Abstract
Sooktyn (SKN), mineralo-herbal drug which is being used largely by the patients for its extremely good therapeutic value to treat the gastric ulcers. The present study was undertaken to evaluate the toxicity studies and antiulcer activity of SKN. Acute and sub-chronic toxicities were studied in male and female Wistar rats. A single acute SKN of 2 000 mg/kg was administered by oral gavage for acute toxicity. Sub-chronic doses were 400 and 800 mg/kg/day. The major toxicological end points examined included animal body weight and food intake, selected tissue weights, and detailed gross necropsy. In addition, we examined blood elements: hematocrit, hemoglobin concentration, erythrocyte count, total leukocyte count and MCH, MCHC and platelets as well as biochemical parameters: urea, sugar, alanine transaminase, aspartate transaminase, alkaline phosphatase, total proteins, and creatinine. Also, anti-ulcer activity was carried out by employing indomethacin, ethanol, pylorus ligation, and hypothermic-stress-induced ulcer models. LD50 may be greater than 2 000 mg/kg (orally) for SKN and there were no signs of toxicity on 28 days sub-chronic oral administration of 400 and 800 mg/kg of SKN in rats on the basis of blood elements and biochemical parameters. The ulcer indices decrease in all ulcer models with 66.62%, 61.24%, 80.18%, and 74.76% in indomethacin, ethanol, pylorus ligation, and hypothermic-stress-induced ulcer models, respectively. The results suggest that SKN has no signs of toxicity at 2 000 mg/kg body weight of rats orally; sub-chronically. The drug is safe and has antiulcer activity.
Keywords: Indomethacin, mineralo-herbal, pylorus ligation, Sooktyn
INTRODUCTION
Gastric ulcers arise due to various factors. Even though the etiology of gastric ulcers is still debated, it is accepted that ulcers are caused due to net imbalances in mucosal aggressive factors and defensive factors. There are variety of drugs like histamine blockers and proton pump inhibitors although has been used for efficient management of gastric ulcer, many of these drugs pose adverse effects like dizziness, drowsiness, gas accumulation, headache, nausea, vomiting, inflammation of the nose, etc. Sooktyn (SKN) is a mineralo-herbal formulation consisting of minerals and herbal plants which has utility in hyperacidity, flatulence, dyspepsia, nausea, vomiting, heart burn, gastro-cardiac symptoms, duodenal and gastric ulcers, gastric irritation and erosion but their scientific evaluation is not made yet.[1–5] Sookty Bhasma, Kapur Kachli are used as antiulcer and local inflammatory agents, respectively, while Jatamansi, Ganthoda, and Khurasani Ajvayan are used as antispasmodic agents.[6] After realizing the potential use of SKN, the present study was undertaken to evaluate the toxicities and antiulcer effect of SKN on different gastric ulcer model in rats.
MATERIALS AND METHODS
Sooktyn (Mineralo-herbal Formulation)
SKN, which is being used largely by the patients for its extremely good therapeutic value and its active constituents as Sookty Bhasma (112.5 mg), Kapur Kachli (80 mg), Jatamansi (40 mg), Ganthoda (30 mg), Khurasani Ajvayan (30 mg), Kelpan Rakh (30 mg), Vachha (10 mg), and Datura Pan (5 mg). It is purchased from the market of Jhansi, UP (India), and manufactured by ALARSIN, 205, GIDC, Pandesara, Alarsin House, A/32, St. No. 3, MIDC Andheri (E), Mumbai, India.
Animals
Swiss albino rats of either sex were obtained from animal house of the department. They were housed in an environmentally regulated room on a 12 hours light: 12 hours dark cycle with 25 ± 2° C and had free access to food and water. The experimental protocol was approved by the Institutional Animal Ethical Committee of University and experiments were conducted according to the CPCSEA, India (CPCSEA-837/ac/2004) guidelines on the use and care of experimental animals.
Experimental Design
Acute oral toxicity
SKN at the dose level 2 000 mg/kg (orally) were used for acute oral toxicity study according to Organization for Economic Cooperation Development (OECD) guideline 423.[7] Three female rats, each sequentially dosed at intervals of 48 hours, were used for the test. Once-daily cage side observations included changes in skin, fur, eyes, mucous membrane (nasal), autonomic (salivation, lacrimation, perspiration, piloerection, urinary incontinence, and defecation), and central nervous system (drowsiness, gait, tremors, and convulsions) changes. Mortality, if any, was determined over a period of 2 weeks.
