Abstract
Mediastinal staging of non-small-cell lung cancer (NSCLC) is of paramount importance. it distinguishes operable from inoperable disease, guides prognosis and allows accurate comparison of outcomes in clinical trials. Noninvasive imaging modalities for mediastinal staging include CT, PET and integrated PET-CT. Mediastinoscopy is considered the current gold standard; however, each of these techniques has limitations in sensitivity or specificity. These inadequacies mean that 10% of operations performed with curative intent in patients with NSCLC are futile, owing to inaccurate locoregional lymph-node staging. endoscopic and endobronchial ultrasound-guided mediastinal lymph-node aspiration are important and promising innovative techniques with reported sensitivities and specificities higher than standard investigations. The role of these techniques in mediastinal lymph-node staging is evolving rapidly and early data suggest that they may diminish the need for invasive surgical staging of the mediastinum. Furthermore, these are outpatient procedures that do not require general anesthesia and may be combined safely in the same sitting, for optimal accuracy of mediastinal staging. we propose a new algorithm for the diagnosis and staging of NSCLC, based on the current evidence, which incorporates endoscopic and endobronchial ultrasound as a first investigation after CT in patients with intrathoracic disease.
Introduction
Mediastinal staging of non-small-cell lung cancer (NSCLC) is a critical process that determines treatment options and prognosis for affected patients as well as allowing accurate comparison of outcomes in clinical trials. In patients with NSCLC who are fit for surgery and have no evidence of extrathoracic disease, the disease status of mediastinal lymph nodes (MLNs) is used to differentiate operable from inoperable candidates. Patients with no evidence of MLN metastases on staging investigations are eligible for surgery; those with mediastinal spread are offered chemotherapy and external-beam radiotherapy or neoadjuvant treatment in the context of a clinical trial.
Inadequacies in the current techniques for the staging of NSCLC result in 21–68% of thoracotomies being futile within 1 year.1,2 Of all NSCLC patients who undergo lung resection, 7–17% will have MLN metastases that are missed by current staging techniques, including PET and mediastinoscopy.1,2 Endoscopic ultrasound and endobronchial ultrasound are new tools for mediastinal staging of NSCLC that allow minimally invasive and accurate sampling of MLNs. We discuss the current methods available for such staging, with a focus on endoscopic and endobronchial ultrasound. We also present a new diagnostic algorithm that incorporates these techniques for intrathoracic staging of NSCLC.
Techniques for mediastinal staging
CT
Contrast-enhanced CT is the first step in the assessment of MLN staging for NSCLC. A threshold value of 1 cm in the length of the shortest nodal axis is generally employed to distinguish potentially malignant MLNs (≥1 cm in diameter) from benign ones (<1 cm in diameter). A meta-analysis of 43 studies that used this criterion demon strated that the sensitivity of CT in the diagnosis of MLN metastasis was as low as 51% with a specificity of 86%, in a population that had a median prevalence of mediastinal metastases of 28%.3 MLNs <1 cm in short-axis length may still harbor malignancy in 20% of cases. Conversely, 40% of enlarged MLNs might be benign,4 particularly in the context of obstructive pneumonitis.5 Relying on CT alone for mediastinal staging would, therefore, both overstage and understage patients, and lead to the potentially catastrophic consequences of missed opportunities to operate or futile surgery. These limitations highlight the importance of pathological confirmation of lymph-node status and that MLN staging cannot be performed by CT alone. However, CT remains an important initial investigation; it delineates the anatomy of the mediastinum, which allows selection of the appropriate invasive staging tool.
PET
Functional imaging with PET and combined PET-CT is a valuable addition to assessments of the mediastinum. PET has superior sensitivity and specificity in mediastinal staging compared with CT3 (Table 1) and has an important clinical role in the diagnosis, staging, re-staging, therapy planning and monitoring of disease in patients with NSCLC. The standardized uptake value (SUV) is a measure of the metabolic activity detected by PET and provides information that can predict treatment response and survival.6,7 A cut-off SUV of 2.5 is generally applied to distinguish neoplastic from normal tissue. However, non-neoplastic lesions, particularly granulomatous disorders and infections, might also generate areas that seem positive for malignancy on PET. An overlap exists, therefore, between true-positive and false-positive findings in MLNs with an SUVmax ≥2.5. PET falsely identifies malignancy in 25% of patients who have MLNs that are enlarged for other reasons.3 Labeling PET-positive (SUV ≥2.5) MLNs as malignant without pathological confirmation may result in a missed opportunity to operate. Consequently, current guidelines3,8,9 advocate that PET-positive MLNs should be invasively investigated before surgery is ruled out.
