Abstract
Research on medicinal plants began to focus on discovery of natural products as potential active principles against various diseases. Medicinal plants are very interesting, have the ability to produce remarkable chemical structures with diverse biological activities. Biophytum sensitivum is used as traditional medicine to cure variety of diseases. During the last few decades, extensive research has been carried out to elucidate the chemistry, biological activities, and medicinal applications of B. sensitivum. Phytochemical analysis have shown that the plant parts are rich in various beneficial compounds which include amentoflavone, cupressuflavone, and isoorientin. Extracts and its bioactive compounds have been known to possess antibacterial, anti-inflammatory, antioxidant, antitumor, radioprotective, chemoprotective, antimetastatic, antiangiogenesis, wound-healing, immunomodulation, anti-diabetic, and cardioprotective activity. The present review has been carried out to shed light on the diverse role of this plant in the management of various ailments facing us.
Keywords: Biophytum sensitivum, medicinal plants, natural products
INTRODUCTION
Plants are naturally gifted. In recent times, research on plants has increased across the globe owing to their immense potential to heal life-threatening diseases. A number of medicinal plants have been evaluated for their therapeutic potentials; most of them have shown their protective effects against various diseases. The secondary metabolites, especially the bioactive compounds present in the plants, provided the basis for several sophisticated traditional medicine systems like Ayurveda, Unani, Folk, and Chinese.[1]
Due to the alarming increase in the rate of advancement of illnesses and their diagnosis, drug discovery from active constituents of the plants is gaining more importance. Drug discovery from medicinal plants involves a multifaceted approach combining botanical, phytochemical, biological, and molecular techniques. Effectiveness of the natural products, mainly the herbal extracts with their proven potential and negligible side effects in therapeutics, has already replaced the synthetically derived chemical substances as in modern allopathic medication system which is regarded as unsafe to human and environment.[2]
Herbal medicines contains one or more herbal substances as active ingredients which is derived from the aerial and non-aerial parts, juices, resins, and oils of the plant either in crude state or as pharmaceutical formulation.[3,4] The chemical constituents of plant may help as cofactors for already available biologically active compounds.[5] Recently, there has been awareness on the understanding of the herbal knowledge for gaining insights into the socioeconomic and cultural components of the society. Advances in quantification and detection of herbal drug molecules provided us the better understanding of the relation between the specific component(s) and its efficacy.[6] Just like conventional molecular drugs, the phytopharmaceuticals are involved in the development and marketing of modern herbal drugs which is clinically effective, inexpensive, and globally competitive.[7] Phytomedicines (can be plants, parts of plants, and isolated bioactive compounds) have been used to treat or prevent various disorders.[6,8] Innumerable drugs from plants found worldwide have been documented in literatures, yet there continues to be untapped knowledge on the curative potential of several plants.
OCCURRENCE AND BOTANICAL DESCRIPTION
Biophytum sensitivum (L: Linnaeus) DC belonging to Division: Magnoliophyta, Class: Magnoliopsida, Order: Oxalidales, Family: Oxalidaceae, found in wet lands of tropical India, South Asia and Africa. Normally, it is present in the shades of trees and shrubs, in grass lands at low and medium altitudes. It is commonly known as Life plant (English). In India, it is also known by various vernacular names, Jhalai (Bengali), Laajjaalu, Lakshmana (Hindi), Hara muni, Jalapushpa (Kannada), Mukkutti (Malayalam), Lajwanti (Marathi), Vipareetalajjaalu, Jhulapushpa (Sanskrit), Nilaccurunki, Tintaanaalee (Tamil), Attapatti, chumi, Jalapuspa, (Telugu). It has been used in traditional medicine for various ailments, especially in Indian medicine.[9,10] The flower of this plant is considered as one of the ten sacred plants which are called as Dasapushpam in tradition and culture of Kerala state in India.[11] Due to seeds remaining dormant, the cultivation of this plant has become very difficult. Shivanna et al. (2008) established a protocol to regenerate B. sensitivum through indirect and direct and somatic embryogenesis from its various explants.[12]
Various crude extract of this plant have shown multifarious activities which includes antioxidants, anti-inflammatory, and antitumor activity. Medicinal plants become the main source for cancer drug development.[13] Pharmacological screening from leaves of the B. sensitivum showed significant antitumor activity in Dalton's Lymphoma Ascites (DLA)-bearing mice[14] and this represents B. sensitivum as a valuable medicinal plant with therapeutic effects. Continued research to elucidate the molecular mechanisms behind the role of antitumor activity is worthwhile. The present article aims to provide a comprehensive review on its state of knowledge about morphology, phytoconstituents, and its various biological activity of B. sensitivum as a revolutionary therapeutic agent to combat life-threatening diseases flourished during the last decade.
PLANT MORPHOLOGY
The little plant grows up to maximum of 20 cm and possess unbranched woody erect stem. Leaves: Leaves abruptly pinnate, leaflets opposite, 6 to 12 pairs, and each leaflet is up to 1.5 cm long, the terminal pair is the largest. Flower: The flowers are many and crowded at the apices of the numerous peduncles, normally yellow, white, or orange with red streak in the center of each of the five petals. The sepals are subulate-lanceolate, striate, and about 7 mm long. Fruits: Fruits are ellipsoid capsules which are shorter than the persistent calyx.[15]
PHYTOCHEMISTRY
The phytochemistry of B. sensitivum showed a wide range of chemical compounds including two biflavones: cupressuflavone and amentoflavone; three flavonoids: luteolin 7- methyl ether, isoorientin and 3’-methoxyluteolin 7-O-glucoside; two acids: 4-Caffeoylquinic acid and 5-Caffeoylquinic acid which were isolated from the aerial parts of B. sensitivum.[16] It also contains 3’, 8”-biapigenin,[17] proanthocyanidins (also known as condensed form of tannins),[18] and some phenolic compounds.[19] These compounds were isolated from the aerial part of this plant [Figure 1].[11]
Figure 1.