Sub-chronic oral toxicity
In this, the animals were divided into three groups of 10 animals each (5 males and 5 females). Group 1 received 0.025% CMC in water and served as control. Groups 2 and 3 received 400 and 800 mg/kg SKN (suspended in 0.025% CMC solution) body weight orally, respectively. The drug was administered daily for 28 days at the same time and observed at least twice for morbidity and mortality. Body weights and food consumption of the animals were evaluated weekly. This sub-chronic oral toxicity study was carried out according to OECD guideline 407.[8,9]
On the 29th day, of the sub-chronic oral toxicity, after an overnight fast, the rats were anesthetized with ether and blood sample for hematological and biochemical analysis were collected by cardiac puncture method into tubes with and without Ethylenediamine tetra acetate (EDTA), respectively. Animals in the study were also subjected to a full, detailed gross necropsy. Organ weights (heart, kidney, liver, spleen, and testis) were also recorded. The liver samples from different groups were preserved in 10% buffered formalin and processed for routine paraffin block preparation. Sections of thickness of about 5 μm were cut and stained with hematoxylin and eosin.
Anti-ulcer activity
Indomethacin-induced ulcer
The experiment was performed by the standard method with some modifications.[10] Wister albino rats were fasted for 18 hours and deprived of water for 12 hours. Animals were divided into four groups (n = 6). The animals in group 1 served as a vehicle control which received 0.025% CMC solution. Animals in groups 2 and 3 were administered with SKN at the doses of 30 and 40 mg/kg p.o., respectively, 1 hour before the indomethacin administration (20 mg/kg p.o.). Group 4 was administered with the reference drug lansoprazole (20 mg/kg p.o.). The animals were sacrificed after 1 hour. Each stomach was then opened along the greater curvature, rinsed with normal saline, and examined grossly. The ulcers were graded using the following scoring system:
0 = normal mucosa
0.5 = blushing
1 = spot ulcers
1.5 = hemorrhage streaks
2 = ulcers > 3 mm but < 5 mm
2.5 = ulcers > 5 mm
Ethanol-induced ulcer
The experiment was performed according to the method of Morimoto and De Andrade.[11,12] After 12 hours of fasting, the rats were randomly divided into four groups of six animals each. The animals in group 1 served as a vehicle control which received 0.025% CMC solution. Animals in groups 2 and 3 were administered with SKN at the doses of 30 and 40 mg/kg p.o., respectively. All the treatments were administered orally. One hour after treatment, all the rats received 1 ml of absolute ethanol to induce gastric ulcer. One hour later, the animals were sacrificed by cervical dislocation, and the stomachs were removed and opened along the greater curvature. The stomachs were gently rinsed with water to remove the gastric contents and ulcers were graded as described previously.
Ulcer induced by pylorus ligation
Pyloric ligation was performed after 18 hours of treatment with respective groups.[13] The animals in group 1 served as a vehicle control which received 0.025% CMC solution. Animals in groups 2 and 3 were administered with SKN at the doses of 30 and 40 mg/kg p.o., respectively. Briefly, the abdomen of the animals was opened under ether anesthesia by a small midline incision below the xiphoid process; pyloric portion of the stomach was slightly lifted out and ligated avoiding damage to its blood supply. The stomach was replaced and the abdominal wall closed by interrupted sutures. The animals were sacrificed 4 hours after pylorus ligation. Stomach was dissected out, and the gastric juice drained into a small beaker and cut opened along the greater curvature and ulcer index was determined as mentioned earlier. The gastric juice was centrifuged for 10minutes at 2 000 rpm. The supernatant was collected and used for the estimation of volume of gastric juice, pH, free acidity, and total acidity. The volume was noted and expressed as ml/100 g/4 hrs and pH was measured using pH meter. Estimation of free and total acidity was carried out by the method of Card using gastric juice.[14] Free acidity and total acidity were determined by titrating with 0.01N sodium hydroxide using Topfer's reagent and phenolphthalein as indicator, respectively. The acidity was expressed as mEq/L/100 g and acid output as mEq/100 g/4 hrs.[15]
Hypothermic restraint stress-induced ulcer
The experiment was performed by the method of Antonio,[16] with some modifications. Rats were fasted for 36 hours and then immobilized in a restraint cage at 4° C for 4 hours. SKN (30 and 40 mg/kg), lansoprazole (20 mg/kg), or the vehicle was administered 1 hour before the stress procedure. The animals were then killed by cervical dislocation for ulcer index determination as described previously.