Table 1.
Comparison of techniques for mediastinal lymph-node staging in non-small-cell lung cancer
| Investigation | Sensitivitya(%) | Specificity (%) |
Advantages | Disadvantages |
|---|---|---|---|---|
| CT | 51 | 86 | Delineates anatomy | Uses 1 cm short-axis diameter cut-off for malignancy 40% of enlarged nodes are benign 20% of normal-sized nodes contain malignancy |
| PET | 74 | 85 | High negative predictive value for stage 1 disease Accurate systemic staging |
25% false-positive rate inaccurate in lymph nodes >1 cm |
| Transbronchial needle aspiration |
78 | 99 | Cost-effective Allows simultaneous airway inspection |
Variability in results and utilization Usually limited to enlarged nodes in stations 4 and 7 |
| Mediastinoscopy | 78 | 100 | Considered gold standard Allows detection of micrometastases and extracapsular extension |
Risks of general anesthesia and surgery Lymph nodes in stations 5, 6, 8, 9 and 11 are not accessible to standard technique |
| Endoscopic ultrasound |
84 | 99.5 | High sensitivity in paraesophageal lymph node stations Access to celiac-axis nodes, liver, left adrenal gland Can detect malignancy in normal-sized nodes Minimally invasive and complementary to endobronchial ultrasound |
Requires specialized training and equipment Lymph node stations 2r, 4r, 6, 10 and 11 and endobronchial tree cannot be assessed |
| Endobronchial ultrasound |
90 | 100 | High sensitivity for majority of mediastinum Can detect malignancy in normal-sized nodes May be easily repeated Minimally invasive and complementary to endoscopic ultrasound |
Requires specialized training and equipment Lymph node stations 5, 6, 8 and 9 cannot be assessed |
Although PET-positive mediastinal lesions require pathological confirmation of malignancy, most clinicians generally consider that PET-negative (SUV ≤2.5) lesions are not malignant, particularly in lymph nodes with a short axis <1 cm.10 However, recent evidence has tempered enthusiasm for this imaging modality because false-negative PET findings can and do occur, and the diagnostic performance of this technique is dependent on nodal size.11,12 MLNs ≥1 cm should be invasively sampled, even if they are judged to be metabolically in active by PET scan.13 Preoperative PET remains justified for patients with enlarged MLNs, however, because of its ability to direct invasive biopsy and also to detect previously unsuspected extrathoracic disease.2 In addition to MLNs ≥1 cm, invasive mediastinal staging remains indicated for any mediastinal disease with high uptake of 18F-labeled fluorodeoxyglucose (FDG), PET-positive hilar disease14 or where low FDG uptake is seen in the primary tumor.
Transbronchial needle aspiration
Blind transbronchial needle aspiration (TBNA) is a safe procedure for staging MLNs and was first described in 1978.15 During standard bronchoscopy, an aspiration needle is introduced into the biopsy channel and blindly punctures the bronchial wall, which allows the MLN to be aspirated. This process can be done most accurately for enlarged lymph nodes in the subcarinal area but lower paratracheal and hilar lymph nodes can also be sampled.16 A positive TBNA result may obviate the need for further invasive tests, particularly when combined with PET.17 A meta-analysis of data on patients who underwent TBNA, however, showed a pooled sensitivity of 39% for this technique, with a false-negative rate of 28%. The prevalence of mediastinal metastases in this population was 34%.18
Rapid on-site evaluation of results obtained with blind TBNA,19 use of CT fluoroscopic guidance,20 performance of up to seven passes of TBNA21 as well as education and experience22 may improve results obtained with this technique. However, its generally low diagnostic yield and negative predictive value mean that TBNA is poorly utilized.23 Further tests are necessary in the event of nondiagnostic or negative samples being obtained.