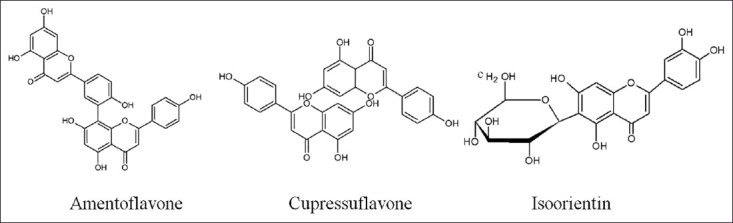
Structures of phytoconstituents isolated from B. sensitivum
TRADITIONAL THERAPY
Tracing the history of traditional practice of B. sensitivum reveals its key action; the plant is bitter, expectorant, stimulant, and tonic in Ayurveda. It has been used as traditional medicine for several purposes. It is recommended to be used in the treatment of stomach ache, asthma, treating insomnia, convulsions, cramps, chest complaints, inflammations, tumors, and remedying chronic skin diseases. Commonly, the whole plant decoction is used for asthma and phthisis and the decoction of root is used for gonorrhea and lithiasis.[9] Specifically, the leaves are diuretic and relieve strangury and commonly known as “Nagbeli,” a folk medicine against “Madhumeha” (Diabetes mellitus), particularly in Eastern Nepal.[20,21] The powdered leaves and seeds were used to apply on wounds.[22] B. sensitivum is one of the plants used against snake envenomation. The whole part of plant is used to counteract the snake venom activity.[23] The major ethnopharmacological uses of B. sensitivum have been depicted in Table 1.
Table 1.
Ethnopharmacological uses of B. sensitivum
PHARMACOLOGICAL POTENTAL OF B. SENSITIVUM
Focusing on the review of research works with various crude extract and its compounds with radioprotection, immunomodulation, antitumor, antioxidant, antibacterial, hypoglycemic, antimetastatic, antiangiogenesis, chemoprevention, anti-diabetic, anti-inflammatory are covered. The widest spectra of pharmacological activities exhibited by B. sensitivum are represented in the Figure 2.
Figure 2.
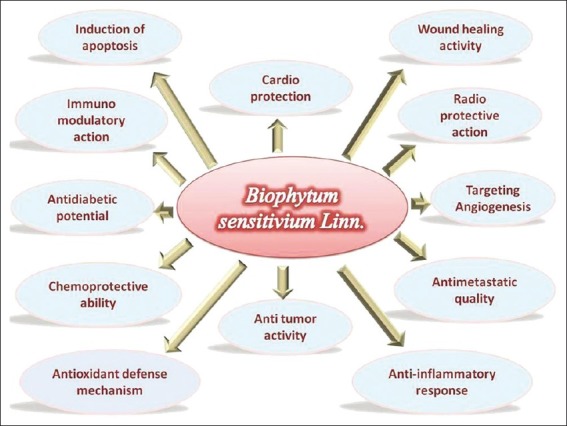
Pharmacological dimensions of B. sensitivum
Radioprotective Action
Over the past 50 years, research in the development of radioprotective agents attained an area of great significance due to its applications in planned radiotherapy and unplanned radiation exposure. Free-radical-induced reproductive cell death is the basis of radiotherapy, and it is clear that the main problem of this approach to fight cancer is to target the ionizing radiation to the tumor cells in order to prevent damage to healthy tissue.[24] The use of B. sensitivum as a radioprotective agent in Swiss albino mice is evaluated. The results of this study showed that B. sensitivum treatment could scavenge the free radicals generated during whole-body radiation exposure and may protect the mice from radiation-induced hemopoietic damage which is mediated through immunomodulation as well as sequential induction of IL-1β, Granulocyte colony stimulating factor (GM-CSF), and IFN-γ.[25]
Induction of apoptosis in B16F-10 melanoma cells
The precisely controlled cellular event apoptosis, also known as programmed cell death, is a well-recognized process essential for normal human embryogenesis as well as to maintain tissue homeostasis and protective processes.[26] The methanolic extract of B. sensitivum and amentoflavone, a biflavonoid present in this plant, were able to inhibit the production of proinflammatory cytokines such as Interleukins (IL-1β and IL-6), GM-CSF, Tumor necrosis factor (TNF-α), and Nitric oxide (NO) by Tumor-associated macrophages.[27,28]
Effect of B. sensitivum on cell-mediated immune response
Guruvayoorappan and Kuttan evaluated the effect of B. sensitivum on mitogen-induced proliferative response of lymphocytes and on Natural Killer (NK) cell-mediated, Antibody-dependent cellular cytotoxicity (ADCC), and Antibody-dependent complement-mediated cytotoxicity (ACC) in Ehrlich ascites carcinoma (EAC)-bearing mice. The administration of B. sensitivum stimulates the immune system, leading to enhanced immune cell proliferation of splenocytes, thymocytes, and bone marrow cells by stimulating the mitogenic potential of various mitogens such as Lipopolysaccharide (LPS), Concanavalin A (Con A), Phytohemagglutinin, and Poke Weed Mitogen. NK cell activity, ADCC, and ACC were found to be enhanced in tumor-bearing animals that suggest the immunomodulatory property of B. sensitivum.[29]
Immunostimulation and Antitumor Activity
The agents that activate the host-defense mechanisms or modulate the immune response by stimulation or suppression are referred as Immunomodulators. The methanolic extract of B. sensitivum was screened for its immunomodulatory and antitumor activity in BALB/c mice. The administration of methanolic extract of B. sensitivum increase the total WBC count and stimulate the hematopoietic system by increasing the count of bone marrow cells and also enhanced the differentiation of stem cells by increasing the presence of γ-esterase-positive bone marrow cells. Increase in circulatory antibody titer and antibody-forming cells indicates its stimulatory effect on the humoral arm of the immune system and production of immune cells by increasing weight of spleen and thymus. The antitumor activities of B. sensitivum extract was determined by both in vitro and in vivo methods. Administration of B. sensitivum inhibited the growth of solid tumor induced by DLA cells and ascites tumor induced by EAC cells. It also decreased cellular Glutathione (GSH), Serum Gamma Glutamyl Transpeptidase (GGT), and NO levels.[30] This was congruent to the report that the aqueous extract of B. sensitivum leaves showed significant antitumor activity against the transplantable murine tumor.[14]
Natural antioxidant defense mechanism
Antioxidative action plays a major role in protection of human beings against oxidative damage caused by the highly reactive unpaired electrons referred as free radicals, which attracted a great deal of attention in recent years. The increased flux of free radicals can react with number of biomolecules including DNA, lipids, and proteins and produce toxic effects.[31] Flavonoids can able to scavenge free radicals by means of directly donating the hydrogen atoms and also by various mechanisms.[32] The possible mechanism by which flavonoids can act is through interaction with various antioxidant enzymes.[33]
The antioxidant potential of B. sensitivum was evaluated in both by in vitro and in vivo. B. sensitivum was found to inhibit in vitro lipid peroxidation and also scavenge superoxide radicals and NO in vitro. Intraperitoneal administration of B. sensitivum in BALB/c mice inhibited Phorbol-12-myristrate-13-acetate-induced superoxide radical generation in macrophages in vivo. The extract also produced a significant increase in catalase activity, Glutathione-S-transferase, GSH, Glutathione reductase and decrease in Glutathione peroxidase.[34] This result strongly supports its usefulness as a natural antioxidant to combat the free radical chain propagation effect. B. sensitivum was reportedly rich in flavonoids which includes Isoorientin, two biflavones: Amentoflavone and Cupressuflavone,[16] and Phenolic compounds;[19] Amentoflavone inhibits the cyclooxygenase-1- and cyclooxygenase-2-catalyzed prostaglandin biosynthesis.[35] A polysaccharide isolated from B. sensitivum was found to be enhancing the complement fixation.[10] These phytocompounds may be responsible for the antioxidant potential of B. sensitivum and further research works need to be initiated to understand the precise mechanism behind the possible role and to find out which active ingredient is responsible for antioxidant action.