RESULTS
Acute Oral Toxicity
In the present study, single dose oral administration of SKN in female rats at 2 000 mg/kg had no effect on mortality, clinical signs, body weight change, or gross observation. Therefore, no acute toxicity was found in rats treated with SKN and lethal doses might be higher than 2 000 mg/kg.
Sub-chronic Oral Toxicity
Body weight and food consumption
There was no significant difference in body weights of male and female rats between their respective control and treatment groups [Figures 1 and 2]. There was no significant difference between food consumption of SKN-treated male and female rats as compared with their respective control.
Figure 1.
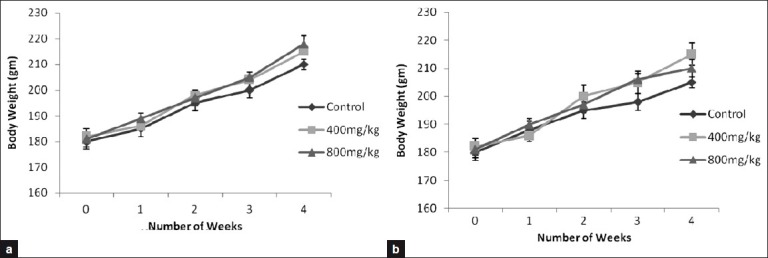
Body weight gain in rats treated orally with vehicle, Sooktyn (SKN, 400 mg/kg), and Sooktyn (SKN, 800 mg/kg) for 28 days. Results are mean ± SEM, n = 5. ANOVA, P > 0.05 between groups in the same day. (a) for male rats and (b) for female rats
Figure 2.
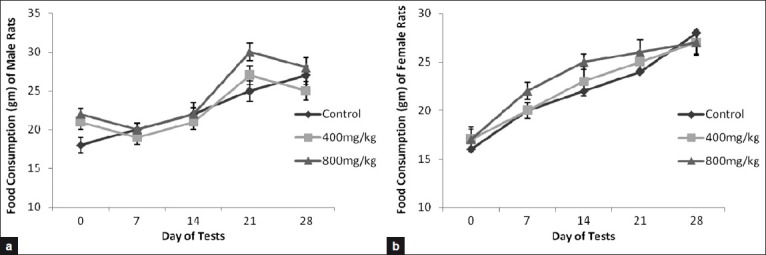
Food consumption by the rats treated orally with vehicle, Sooktyn (SKN, 400 mg/kg), and Sooktyn (SKN, 800 mg/kg) for 28 days. Results are mean ± SEM, n = 5. ANOVA, P > 0.05 between groups in the same day. (a) for male rats and (b) for female rats
Hematological and Biochemical Analysis
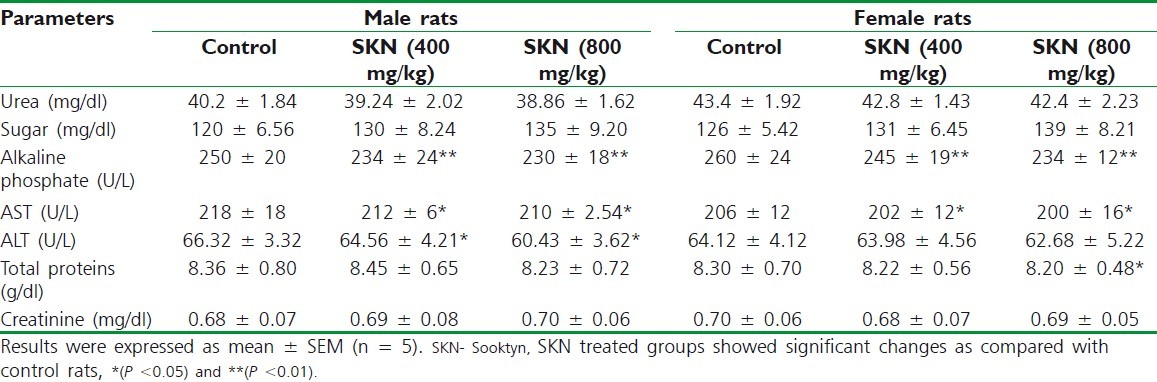
The status of bone marrow activity and intravascular effects were monitored by hematological examination as summarized in Table 1 and biochemical parameters such as, urea, sugar, alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), total proteins, and creatinine were studied and are presented in Table 2.
Table 1.
