–403. doi: 10.1128/jvi.73.5.4393-4403.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe BR, Vojdani F, Buffa V, Shattock RJ, Montefiori DC, Bakke J, Mirsalis J, d’Andrea AL, Hume SD, Bratcher B, Saucedo CJ, McMahon JB, Pogue GP, Palmer KE. Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc Natl Acad Sci U S A. 2009;106(15):6099–104. doi: 10.1073/pnas.0901506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmann S, Baribaud F, Doms RW. DC-SIGN and DC-SIGNR: helping hands for HIV. Trends Immunol. 2001;22(12):643–6. doi: 10.1016/s1471-4906(01)02081-6. [DOI] [PubMed] [Google Scholar]
- Pusch O, Boden D, Hannify S, Lee F, Tucker LD, Boyd MR, Wells JM, Ramratnam B. Bioengineering lactic acid bacteria to secrete the HIV-1 virucide cyanovirin. J Acquir Immune Defic Syndr. 2005;40(5):512–20. doi: 10.1097/01.qai.0000187446.76579.d3. [DOI] [PubMed] [Google Scholar]
- Sanders RW, Venturi M, Schiffner L, Kalyanaraman R, Katinger H, Lloyd KO, Kwong PD, Moore JP. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J Virol. 2002;76(14):7293–305. doi: 10.1128/JVI.76.14.7293-7305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan CN, Pantophlet R, Wormald MR, Ollmann Saphire E, Stanfield R, Wilson IA, Katinger H, Dwek RA, Rudd PM, Burton DR. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1-->2 mannose residues on the outer face of gp120. J Virol. 2002;76(14):7306–21. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol. 2003;1(1):25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- Stein SEG. Transmission and epidemiology. In: Richman DD, editor. Human immunodeficiency virus. International Medical Press; 2003. pp. 5:1–5:22. [Google Scholar]
- Tsai CC, Emau P, Jiang Y, Agy MB, Shattock RJ, Schmidt A, Morton WR, Gustafson KR, Boyd MR. Cyanovirin-N inhibits AIDS virus infections in vaginal transmission models. AIDS Res Hum Retroviruses. 2004;20(1):11–8. doi: 10.1089/088922204322749459. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Emau P, Jiang Y, Tian B, Morton WR, Gustafson KR, Boyd MR. Cyanovirin-N gel as a topical microbicide prevents rectal transmission of SHIV89.6P in macaques. AIDS Res Hum Retroviruses. 2003;19(7):535–41. doi: 10.1089/088922203322230897. [DOI] [PubMed] [Google Scholar]
- van Harmelen J, Williamson C, Kim B, Morris L, Carr J, Karim SS, McCutchan F. Characterization of full-length HIV type 1 subtype C sequences from South Africa. AIDS Res Hum Retroviruses. 2001;17(16):1527–31. doi: 10.1089/08892220152644232. [DOI] [PubMed] [Google Scholar]
- Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. Antibody neutralization and escape by HIV-1. Nature. 2003;422(6929):307–12. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- Witvrouw M, Fikkert V, Hantson A, Pannecouque C, O’Keefe BR, McMahon J, Stamatatos L, de Clercq E, Bolmstedt A. Resistance of human immunodeficiency virus type 1 to the high-mannose binding agents cyanovirin N and concanavalin A. J Virol. 2005;79(12):7777–84. doi: 10.1128/JVI.79.12.7777-7784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong C, O’Keefe BR, Botos I, Wlodawer A, McMahon JB. Overexpression and purification of scytovirin, a potent, novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Protein Expr Purif. 2006;46(2):233–9. doi: 10.1016/j.pep.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Zhang M, Gaschen B, Blay W, Foley B, Haigwood N, Kuiken C, Korber B. Tracking global patterns of N-linked glycosylation site variation in highly variable viral glycoproteins: HIV, SIV, and HCV envelopes and influenza hemagglutinin. Glycobiology. 2004;14(12):1229–46. doi: 10.1093/glycob/cwh106. [DOI] [PubMed] [Google Scholar]
- Zhou JY, Montefiori DC. Antibody-mediated neutralization of primary isolates of human immunodeficiency virus type 1 in peripheral blood mononuclear cells is not affected by the initial activation state of the cells. J Virol. 1997;71(3):2512–7. doi: 10.1128/jvi.71.3.2512-2517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziolkowska NE, O’Keefe BR, Mori T, Zhu C, Giomarelli B, Vojdani F, Palmer KE, McMahon JB, Wlodawer A. Domain-swapped structure of the potent antiviral protein griffithsin and its mode of carbohydrate binding. Structure. 2006;14(7):1127–35. doi: 10.1016/j.str.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziolkowska NE, Wlodawer A. Structural studies of algal lectins with anti-HIV activity. Acta Biochim Pol. 2006;53(4):617–26. [PubMed] [Google Scholar]