Antibacterial property
The use of B. sensitivum as an anti-infective agent was demonstrated recently. The leaves extracts of B. sensitivum (methanol, chloroform, acetone, and petroleum ether) was evaluated for its antibacterial activity by them against several human pathogenic bacterial strains (Bacillus subtilis, Staphylococcus aureus, Streptococcus pneumonia, Klebsiella pneumoniae, Salmonella typhi, Proteus vulgaris, and Escherichia coli). All the extracts showed various remarkable levels of activity on different test organisms and their activity is quite comparable with the standard antibiotics. This discovery indicates a potent remedy from B. sensitivum that will be a great advancement in bacterial infection therapies.[36]
Cardioprotection
Coronary heart disease is characterized by the presence of high blood cholesterol which is the main risk factor for this major life-threatening disease.[37] The possible hypocholesterolemic effect of B. sensitivum leaf water extract in inbred male albino rabbits was assessed. Simultaneous administration of B. sensitivum produced a beneficial effect by improving all the parameters of lipid profile which include Very low-density lipoprotein and Low-density lipoprotein, except High-density lipoprotein, and also it causes slight lowering of plasma glucose, but without any risk of causing severe hypoglycemia.[38]
Antimetastatic quality
The eradication or prevention of highly complex metastatic process, the major cause of mortality in various cancer patients, remains current clinical challenge in the investigation of cancer research. This multistep process involves up and down regulations in the expression of specific genes and various cytophysiological changes. Cancer cells from the primary neoplasm enter into the blood vessels or lymphatics and circulate in the body fluids, then form a new colony at a distant site.[39,40] The treatment with B. sensitivum in B16F-10 melanoma-induced experimental lung metastasis in C57BL/6 mice significantly reduced lung collagen hydroxyproline, hexosamine, uronic acid levels, serum sialic acid, and gamma glutamyl transpeptidase. It inhibits the expression level of several proinflammatory cytokines (IL-1, IL-6, GM-CSF, and TNF) and the proteolytic enzymes matrix metalloproteinases. It also inhibits the activation and nuclear translocation of p65, p50, c-Rel subunits of nuclear factors such as c-fos, activated transcription factor-2, and cyclic adenosine monophosphate response element binding protein in B16F-10 melanoma cells.[41]
Targeting angiogenesis
Understanding the key molecular pathways and signaling molecules involved in the formation of new capillaries from preexisting vasculature by the tumor cells has been a highly active area in the investigation of cancer therapy over the past two decades.[42] Angiogenesis is one of the essential hallmarks required for the transformation of normal cell to a cancer cell. Targeting of angiogenesis has become a promising strategy for cancer treatment by developing angiogenesis inhibitors. Inhibition of angiogenesis suppresses the growth of tumor by switching off the supply of oxygen and nutrients.[43]
B. sensitivum displayed antiangiogenic activity in both in vitro and in vivo models. Intraperitoneal administration of methanol extract of B. sensitivum at a concentration of 50 mg/kg inhibited the tumor-directed capillary formation induced by B16-F10 melanoma cells and increased the level of proinflammatory cytokines such as IL-1β, IL-6, TNF-α, GM-CSF, and VGEF (Vascular Endothelial Growth Factor), the direct endothelial cell-proliferating agent. It also increased production of IL-2 and tissue inhibitor of metalloprotease-1 in B16-F10-injected and treated C57BL/6 animal models. The extract at concentrations of 1 μg/ml, 5 μg/ml, and 10 μg/ml inhibited the VGEF-induced vessel sprouting in rat aortic ring assay and inhibited the VGEF-induced proliferation, cell migration, and capillary-like tube formation of primary cultured human endothelial cells and VGEF mRNA levels in B16-F10 melanoma cells. Antiangiogenic activity of B. sensitivum was supported by its significant modulation of inflammatory cytokines and inhibition of VGEF expression levels.[44]
Chemoprotective ability
The potential and powerful approach of modern chemotherapy uses natural, synthetic, or biological chemical agents to reverse, control, or prevent the multistep process of carcinogenesis by altering its molecular and cellular events.[45,46] The ethanolic extract of B. sensitivum was studied against cyclophosphamide (CTX)-induced toxicity in mice. Intraperitoneal administration of the extract with CTX significantly increased the total WBC count, bone marrow cellularity, alpha-esterase-positive cells, and weight of lymphoid organs. Reduction of GSH in liver and in intestinal mucosa of CTX-treated controls was significantly reversed by B. sensitivum administration with amelioration of changes in serum and liver Alkaline Phosphatse, Glutamic pyruvate transaminase, , and lipid peroxidation. The level of the proinflammatory cytokine, TNF-alpha, which was elevated during CTX administration, was significantly reduced by the administration of B. sensitivum extract. The lowered levels of cytokines IFN-gamma, IL-2, and GM-CSF after CTX treatment were also found to be increased by B. sensitivum extract administration. Even though it did not compromise the antineoplastic activity of CTX, there is a synergistic action of CTX and B. sensitivum in reducing the solid tumors in mice was found.[47]
Antidiabetic potential
The inherited or acquired deficiency in production of insulin leads to hyperglycemic condition or Diabetes mellitus, as a result of high blood sugar which in turn damage many of body systems. The prevalence of diabetes mellitus is alarmingly increasing worldwide from 2.8% in 2000 to 4.4% in 2030.[48] The administration of B. sensitivum reduced the activities of gluconeogenic enzymes present in glucose homeostasis and increased the level of plasma insulin which enhances the glycogenesis in streptozotocin and nicotinamide-induced diabetic rats have recently been reported.[49] The insulinotropic effect was investigated in alloxan-induced diabetic rabbits and the results showed significant hypoglycemic effect possibly due to stimulating action of synthesis of insulin from beta cells of islets of Langerhans of pancreas.[50,51]
Anti-inflammatory response