Hematological parameters for rats after 28 days treatment with vehicle and Sooktyn at two doses in male and female rats
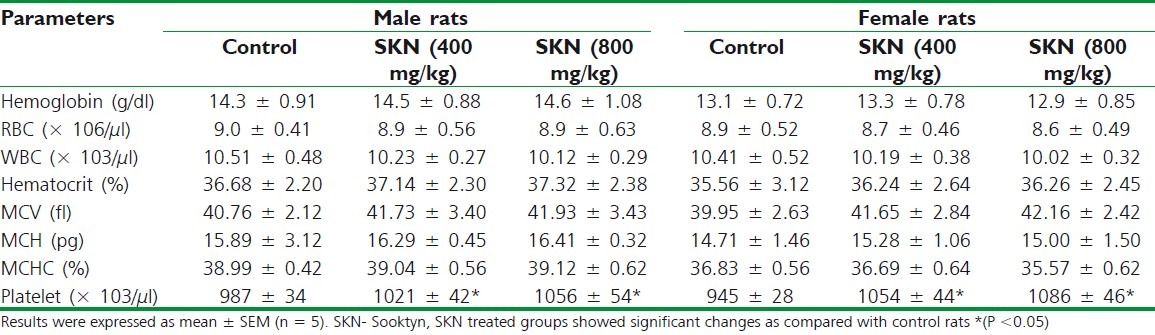
Table 2.
Biochemical parameters for rats after 28 days treatment with vehicle and Sooktyn at two doses in male and female rats
Organs weight
The absolute organ weights in all treated groups of both sexes at the doses level of 400 and 800 mg/kg/day of SKN in the repeated dose 28 days oral toxicity study were not significantly different from their respective control groups with the exception of the liver weight of female rats that was slightly higher than the controls at dose level 800 mg/kg/day. The results are presented in Figure 3.
Figure 3.
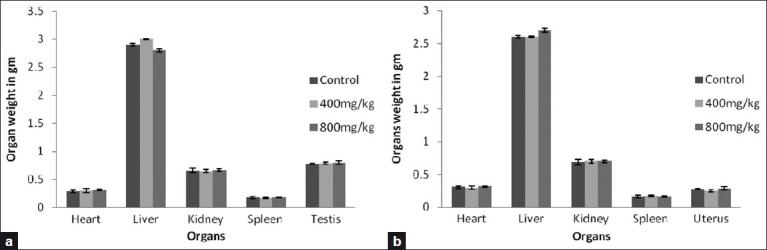
Effects of vehicle and the Sooktyn (SKN, 400 mg/kg) and sooktyn (SKN, 800 mg/kg) orally, on the organ weights of the rats for repeated oral toxicity study for 28 days. Each column and vertical bar represents the mean ± SEM of five animals. ANOVA, P > 0.05 between groups in the same day. (a) for male rats and (b) for female rats
Histopathological studies
There were no significant changes in liver cells in control and treated male and female rats. Sections of liver are shown in Figures 4 and 5.
Figure 4.
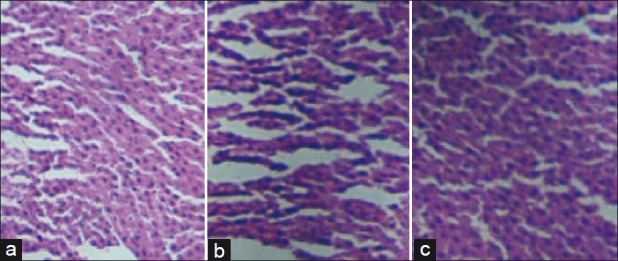
Photomicrographs of liver histopathology from representative male rats: (a) Control group. (b) Sooktyn (SKN) (400 mg/kg/day) group, and (c) SKN (800 mg/kg/day) group (hematoxylin-eosin stain)
Figure 5.