Mediastinoscopy
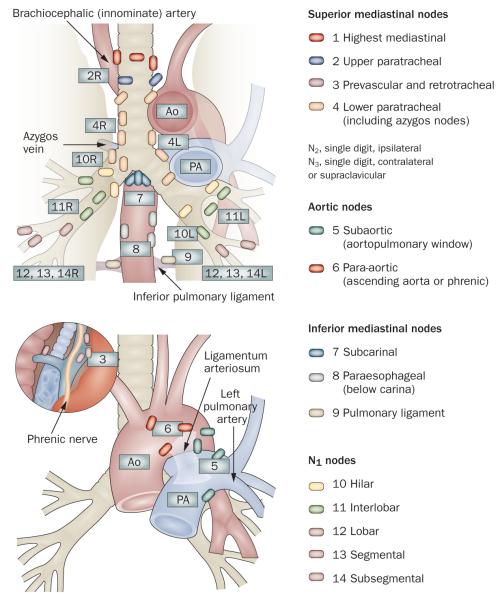
Mediastinoscopy is currently considered the gold standard technique for preoperative MLN staging in patients with NSCLC, and affords access to the paratracheal nodes (stations 2R, 2L, 4R, 4L), pretracheal nodes (station 3) and anterior subcarinal nodes (station 7). However, lymph nodes in the aortopulmonary window (station 5), anterior mediastinal nodes (station 6), posterior subcarinal nodes (station 7) and inferior mediastinal nodes (stations 8 and 9) are not accessible by this technique (Figure 1).24 Micrometastases in normal-sized lymph nodes can be detected in biopsy samples; in addition, the surgeon can determine whether extracapsular spread has occurred, which confers inoperability. However, the sensitivity of detection for mediastinal metastases in patients with NSCLC is low at 78% (range 67–92%) and has a false-negative rate of 10%.25,13 This inaccuracy is, in part, explained by the limited access of mediastinoscopy to the mediastinum.
Figure 1.
Mediastinal lymph node stations, according to American Thoracic Society Regional Lymph Node Station criteria. © 1997 American College of Chest Physicians. Reproduced with permission from the American College of Chest Physicians via the Copyright Clearance Center.24
Mediastinoscopy is also considerably underutilized and poorly performed in routine clinical practice. Little and coauthors collated data on 11,668 patients who underwent thoracotomy for lung cancer in 729 hospitals in the US. Only 27% of these patients had preoperative mediastinoscopy, and in only 47% of those procedures were lymphoid tissue samples obtained.26 Smulders and colleagues27 demonstrated that only 40% of mediastinoscopies were performed according to standard techniques in four hospitals in the Netherlands (three community hospitals and a non-university teaching hospital). Mediastinoscopy thus tends to be underemployed in the staging of lung cancer. Furthermore, when it is carried out, only half of the procedures obtain diagnostic tissue. Despite these limitations and a lack of standardization of the technique, mediastinoscopy currently remains the gold standard technique for staging mediastinal lymphadenopathy.
Endoscopic ultrasound
Endoscopic ultrasound (EUS)-guided fine-needle aspiration (FNA) of mediastinal lymphadenopathy has been available for over a decade.28 under conscious sedation, an endoscope is placed in the patient’s esophagus. Radial echoendoscopes provide cross-sectional imaging but do not allow tissue samples to be obtained; these devices have been superseded by an integrated linear ultrasound probe that allows visualization of the mediastinum (Figure 2). Aspiration with a 22-gauge needle is performed through the wall of the esophagus under direct vision. Owing to the anatomical location of the esophagus, EUS-FNA can be used to sample mediastinal lymph nodes in stations 2L, 4L, 5, 7, 8 and 9. Furthermore, aspiration of the celiac axis nodes, left lobe of the liver and left adrenal gland via EUS can provide additional important staging information.
Figure 2.

Devices used in endoscopic and endobronchial ultrasound. Endoscopic ultrasound probe (left) and endobronchial ultrasound scope (right).
Samples obtained by EUS-FNA with a 22-gauge needle are suitable for cytopathological analysis. Occasionally, core samples obtained by EUS-FNA can be sent for histopathological investigation; however, core tissue samples are more reliably obtained using a 19-gauge Tru-Cut® needle (Alliance Corp., McGaw Park, IL). This method requires that the mediastinal lymph node be at least 2 cm in diameter in the direction of the biopsy. Adding Tru-Cut® biopsy to FNA may improve the diagnostic accuracy and the adequacy of sampling.29 Conflicting evidence exists on the importance of on-site evaluation of biopsy samples,30,31 but their diagnostic yield can be maximized by performing 3–5 passes.31,32
Cohort studies have demonstrated that EUS-FNA is a safe and efficacious procedure for the mediastinal staging of NSCLC. A meta-analysis of 18 studies33 that included a total of 1,201 patients demonstrated a pooled sensitivity of 83% (range 45–100%), a pooled specificity of 97% (range 88–100%) and a false-negative rate of 22%. The median prevalence of mediastinal disease in this cohort was 61%. These studies only included patients with mediastinal lymph nodes that were accessible to EUS-FNA.