Inflammation is the important cause known to be responsible for association with several diseases like Cancer, Diabetes, Arthritis, Parkinson's disease, Alzheimer's disease, and Ulcerative colitis.[52–56] The anti-inflammatory action of B. sensitivum was investigated in aqueous and methanol extracts of the aerial parts and roots in the carrageenin-induced rat paw edema. The inhibition of edema was found to be maximum in aqueous extract of aerial parts and roots and methanol fraction of roots than the methanol fraction of aerial parts.[57] B. sensitivum inhibits the production of NO, proinflammatory cytokines like IL-1β, IL-6, and TNF-α, the important targets for treatment of inflammatory disorders in LPS or Con A-stimulated primary macrophages. The gene expression profile showed that B. sensitivum downregulate the expression of Inducible NO synthase and COX-2 and acts as potential inhibitor in inflammatory conditions.[58]
Amentoflavone–A potent biflavonoid in B. sensitivum
Amentoflavone is a potent biflavonoid present in B. sensitivum and other numerous plant species which include Putranjiva roxburghii Wall. (family: Euphorbiaceae), Mangifera indica Linn. (family: Anacardiaceae), Ginkgo biloba Linn. (family: Ginkgoaceae), Selaginella sinensis, Selaginella willdenowii, Selaginella tamariscina (family: Slaginellaceae)[59,66,71] Antidesma laciniatum (family: Euphorbiaceae),[60] Trattinickia rhoifolia (family: Burseraceae),[61] Thuja orientalis (family: Cupressaceae),[62] Nandina domestica (family: Berberidaceae).[63] Cycas rumphii (family: Cycadaceae), and Trifolium alexandrinum (family: Fabaceae).[64]
Amentoflavone, the active ingredient of B. sensitivum and other plants, has been shown to exhibit various pharmacological activities such as antiviral,[65,66] anti-inflammatory,[67] antidepressant,[68] antioxidant,[69] analgesic activities,[70] Inhibitor of phospholipase Cγ1[71] irreversible inhibitor of lymphocyte proliferation,[72] inhibitor of NO synthase in macrophages,[73] inhibitor of cAMP phosphodiesterase in rat adipose tissues,[74] inhibits nonenzymatic lipid peroxidation,[75] a potent caffeine-like Ca2+ releaser in skeletal muscle sarcoplasmic reticulum,[58] inhibits the papain super family member of cysteine proteases–Cathepsin B,[76] a potent scavenger of superoxide,[77] and inhibitory effect on the degradation of IkBα and NF-kB translocation into the nucleus.[78] It has potential to activate the proliferation of lymphoid cells and effector cell functions by increasing the production of IL-2 and IFN-γ and could impede the level of markers of proliferating tumor cells, serum sialic acid and serum γ-GGT.[79] It also has the ability to inhibit the production of proinflammatory cytokines such as IL-1β, IL-6, GM-CSF, and TNF-α in B16F-10 cells and peritoneal macrophages;[80] antiangiogenic qualities by distracting the integrity of endothelial cells and by modulating the endogenous factors required for the neovascularization process.[81] In view of the above interpretations, Amentoflavone become the potent bioactive principle from B. sensitivum and other several plants; it is evident to accept its worthwhile contribution to healthcare.
Other active ingredients
Isoorientin is one of the main biologically active phytochemical components of B. sensitivum. It is also reported to be present in different plants species such as Gentiana olivieri (family: Gentianaceae),[82] Cucumis sativus, Cucumis metuliferus, Cucumis myriocarpus (family: Cucurbitaceae),[83] Cymbopogon citrates (family: Poaceae),[84] Centaurea gigantean (family: Asteraceae),[85] Clematis rehderiana (family: Ranunculaceae),[86] Commelina communis (family: Commelinacae),[87] Sasa borealis (family: Poaceae),[88] Daphne gnidium,[89] Passiflora edulis (family: Passifloraceae),[90] Caraipa densifolia,[91] Cecropia pachystachya (family: Cecropiaceae),[92] Patrinia villosa[93], Arum palaestinum (family: Araceae),[94] and Zea Mays (family: Poaceae).[95] Isoorientin can also synthesized from commercially available Phloroacetophenone.[96] Literatures on biological activities of isoorientin shows Hypoglycemic and antihyperlipidemic effects,[82] Antinociceptive, anti-inflammatory and gastroprotective activities,[97] antioxidant potential,[98] and myolytic activity on uterine smooth muscle of rats and guinea pigs.[94] Another component present in B. sensitivum is luteolin 7-methyl ether. It is also present in Blumea balsamifera (family: Asteraceae) and exhibited strong cytotoxicity against human lung cancer cell lines (NCI-H187).[99] As documented by several workers, further work on clinical investigations of these biologically active phytocomponents seems to be highly essential.
CONCLUSION
Medicinal plants still remains as thriving source of life-saving drugs for the large majority of people treating health problems. During the past two decades, remarkable progress in medicinal plants research such as chemical characterization, biological, pharmacological, and toxicological activity of the plants has been witnessed. In the present review, the proven scientific validation for the plant B. sensitivum reported in the last decade was highlighted. The bioactivity of the various extracts of B. sensitivum corresponding to its traditional application can validate the traditional folk medicinal usage of the plant. Furthermore, the theories, beliefs, and experimental studies will hopefully broaden our knowledge base needed for designing beneficial multi-target therapeutic agents from the B. sensitivum in near future directions. Studies in the recent past indicate its potential effectiveness of pure chemical compounds attributed to it and its diverse medicinal activities. From this, it is confirmed that B. sensitivum with its active ingredients can be implicated in the maintenance of health as well as in prevention, treatment, or improvement of several disease areas. However, further exploration for development of new drug molecules and to elucidate the mechanism responsible for its therapeutic action is of paramount importance. Further research works need to be initiated to look for the possible role of this plant extract and its chemical constituents to variety of diseases in human models.
ACKNOWLEDGMENT
The authors acknowledge funding from the DST, New Delhi, India (Grant No: SR/FT/LS-30/2009). The valuable guidance of Dr. V. M. Berlin Grace, Head, Department of Biotechnology and Dr. M. Patrick Gomez, Director, School of Biotechnology and Health Sciences, Karunya University, is gratefully acknowledged.
Footnotes
Source of Support: DST, New Delhi, India (Grant No: SR/FT/LS-30/2009).
Conflict of Interest: Nil.