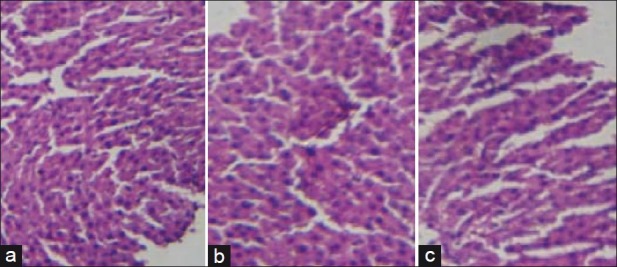
Photomicrographs of liver histopathology from representative female rats: (a) Control group. (b) SKN (400 mg/ kg/day) group, and (c) Sooktyn (SKN) (800 mg/kg/day) group (hematoxylin-eosin stain)
Anti-ulcer Activity
Indomethacin-induced ulcer
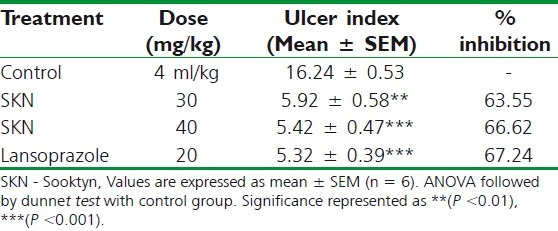
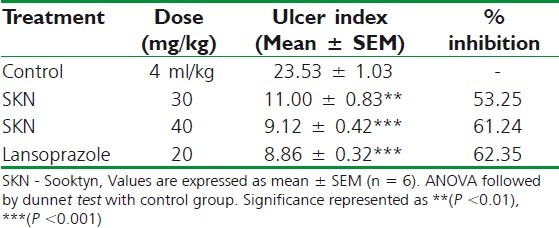
As tabulated in Table 3, the administration of indomethacin produced lesions in the gastric mucosa (16.24 ± 0.53) in control animals that were pre-treated with 0.025% CMC suspension, which were reduced in the animals pre-treated with SKN 30 mg/kg (5.92 ± 0.58; P < 0.05), SKN 40 (5.42 ± 0.47; P < 0.001), or 20 mg/kg lansoprazole (5.32 ± 0.39; P < 0.001).
Table 3.
Effect of Sooktyn on indomethacininduced ulceration in rats
Ethanol-induced ulcer
In the ethanol-induced ulcer model, it was observed that the treatment with SKN (30 and 40 mg/kg) and lansoprazole (20 mg/kg) reduces the ulcer index in comparison with negative control group (P < 0.05). The percentages of inhibition of ulcers were 71.3 ± 5.5, 72.7 ± 6.3, 76.5 ± 7.1, and 92.3 ± 7.5 for the groups treated with 30 and 40 mg/kg of SKN and positive control (lansoprazole), respectively. These results are summarized in Table 4.
Table 4.
Effect of Sooktyn on ethanol-induced ulceration in rats
Ulcer induced by pylorus ligation
Pylorus ligation-induced ulcers were reduced in the animals pre-treated with SKN 30 mg/kg (4.62 ± 0.36; P < 0.01), SKN 40 (3.62 ± 0.41; P < 0.001), or 20 mg/kg lansoprazole (3.18 ± 0.54; P < 0.001), when compared with the control animals that were pre-treated only with the vehicle. Results are presented in Table 5 and Figure 6.
Table 5.
Effect of Sooktyn on pylorus ligationinduced ulceration in rats
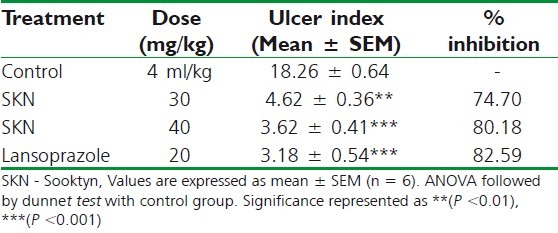
Figure 6.
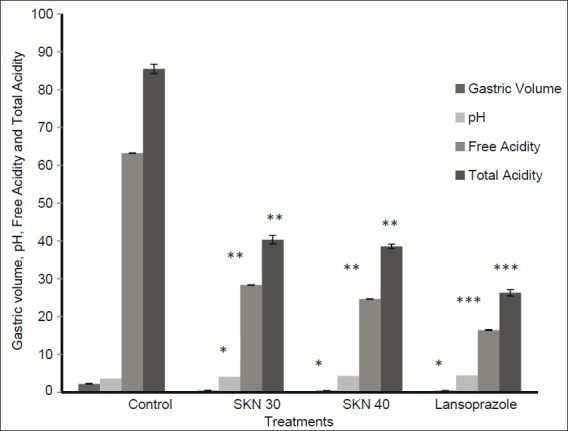
Effects of vehicle, Sooktyn (SKN, 30 mg/kg), and Sooktyn (SKN, 40 mg/kg) given orally to the respective groups. Columns for gastric volume (ml/100 g/4 hrs), pH, free acidity (mEq/L/100 g), total acidity (mEq/L/100 g) and vertical bar represents the mean±SEM of six animals. ANOVA, *(P < 0.05), **(P < 0.01) and ***(P < 0.001) against their control
Hypothermic restraint stress-induced ulcer
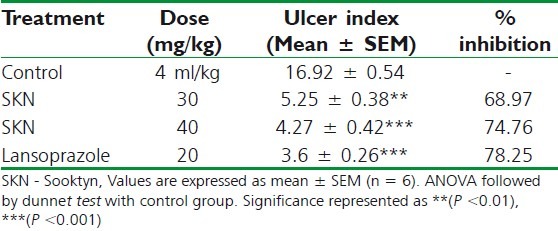
In the hypothermic restraint stress ulcer model, a significant reduction in ulcer index was observed in animals treated with SKN (30 and 40 mg/kg) and lansoprazole (20mg/kg), in comparison with negative control group. The percentages of inhibition of ulcers were 68.97, 74.76, and 78.25 for the groups treated with 30 and 40 mg/kg of SKN and positive control (lansoprazole), respectively. These results are summarized in Table 6.