Randomized trials of EUS-FNA in NSCLC
The importance of EUS in lung cancer staging is yet to be fully addressed in randomized trials. Tournoy et al. recruited 40 individuals who required invasive mediastinal staging and randomly allocated them to undergo mediastinoscopy (21 patients) or EUS-FNA (19 patients).34 Negative EUS-FNA results were followed by mediastinoscopy. Patients with negative mediastinoscopy results underwent thoracotomy and definitive MLN sampling. The authors showed that only 32% of patients allocated to EUS required mediastinoscopy (P <0.001). EUS, therefore, significantly reduced the need for mediastinoscopy in patients with NSCLC who required invasive MLN staging. Another randomized study compared conventional work-up to a strategy where all patients were offered EUS-FNA in addition to conventional work-up.35 Of 104 patients, 51 underwent conventional work-up and 54 were allocated to routine EUS-FNA and conventional work-up. PET was only available for 30–50% of these patients. Preliminary results demonstrated that the number of futile thoracotomies was significantly reduced by the addition of EUS-FNA to conventional work-up: after a median follow-up of 1.3 years, 13 (25%) thoracotomies were futile in the conventional management group, compared with 5 (9%) in the routine EUS-FNA group.35 Unfortunately, health-care costs in these two randomized trials were not reported. These studies do suggest that EUS-FNA may reduce the number of mediastinoscopies and futile thoracotomies in patients who require invasive MLN staging. Further randomized studies that include economic analyses are awaited.
EUS-FNA in specific groups of patients
Although randomized, controlled trials of unselected patients might provide the highest level of evidence in support of a new procedure,36 cohort studies are important to demonstrate efficacy in different situations.37 Singh and colleagues employed EUS-FNA as a first test after CT in 93 patients.38 The authors sampled the MLNs, celiac axis nodes, left lobe of the liver and left adrenal nodes in a single procedure, which enabled both tissue diagnosis and staging to be performed during a single test in 70% of cases. Metastases to celiac axis nodes were detected in 11% of cases, half of which had not been suspected from CT findings. The study also highlighted the improved accuracy of EUS-FNA compared with CT and PET for the detection of metastases from lung cancer and the poor prognosis of patients with celiac axis nodal involvement.39
Annema et al.39 showed that adding EUS-FNA to routine mediastinoscopy in 100 patients changed preoperative staging in 16% of cases, because EUS-FNA enabled minimally invasive sampling of posterior areas of the mediastinum that could not be reached by standard mediastinoscopy. If EUS is performed after CT, PET and negative mediastinoscopy, malignant N2 or N3 disease may still be detected in 37% of patients.40 EUS-FNA detected metastases in lymph nodes that were inaccessible to mediastinoscopy, which reinforces the importance of test selection based on lymph-node distribution seen on noninvasive imaging.
Metastatic disease in MLNs that are <1 cm in short-axis length can also be detected by EUS-FNA.41,42 Although the sensitivity of this technique can be reduced in such small nodes,33 the detection of mediastinal metastases in posterior lymph nodes of this size on CT scan is an important finding. Since posterior lymph nodes are inaccessible to standard mediastinoscopy, these patients would otherwise have undergone futile thoracotomy.
In patients with PET-positive (FDG-avid) mediastinal lymphadenopathy, EUS-FNA has high sensitivity for the diagnosis of metastases.43,44 In one study, EUS-FNA of PET-positive nodes was a cost-effective strategy that reduced the need for surgical staging procedures by more than 50%, which saved 40% of staging costs.44 Studies that compared the performance of CT, PET and EUS-FNA as staging modalities concluded that EUS-FNA gave the most accurate results.45,46 However, the high negative predictive value of combined CT and PET meant that routine use of preoperative EUS-FNA in this group of patients was not justified.47
EUS-FNA represents an important advance in the accurate mediastinal staging of NSCLC. It is a particularly useful method for sampling posterior or left-sided MLNs, regardless of their size. However, issues of utilization, cost, availability, training and expertise in this procedure remain. A recent survey in the US indicated that over 60% of oncologists felt that EUS would not improve staging of NSCLC.48 Furthermore, in centers where EUS was available, less than 20% of oncologists would employ it for lung-cancer staging. Incorporation of EUS-FNA into guidelines for lung-cancer staging9,13 and increased use of a multi-disciplinary approach to lung cancer management should encourage the use of EUS-FNA in clinical practice.