REFERENCES
- 1.Ameenah Gurib-Fakim. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol Aspects Med. 2006;27:1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Balunas Mj, Kinghorn AD. Drug discovery from medicinal plants. Life Sci. 2005;78:431–41. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Barboza GE, Cantero JJ, Nunez C, Paccioroni A, Ariza-Espinar L. Medcinal plants - A general review and a phytochemical and ethnopharmacological screening of the native Argentina flora. Tomo. 2009;34:7–365. [Google Scholar]
- 4.Silano M, De Vincenzi M, De Vincenzi A, Silano V. The new European legislation on Traditional Herbal Medicines: Main features and perspectives. Fitoterapia. 2004;75:107–16. doi: 10.1016/j.fitote.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Nahrstedt A, Butterweck VL. Lessons learned from herbal medicinal plants: The example of St.John's Wort. J Nad Prod. 2010;73:1015–21. doi: 10.1021/np1000329. [DOI] [PubMed] [Google Scholar]
- 6.Steenkamp V. Phytomedicines for the prostate. Fitoterapia. 2003;74:545–52. doi: 10.1016/s0367-326x(03)00155-2. [DOI] [PubMed] [Google Scholar]
- 7.Dev S. Impact of natural products in modern drug development. Indian J Ex biol. 2010;48:191–8. [PubMed] [Google Scholar]
- 8.Sahoo N, Manchikanti P, Dey S. Herbal drugs: Standards and regulation. Fitoterapia. 2010;81:462–71. doi: 10.1016/j.fitote.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Jirovetz L, Buchbauer G, Wobus A, Shafi MP, Jose B. Medicinal used plants from India: Analysis of the essential oil of air- dried Biophytum sensitivum (L.) DC. Sci Pharm. 2004;72:87–96. [Google Scholar]
- 10.Inngjerdingen KT, Colibaly A, Diallo D, Michaelsen TE, Paulsen BS. A Complement fixing polysaccharide from Biophytum petersianum Klotzch, a medicinal plant from Mali, West Africa. Biomacromolecules. 2006;7:48–53. doi: 10.1021/bm050330h. [DOI] [PubMed] [Google Scholar]
- 11.Varghese KJ, Anila J, Nagalekshmi R, Resiya S, Dasapushpam JS. The traditional uses and the therapeutic potential of ten sacred plants of Kerala state in India.International journal of pharmaceutical sciences and research. 2010:50–59. [Google Scholar]
- 12.Shivanna MB, Vasanthakumari MM, Manala MC. Regeneration of B.sensitivum (Linn), DC through organogenesis and somatic embryogenesis. Indian J Biotechnol. 2009;8:127–31. [Google Scholar]
- 13.Tian Z, Si J, Chang Q, Zhou L, Chen S, Xiao P, Wu E. Antitumor activity and mechanisms of action of total glycosides from aerial part of Cimicifuga dahurica targeted against hepatoma. BMC Cancer. 2007;7:237. doi: 10.1186/1471-2407-7-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhaskar VH, Rajalakshmi V. Anti tumour activity of aqueous extract of Biophytum sensitivum Linn. Ann. Biol Res. 2010;3:76–80. [Google Scholar]
- 15.Kirtikar KR, Basu BD. Dehradun, India: International book distributors; 2005. Indian medicinal plants; p. 3. [Google Scholar]
- 16.Lin YL, Wang WY. Chemical constituents of Biophytum sensitivum. Chin Pharm Jr. 2003;55:71–5. [Google Scholar]
- 17.Jachak SM, Bucar F, Kartnig TH, Shubert-Zsilavecz M. Prague, Czech Republic: 44th Annual Congress of the society for Medicinal Plant Research; 1996. C-Glycosylflavones from B. sensitivum leaves; p. 188. [Google Scholar]
- 18.Bucar FS, Jachak M, Kartnig TH, Noreen Y, Bohlin L, Schubert-Zsilavecz M. Sweden: Swedish Academy of Pharmaceutical Sciences; 1997. Phenolic compounds of Biophytum sensitivum and their activities on COX catalyzed prostaglandin biosynthesis, International Symposium of Bioassay methods in Natural Product Research and Drug Development, Uppsala University, Uppsala; p. 49. [Google Scholar]
- 19.Bucar FS, Jachak M, Kartnig TH, Schubert-Zsilavecz M. Phenolic compounds from Biophytum sensitivum Pharmazie. 1998;53:651–653. [Google Scholar]
- 20.Puri D, Baral N, Upadhyaya BP. Indigenous plant remedies in Nepal used in heart diseases. J Nepal Med Assoc. 1997;36:334–7. [Google Scholar]
- 21.Pant PC, Joshi MC. Studies on some controversial indigenous herbal drugs based on ethnobotanical research: A review. J Res Edu in Indian Med. 1993;12:19–29. [Google Scholar]
- 22.The Wealth of India: First Supplement Series, NISC, CSIR, India, 1 A-Ci. 2000:140. [Google Scholar]
- 23.Gomes A, Das R, Sarkhel S, Mishra R, Mukherjee S, Bhattacharya S, et al. Herbs and Herbal constituents active against Snake bite. Indian J Ex boil. 2010;48:865–78. [PubMed] [Google Scholar]
- 24.Paul P, Unnikrishnan MK, Nagappa AN. Phytochemicals as radioprotective agents A review. IJNPR. 2011;2:137–50. [Google Scholar]
- 25.Guruvayoorappan C, Kuttan G. Protective effect of B.sensitivum (L.) DC on Radiation- Induced Damage. Immunopharmacol Immunotoxicol. 2008;30:815–35. doi: 10.1080/08923970802439480. [DOI] [PubMed] [Google Scholar]
- 26.Woodle ES, Kulkarni S. Programmed cell death. Transplantation. 1998;66:681–91. doi: 10.1097/00007890-199809270-00001. [DOI] [PubMed] [Google Scholar]
- 27.Guruvayoorappan C, Kuttan G. Apoptotic effect of Biophytum sensitivum on B16F-10 cells and its regulatory effects on nitric oxide and cytokine production on tumor- associated macrophages. Integr Cancer Ther. 2007;6:373–80. doi: 10.1177/1534735407309484. [DOI] [PubMed] [Google Scholar]