Table 6.
Effect of Sooktyn on hypothermicstress-induced ulceration in rats
DISCUSSION
To determine the safety of drugs composed of minerals and plants for human use, toxicological evaluation is carried out in various experimental animals to predict toxicity and to provide guidelines for selecting a “safe” dose in human beings. The highest overall concordance of toxicity in animals with human beings is with hematological, gastrointestinal, and cardiovascular adverse effects,[17] while certain adverse effects in human beings, especially hypersensitivity and idiosyncratic reactions, are poorly correlated with toxicity observed in animals. Nevertheless, the evaluation of adverse effects of sub-chronic studies in experimental animals may be more relevant in determining the overall toxicity of the plant/mineralo-herbal preparation. Acute toxicity studies have to be conducted first to select proper dose(s) for sub-chronic studies; the doses selected for sub-chronic toxicity studies should be at and above the suggested human dose. The relative organ weight changes observed included increased heart and lungs weight in male and female rats. The slight changes in the absolute and relative organ weights were considered not to be treatment related because the values were within the normal laboratory range and no abnormality was noted with respect to gross or histopathological examinations of all organs examined.
Analysis of blood parameters is relevant to risk evaluation as the changes in the hematological system have a higher predictive value for human toxicity, when the data are translated from animal studies.[17] The hematological profile of treated rats showed no significant difference with control group. Although not significant, a global increase was observed in hematocrit, hemoglobin concentration, and platelets, implying that there may be a possible increase in erythropoiesis with increasing doses of SKN. Sub-chronic administration of SKN caused significant decrease in the levels of total protein, AST, ALT, and ALP. These observations of significant decrease in the levels of liver enzymes may indicate that SKN has liver protective effects.[18,19]
Nonsteroidal antiinflammatory drugs (NSAIDs) (indomethacin) are known to induce ulcers during the course of anti-inflammatory therapy, by inhibiting prostaglandin synthetase through the cycloxygenase pathway.[20] In the stomach, prostaglandins play a vital protective role, stimulating the secretion of bicarbonate and mucus, maintaining mucosal blood flow, and regulating mucosal cell turnover and repair.[12] Thus, the suppression of prostaglandin synthesis by NSAIDs results in increased susceptibility to mucosal injury and gastro-duodenal ulceration. It was observed that SKN displayed significant reduction of the ulcer index in the indomethacin-induced ulcer model. These results suggest the possible involvement of prostaglandins and/or mucus in the antiulcer effect of the extract.
However, an important anti-ulcerogenic effect was observed in the ethanol-induced ulcers. Ethanol-induced gastric damage may be due to stasis in gastric blood flow, which contributes to the development of hemorrhage and necrotic aspects of tissue injury. This action is direct upon the gastric epithelium, also causing perturbation of mast cells and release of a vasoactive mediator such as histamine. Some scientist believes that changes in gastric circulation after ethanol administration remain unknown, but it has been reported that microcirculation damage can be prevented by prostaglandin administration.[21,22] On the other hand, ethanol-induced gastric lesions are thought to arise as a result of direct damage to gastric mucosal cells, resulting in the development of free radicals and hyperoxidation of lipids.[23,24] This suggests that antioxidant compounds could be active in this experimental model, producing an antiulcerogenic effect, because these substances are capable of settling on the membrane and counteracting lipid peroxide formation.[25]
Another important protective factor is the inhibition of acid secretion,[26] since when levels of acid overwhelm mucosal defense mechanisms, this leads to ulcer formation.[27] In this, oral administration of SKN was tested in the pylorus ligature model, where SKN reduced the ulcer index. Also, gastric volume (ml/100 g), pH, free acidity (mEq/L/100 g), and total acidity (mEq/L/100 g) were reduced during 4 hours of pylorus ligature in rats treated either with SKN or lansoprazole.