- 28.Guruvayoorappan C, Kuttan G. Amentoflavone stimulates apoptosis in B16F-10 melanoma cells by regulating bcl-2, p53 as well as caspase-3 genes and regulates the nitric oxide as well as proinflammatory cytokine production in B16F-10 melanoma cells, tumor associated macrophages and peritoneal macrophages. J Exp Ther Oncol. 2008;7:207–18. [PubMed] [Google Scholar]
- 29.Guruvayoorappan C, Kuttan G. Effect of Biophytum sensitivum on Cell-Mediated Immune response in Mice. Immunopharmacol Immunotoxicol. 2007;29:337–50. doi: 10.1080/08923970701606163. [DOI] [PubMed] [Google Scholar]
- 30.Guruvayoorappan C, Kuttan G. Immunomodulatory and Anti tumour activity of Biophytum sensitivum Extract. Asian Pac J Cancer Prev. 2007;8:27–32. [PubMed] [Google Scholar]
- 31.Wiseman H, Haliwell B. Damage to DNA by reactive oxygen and nitrogen species: Role of inflammatory disease and progression to cancer. Biochem J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prochazkova D, Bousova I, Wilhelmova N. Antioxidant and prooxidant properties. Fitoterapia. 2011;82:513–23. doi: 10.1016/j.fitote.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 33.Nijveldt RJ, Van nood E, Van Hoom DE, Boelens PG, Van Norren K, Van Leeuwen PA. Flavonoids: A review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–25. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 34.Guruvayoorappan C, Afira AH, Kuttan G. Antioxidant potential of B.sensitivum extract in vitro and in vivo. J Basic Clinl Physiol Pharmacol. 2006;17:255–67. doi: 10.1515/jbcpp.2006.17.4.255. [DOI] [PubMed] [Google Scholar]
- 35.Bucar F, Jachak SM, Noreen Y, Kartnig T, Perera P, Bohlin L, et al. Amentoflavone from B.sensitivum and its effect COX-1/COX-2 catalysed prostaglandin synthesis. Planta Med. 1998;64:373–4. doi: 10.1055/s-2006-957455. [DOI] [PubMed] [Google Scholar]
- 36.Natarajan D, Shivakumar MS, Srinivasan R. Antibacterial activity of leaf extract of Biophytum sensitivum (L.) DC. J Pharm Sci Res. 2010;2:717–20. [Google Scholar]
- 37.Zaloga GP, Harvey KA, Stillwell W, Siddiqui R. Trans fatty acids and coronary heart disease. Nutr Clin Pract. 2006;21:505–12. doi: 10.1177/0115426506021005505. [DOI] [PubMed] [Google Scholar]
- 38.Puri D. Hypocholestrolemic effect of Biophytum sensitivum leaf water extract. Pharm Biol. 2003;41:253–8. [Google Scholar]
- 39.Fidler IJ, Hart IR. Biological diversity in metastatic neoplasm, origin and implications. Science. 1982;217:998–1001. doi: 10.1126/science.7112116. [DOI] [PubMed] [Google Scholar]
- 40.Weiss L. Metastatic inefficiency. Adv Cancer Res. 1990;54:159–211. doi: 10.1016/s0065-230x(08)60811-8. [DOI] [PubMed] [Google Scholar]
- 41.Guruvayoorappan C, Kuttan G. Antimetastatic effect of B.sensitivum is exerted through its cytokine and immunomodulatory activity and its regulatory effect on the activation and nuclear translocation of transcription factors in B16F-10 melanoma cells. J Exp Ther Oncol. 2007;7:49–63. [PubMed] [Google Scholar]
- 42.Tortora G, Melisi D, Ciardiello F. Angiogenesis: A Target for Cancer Therapy. Curr Pharm Des. 2004;10:11–26. doi: 10.2174/1381612043453595. [DOI] [PubMed] [Google Scholar]
- 43.Cook KM, Figg WD. Angiogenesis Inhibitors: Current Stratergies and Future Prospects. Cancer J Clin. 2010;60:222–43. doi: 10.3322/caac.20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guruvayoorappan C, Kuttan G. Anti-angiogenic effect of Biophytum sensitivum is exerted through its cytokine modulation activity and inhibitory activity against VEGF mRNA expression and endothelial cell migration and capillary tube formation. J Exp Ther Oncol. 2007;6:241–50. [PubMed] [Google Scholar]
- 45.Tsao AS, Kim ES, Hong WK. Chemoprevention of Cancer. Cancer J Clin. 2004;54:150–80. doi: 10.3322/canjclin.54.3.150. [DOI] [PubMed] [Google Scholar]
- 46.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–80. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 47.Guruvayoorappan C, Kuttan G. Evaluation of chemoprotective effect of Biophytum sensitivum (L.) DC extract against cyclophosphamide induced toxicity in Swiss albino mice. Drug metabol Drug Interact. 2007;22:131–50. doi: 10.1515/dmdi.2007.22.2-3.131. [DOI] [PubMed] [Google Scholar]
- 48.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 49.Prabu KA, Kumarappan CT, Christudas S, Kalaichelvan VK. Effect of B.sensitivum on streptozotocin and nicotinamide induced diabetic rats. Asian Pac J Trop Med. 2012;2:31–35. doi: 10.1016/S2221-1691(11)60185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puri D, Baral N. Hypoglycemic effect of B.sensittivum in the alloxan diabetic rabbits. Indian J Physiol Pharmacol. 1998;42:401–6. [PubMed] [Google Scholar]
- 51.Puri D. The insulinotropic activity of a Nepalese medicinal plant Biophytum sensitivum preliminary experimental study. J Ethnopharmacol. 2001;78:89–93. doi: 10.1016/s0378-8741(01)00306-3. [DOI] [PubMed] [Google Scholar]
- 52.Virani SS, Polsani VR, Nambi V. Novel markers of inflammation in artherosclerosis. Curr Atheroscler Rep. 2008;10:164–70. doi: 10.1007/s11883-008-0024-0. [DOI] [PubMed] [Google Scholar]
- 53.Maiti R, Agarwal NK. Artherosclerosis in Diabetes Mellitus: Role of inflammation. Indian J Med Sci. 2007;5:292–306. [PubMed] [Google Scholar]
- 54.Bartsch H. Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat Res. 1994;2:253–64. doi: 10.1016/0027-5107(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 55.Stevens RJ, Douglas KM, Saratzis AN, Kitas GD. Inflammation and artherosclerosis in rheumatoid arthritis. Expert Rev Mol Med. 2005;7:1–24. doi: 10.1017/S1462399405009154. [DOI] [PubMed] [Google Scholar]
- 56.da Silva MS, Sánchez-Fidalgo S, Talero E, Cárdeno A, da Silva MA, Villegas W, et al. Anti-inflammatory intestinal activity of Abarema cochliacarpos (Gomes) Barneby, Grimes in TNBS colitis model. J Ethnopharmacol. 2010;128:467–75. doi: 10.1016/j.jep.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 57.Jachak SM, Bucar F, Kartnig Th. Anti-inflammatory activity of extracts of B.sensitivum in Carrageenin-induced Rat Paw Oedema. Phytother Res. 1999;13:73–4. doi: 10.1002/(SICI)1099-1573(199902)13:1<73::AID-PTR374>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 58.Guruvayoorappan C, Kuttan G. Methanol extract of B.sensitivum alters the cytokine profiles and inhibits iNOS and COX-2 expression in LPS/Con A stimulated macrophages. Drug Chem toxicol. 2008;31:175–88. doi: 10.1080/01480540701688915. [DOI] [PubMed] [Google Scholar]
- 59.Ravishankara MN, Pillai DA, Padh H, Rajani M. A Sensitive HPTLC Method for estimation of Amentoflavone, a Bioactive Principle from Biophytum sensitivum (Linn.) DC. and Putranjiva roxburghii Wall. J Planar Chromat. 2003;16:3–6. [Google Scholar]
- 60.Alembert T, Tchinda, Teshome A, Dagne E, Arnold N, Ludger A. Wessjohann.Squalene and Amentoflavone from Antidesma Laciniatum. B Chem Soc Ethiopia. 2006;20:325–8. [Google Scholar]
- 61.Ubaldo J SS, Porcor CR. Chemical constituents of the leaves from Trattinickia rhoifolia. Avances en Quimica. 2010;5:63–5. [Google Scholar]
- 62.Xu GH, Ryoo IJ, Kim YH, Choo SJ, Yoo ID. Free Radical Scavenging and Anti elastase activities of Flavonoids from the Fruits of Thuja orientalis. Arch Pharmacal Res. 2009;32:275–82. doi: 10.1007/s12272-009-1233-y. [DOI] [PubMed] [Google Scholar]
- 63.Suzuki A, Matsunaga K, Mimaki Y, Sashida Y, Ohizumi Y. Properties of Amentoflavone, a potent caffeine like Ca 2+ releaser in skeletal muscle sarcoplasmic reticulum. Eur J Pharmacol. 1999;372:97–102. doi: 10.1016/s0014-2999(99)00144-2. [DOI] [PubMed] [Google Scholar]
- 64.Uddin Q, Malik A, Azam S, Hadi N, Azmi AS, Parveen N, et al. The Biflavonoid, Amentoflavone degrades DNA in the presence of copper ions. Toxicol in vitro. 2004;18:435–40. doi: 10.1016/j.tiv.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 65.Lin YM, Flavin MT, Schure R, Chen FC, Sidewell R. Antiviral activities of bioflavonoids. Planta Med. 1999;65:120–5. doi: 10.1055/s-1999-13971. [DOI] [PubMed] [Google Scholar]
- 66.Ma SC, But PP, Ooi VE, He YH, Lee SH. Antiviral Amentoflavone from Selaginella sinensis. Biol Pharm Bull. 2001;24:311–2. doi: 10.1248/bpb.24.311. [DOI] [PubMed] [Google Scholar]
- 67.Kim HK, Son KH, Chang HW, Kang SS, Kim HO. Amentoflavone, a plant bioflavone: a new potential anti-inflammatory agent. Arch Pharm Res. 1998;21:406–10. doi: 10.1007/BF02974634. [DOI] [PubMed] [Google Scholar]
- 68.Baureithel KH, Buter KB, Engesser A, Bukard W, Schaffner W. Inhibition of enzodiazepine binding in vitro by Amentoflavone, a constituent of various species of Hypericum. Pharm Acta Helv. 1997;72:153–7. doi: 10.1016/s0031-6865(97)00002-2. [DOI] [PubMed] [Google Scholar]
- 69.Cholbi MR, Paya M, Alcaraz MJ. Inhibitory effects of phenolic compounds on CCl4 – induced microsomal lipid peroxidation. Experientia. 1991;72:153–7. doi: 10.1007/BF01945426. [DOI] [PubMed] [Google Scholar]
- 70.Da Silva KL, Dos Santos AR, Mattos PE, Yunus RA, Delle Monache F, Cechine-Filho V. Chemical composition and analgesic acivity of Calophyllum brasiliense leaves. Therapie. 2001;56:431–4. [PubMed] [Google Scholar]
- 71.Lee H, Oh W, Kim B, Ahn S, Kang D, Shin D, et al. Inhibition of phospholipase Cγ1 activity by Amentoflavone isolated from Selaginella tamariscina. Planta Med. 1996;62:293. doi: 10.1055/s-2006-957887. [DOI] [PubMed] [Google Scholar]
- 72.Lee SJ, Choi JH, Son KH, Chang HW, Kang S, Kim HP. Suppression of mouse lymphocyte proliferation in vitro by naturally occuring bioflavonoids. Life Sci. 1995;57:551–8. doi: 10.1016/0024-3205(95)00305-p. [DOI] [PubMed] [Google Scholar]
- 73.Woo ER, Lee JY, Cho IJ, Kim SG, Kang KW. Amentoflavone inhibits the induction of nitric oxide synthase by inhibiting NF-kB activation in macrophages. Pharmacol Res. 2005;51:539–46. doi: 10.1016/j.phrs.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 74.Saponara R, Bosisio E. Inhibition of rat adipocyte cAMP phosphodiesterase by bioflavones of Ginkgo biloba L. J Nat Prod. 1998;61:1386–7. doi: 10.1021/np970569m. [DOI] [PubMed] [Google Scholar]
- 75.Mora A, Paya M, Rios JL, Alcaraz MJ. Structure activity relationships of polymethoxyflavones ad other flavonoids as inhibitors of non enzymic lipid peroxidation. Biochem Pharmacol. 1990;40:793–7. doi: 10.1016/0006-2952(90)90317-e. [DOI] [PubMed] [Google Scholar]
- 76.Pan X, Tan N, Zeng G, Zhang Y, Jia R. Amentoflavone and its derivatives as novel natural inhibitors of human Cathepsin B. Bioorg Med Chem. 2005;13:5819–25. doi: 10.1016/j.bmc.2005.05.071. [DOI] [PubMed] [Google Scholar]
- 77.Huguet AI, Manez S, Alcaraz MJ. Superoxide scavenging properties of flavonoids in a non enzymic system. Z Naturforsch C. 1990;45:19–24. doi: 10.1515/znc-1990-1-205. [DOI] [PubMed] [Google Scholar]
- 78.Banerjee T, Valacchi G, Ziboh VA, van der Vliet A. Inhibition of TNF alpha a induced cyclooxygenase-2 expression by amentoflavone through suppression of NK-ê êB activation in A549 cells. Mol Cell Biochem. 2002;238:105–10. doi: 10.1023/a:1019963222510. [DOI] [PubMed] [Google Scholar]