Hypothermic restraint-stress-induced ulcer is a well-accepted model for the induction of gastric ulcer. In this, incidence of ulcers is mainly due to increased acid secretion and generation of free radicals. SKN has significantly decreased the ulcer index in this model when compared with control and might be anti-stress and antioxidant activity of SKN which could be suggestive of the free radical scavenging effect of the same.[28]
Conclusively, the results suggest that SKN has no signs of toxicity at 2 000 mg/kg body weight of rats orally; sub-chronically. The drug is safe and has antiulcer activity.
ACKNOWLEDGEMENT
Authors express their sincere thanks to Prof. R M Dubey, Vice Chancellor, IFTM University, Moradabad, for providing research facilities in the laboratories of the University and his constant encouragement to carry out the research work.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Sairam K, Priyambada S, Aryya NC, Goel RK. Gastroduodenal ulcer protective activity of Asparagus racemosus: an experimental, biochemical and histological study. J Ethnopharmacol. 2003;86:1–10. doi: 10.1016/s0378-8741(02)00342-2. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez-Mendoza ME, Reyes-Trejo B, Sanchez-Gomez P, Rodriguez-Silverio J, Castillo-Henkel C, Cervantes-Cuevas H, et al. Bioassay-guided isolation of an anti-ulcer chromene from Eupatorium aschenbornianum: Role of nitric oxide, prostaglandins and sulfydryls. Fitoterapia. 2010;81:66–71. doi: 10.1016/j.fitote.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Tsukimi Y, Okabe S. Recent advances in gastrointestinal pathophysiology: role of heat shock proteins in mucosal defense and ulcer healing. Biol Pharm Bull. 2001;24:1–9. doi: 10.1248/bpb.24.1. [DOI] [PubMed] [Google Scholar]
- 4.Calatayud S, Barrachina D, Esplugues JV. Nitric oxide: Relation to integrity, injury, and healing of the gastric mucosa. Microsc Res Tech. 2001;53:325–35. doi: 10.1002/jemt.1100. [DOI] [PubMed] [Google Scholar]
- 5.Chandra P, Sachan N, Gangwar AK, Sharma PK. Comparative study of mineralo-herbal drugs (Kamadugha and Sutshekhar Rasa Sada) on gastric ulcer in experimental rats. J Pharmacy Res. 2010;3:1659–62. [Google Scholar]
- 6.Kokate CK, Purohit AP, Gokhale SB. 45th ed. Pune: Nirali Prakashan; 2010. Pharmacognosy. [Google Scholar]
- 7.Paris: Organization for Economic Co-operation and Development; 2002. Acute oral toxicity, Acute oral toxic class method guideline-423. [Google Scholar]
- 8.Paris: Organization for Economic Co-operation and Development; 1995. Repeated dose 28-day oral toxicity test method guideline-407. [Google Scholar]
- 9.Chandra P, Sachan N, Ghosh AK, Kishore K. Acute and Sub-chronic Oral Toxicity Studies of a Mineralo-herbal Drug Amlena on Experimental Rats. Int J Pharm Res Innov. 2010;1:15–8. [Google Scholar]
- 10.Gupta M, Mazumder UK, Manikandan L, Bhattacharya S, Senthilkumar GP, Suresh R. Anti-ulcer activity of ethanol extract of Terminalia pallida Brandis. in Swiss albino rats. J Ethnopharmacol. 2005;97:405–8. doi: 10.1016/j.jep.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Morimoto Y, Shimohara K, Oshima S, Sukamoto T. Effects of the new anti-ulcer agent KB-5492 on experimental gastric mucosal lesions and gastric mucosal defensive factors, as compared to those of terprenone and cimetidine. Jpn J Pharmacol. 1991;57:495–505. doi: 10.1254/jjp.57.495. [DOI] [PubMed] [Google Scholar]
- 12.De-Andrade SF, Lemos M, Comunello E, Noldin VF, Filho VC, Niero R. Evaluation of the antiulcerogenic activity of Maytenus robusta (Celastraceae) in different experimental ulcer models. J Ethnopharmacol. 2007;113:252–7. doi: 10.1016/j.jep.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Shay KS, Fels SS, Meranze D, Gruenstein M, Siplet H. A simple method for the uniform production of gastric ulceration. Gastroenterol. 1945;5:43–55. [Google Scholar]
- 14.Card WI, Marks IN. The relationship between the acid output of the stomach following “maximal” histamine stimulation and the parietal cell mass. Clin Sci. 1960;19:147–63. [PubMed] [Google Scholar]
- 15.Devi RS, Narayan S, Vani G, Devi CS. Gastroprotective effect of Terminalia arjuna bark on diclofenac sodium induced gastric ulcer. Chem Biol Interact. 2007;167:71–83. doi: 10.1016/j.cbi.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Antonio JM, Gracioso JS, Toma W, Lopez LC, Oliveira F, Brito AR. Antiulcerogenic activity of ethanol extract of Solanum variabile (false “jurubeba”) J Ethnopharmacol. 2004;93:83–8. doi: 10.1016/j.jep.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 17.Olson H, Betton G, Robinson D, Thomas K, Monro A, Kolaja G, et al. Concordance of toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol. 2000;32:56–67. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]
- 18.Burger C, Fischer DR, Cordenunzzi DA, Batschauer AP, Filho VC, Soares AR. Acute and subacute toxicity of the hydroalcoholic extract from Wedelia paludosa (Acmela brasiliensis) (Asteraceae) in mice. J Pharm Sci. 2005;8:370–3. [PubMed] [Google Scholar]
- 19.Witthawaskul P, Panthong A, Kanjanapothi D, Taesothikul T, Lertprasertsuke N. Acute and sub acute toxicities of saponin mixture isolated from Scheffera leucantha Viguier. J Ethnopharmacol. 2003;89:115–21. doi: 10.1016/s0378-8741(03)00273-3. [DOI] [PubMed] [Google Scholar]
- 20.Rainsford KD. The effect of 5-lipoxygenase inhibitors and leukotriene antagonists on the development of gastric lesions induced by nonsteroidal anti-inflammatory drugs in mice. Agents Actions. 1987;21:316–9. doi: 10.1007/BF01966502. [DOI] [PubMed] [Google Scholar]
- 21.Guth PH, Paulsen G, Nagata H. Histologic and microcirculatory changes in alcohol-induced gastric lesions in the rat: effect of prostaglandin cytoprotection. Gastroenterol. 1984;87:1083–90. [PubMed] [Google Scholar]
- 22.Oates PJ, Hakkinen JP. Studies on the mechanisms of ethanol-induced gastric damage in rats. Gastroenterol. 1988;94:10–21. doi: 10.1016/0016-5085(88)90604-x. [DOI] [PubMed] [Google Scholar]
- 23.Pihan G, Regill C, Szabo S. Free radical and lipid peroxidation in ethanol- or aspirin-induced gastric mucosal injury. Dig Dis Sci. 1987;32:1395–401. doi: 10.1007/BF01296666. [DOI] [PubMed] [Google Scholar]
- 24.Puurunen J, Huttunen P, Hirvonen J. Is ethanol-induced damage of the gastric mucosa a hyperosmotic effect? Comparative studies on the effects of ethanol, some other hyperosmotic solutions and acetylsalicylic acid on rat gastric mucosa. Acta Pharmacol Toxicol (Copenh) 1980;47:321–7. doi: 10.1111/j.1600-0773.1980.tb01567.x. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez FG, Portela TY, Stipp EJ, Di Stasi LC. Antiulcerogenic and analgesic effects of Maytenus aquifolium, Sorocea bomplandii and Zolernia ilicifolia. J Ethnopharmacol. 2001;77:41–7. doi: 10.1016/s0378-8741(01)00268-9. [DOI] [PubMed] [Google Scholar]
- 26.Konturek SJ, Konturek PC, Brzozowski T, Konturek JW, Pawlik WW. From nerves and hormones to bacteria in the stomach; nobel prize for achievements in gastrology during last century. J Physiol Pharmacol. 2005;56:507–30. [PubMed] [Google Scholar]
- 27.Schubert ML. Gastric secretion. Curr Opin Gastroenterol. 2004;20:519–25. doi: 10.1097/00001574-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Dharmani P, Kuchibhotla VK, Maurya R, Srivastava S, Sharma S, Palit G. Evaluation of anti-ulcerogenic and ulcer-healing properties of Ocimum sanctum Linn. J Ethnopharmacol. 2004;93:197–206. doi: 10.1016/j.jep.2004.02.029. [DOI] [PubMed] [Google Scholar]