- 79.Guruvayoorappan C, Kuttan G. Amentoflavone, a bioflavonoid from Biophytum sensitivum augments lymphocyte proliferation, natural killer cell and antibody dependent cellular cytotoxicity through enhanced production of IL-2 and IFN-γ and restrains serum sialic acid and gamma glutamyl transpeptidase production in tumor bearing animals. J Exp Ther Oncol. 2007;6:285–95. [PubMed] [Google Scholar]
- 80.Guruvayoorappan C, Kuttan G. Amentoflavone stimulates apoptosis in B16F-10 melanoma cells by regulating bcl-2, p53 as well as caspase-3 genes and regulates the nitric oxide as well as proinflammatory cytokine production in B16F-10 melanoma cells, tumor associated macrophages and peritoneal macrophages. J Exp Ther Oncol. 2008;7:207–18. [PubMed] [Google Scholar]
- 81.Guruvayoorappan C, Kuttan G. Inhibition of tumor specific angiogenesis by Amentoflavone. Biochemistry Moscow. 2008;73:209–18. doi: 10.1134/s0006297908020132. [DOI] [PubMed] [Google Scholar]
- 82.Sezik E, Aslan M, Yesilada E, Ito S. Hypoglycemic activity of Gentiana olivieri and isolation of the active constituent through bioassay - directed fractionation techniques. Life Sci. 2005;76:1223–38. doi: 10.1016/j.lfs.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 83.Krauze-Baranowska M, Cisowski W. Flavanoids from some species of the genus Cucumis. Biochem Syst Ecol. 2005;29:321–4. doi: 10.1016/s0305-1978(00)00053-3. [DOI] [PubMed] [Google Scholar]
- 84.Orrego R, Leiva E, Cheel J. Inhibitory effect of three C-glycosylflavanoids from Cymbopogon citrates on Human Low Density Lipoprotein oxidation. Molecules. 2009;14:3906–13. doi: 10.3390/molecules14103906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shoeb M, Jaspars M, Macmanus SM, Celik S, Nahar L, Thoo lin PK, et al. Anticolon cancer potential of phenolic compounds from aerial parts of Centaurea gigante (Asteraceae) J Nat Med. 2007;61:164–9. [Google Scholar]
- 86.Du ZZ, Yang XW, Han H, Cai XH, Luo XD. A new flavones C-Glycoside from Clematis rehderiana. Molecules. 2010;15:672–9. doi: 10.3390/molecules15020672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shibano M, Kakutani K, Taniguchi M, Yasuda M, Baba K. Antioxidant constituents in the dayflower Commelina communis and their alpha glucosidase inhibitory activity. J Nat Med. 2008;62:349–53. doi: 10.1007/s11418-008-0244-1. [DOI] [PubMed] [Google Scholar]
- 88.Park HS, Lim JH, Kim HJ, Choi HJ, Lee IS. Antioxidant flavones Glycosides from the leaves of Sasa borealis. Arch Pharm Res. 2007;30:161–6. doi: 10.1007/BF02977689. [DOI] [PubMed] [Google Scholar]
- 89.Deiana M, Rosa A, Casu V, Cottiglia F, Bonsignore L, Dessi MS. Chemical composition and antioxidant activity of extracts from Daphne gnidium L. Journal of American Oil Chemists Society. 2003;80:65–70. [Google Scholar]
- 90.Li H, Zhou P, Yang Q, Shen Y, Deng J, Li L, et al. Comparitive studies on anxiolytic and favonoid compositions of Passiflora edulis ‘edulis’and Passiflora edulis ‘flavicarpa’. J Ethnopharmacol. 2011;133:1085–90. doi: 10.1016/j.jep.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 91.Silveira Da CV, Trevisan MT, Rios JB, Erben G, Haubner R, Pfundstein B, et al. Secondary plant substances in various extracts of the leaves, fruits stem, bark of Caraipa densifolia Mart. Food Chem Toxi. 2010;48:1597–606. doi: 10.1016/j.fct.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 92.Aragao DM, Guarize L, Lanini J, Da Costa JC, Garcia R M, Scio E. Hypoglycemic effects of Cecropia pachystachya in normal and alloxan induce diabetic rats. J Ethnopharmacol. 2010;128:628–33. doi: 10.1016/j.jep.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 93.Peng J, Fan G, Hong Z, Chai Y, Wu Y. Preparative separation of isovitexin and isorientin from Patrinia villosa Juss by high spped counter current chromatography. J Chromatogr A. 2005;1074:111–5. doi: 10.1016/j.chroma.2005.03.067. [DOI] [PubMed] [Google Scholar]
- 94.Afifi FU, Khalil E, Abdalla S. Effect of isorientin isolated from Arum palaestinum on uterine smooth muscle of rats and guinea pigs. J Ethnopharmacol. 1999;65:173–7. doi: 10.1016/s0378-8741(98)00147-0. [DOI] [PubMed] [Google Scholar]
- 95.Liu J, Wang C, Wang Z. The antioxidant and free radical scavenging activities of extract and fractions from corn silk (Zea mays L) and related flavone glycosides. Food Chem. 2011;126:261–9. [Google Scholar]
- 96.Kumazawa T, Minatogawa T, Matsuba S, Sato S, Onodera J. An effective synthesis of isoorientin: The regioselective synthesis of a 6-C-glucosylflavone. Carbohydr Res. 2000;329:507–13. doi: 10.1016/s0008-6215(00)00226-3. [DOI] [PubMed] [Google Scholar]
- 97.Küpeli E, Aslan M, Gürbüz I, Yesilada E. Evaluation of invivo Biological activity profile of Isoorientin. Z Naturforsch C. 2004;59:787–90. doi: 10.1515/znc-2004-11-1204. [DOI] [PubMed] [Google Scholar]
- 98.Lim JH, Park HS, Choi JK, Lee IS, Choi HJ. Isoorientin Induces Nrf2 pathway driven antioxidant response through Phosphatidylinositol 3- kinase signaling. Arch Pharm Res. 2007;12:1590–8. doi: 10.1007/BF02977329. [DOI] [PubMed] [Google Scholar]
- 99.Saewan N, Koysomboon S, Chantrapromma K. Anti-tyrosinase and anti-cancer activities of flavonoids from Blumea balsamifera DC. J Med Plants Res. 2011;5:1018–25. [Google Scholar]