Endobronchial ultrasound
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is a new and minimally invasive technique that represents a significant advance in the mediastinal staging of NSCLC. The procedure was initially performed by placing a catheter with a radial ultrasound mini-probe at the tip, in the working channel of a bronchoscope. When the lymph node to be sampled had been located, the catheter was withdrawn and replaced with a TBNA needle. The lymph node was then sampled blindly with TBNA. Recently, an integrated, curvilinear, ultrasound bronchoscope has been developed. This device allows TBNA to be performed with a 22-gauge needle under real-time ultrasonographic guidance (Figure 3), which has superseded the radial mini-probe technique. These technological advances allow the pulmonologist and thoracic surgeon for the first time to sample the mediastinum in a minimally invasive manner with high sensitivity.49
Figure 3.
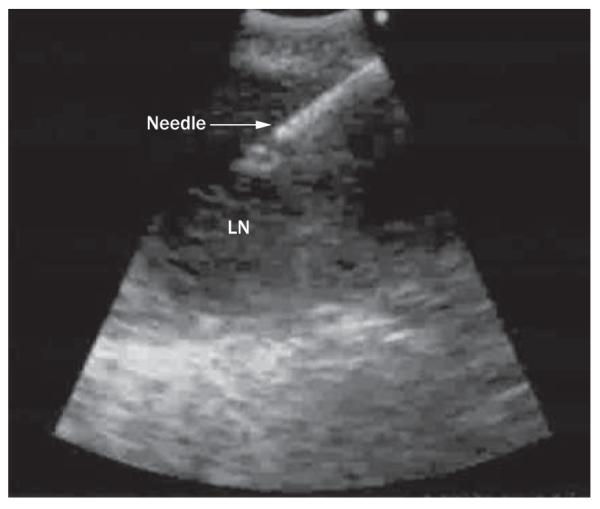
An ultrasonographic image obtained during endobronchial ultrasound-guided transbronchial needle aspiration. Abbreviation: LN, lymph node.
Lymph nodes at stations 1, 2, 3, 4, 7, 10 and 11 are readily accessible to this technique, an increased range compared with standard mediastinoscopy. EBUS-TBNA routinely provides samples from the posterior subcarinal space and hilar areas that are out of reach of cervical mediastinoscopy. However, randomized studies that compared mini-probe ultrasound-guided TBNA with the blind sampling technique50,51 have produced conflicting results. One such study suggested that the diagnostic yield of mini-probe EBUS was similar to that of blind TBNA,50 whereas another trial concluded that mini-probe EBUS had a significantly improved diagnostic yield.51 This latter study noted a benefit of mini-probe EBUS versus blind TBNA in all lymph node stations except in the subcarinal region. However, the trial may not have been sufficiently powered to detect a small improvement in yield from enlarged subcarinal glands, a region in which blind TBNA traditionally has a high yield. Importantly, data now exist that confirm the theoretical benefit of real-time linear EBUS-TBNA over the blind method. A single-center study conducted in the US prospectively examined 138 consecutive patients with suspected or proven lung cancer, 30% of whom had mediastinal metastases.52 Each patient sequentially underwent blind TBNA, EBUS-TBNA and EUS-FNA. The study demonstrated that linear real-time EBUS-TBNA had a significantly superior sensitivity over standard TBNA in the detection of mediastinal disease (69% versus 36%).52
To date, no completed randomized trials have investigated the effects of EBUS-TBNA on patients’ outcomes and health-care costs. The published study with the largest cohort of patients included 502 individuals from Germany, the US and Denmark,53 in whom EBUS-TBNA had a sensitivity of 93.5%. Meta-analysis of the diagnostic accuracy of EBUS-TBNA in 918 patients treated in expert centers has demonstrated a pooled sensitivity of 90%, with a false-negative rate of 20% when the disease prevalence was 68%.13 The analysis did, however, include 87 patients who had mini-probe-guided TBNA. No complications of EBUS-TBNA have been reported.
Given the high diagnostic accuracy of EBUS-TBNA, the logical approach is to compare it to the gold standard of mediastinoscopy. One study suggested that EBUS-TBNA is superior to mediastinoscopy for cancer staging in patients with enlarged MLNs.54 This study included 66 consecutive patients in whom lung cancer was suspected on the basis of clinical and CT findings. All patients had enlarged mediastinal adenopathy (≥10 mm short-axis diameter) confined to lymph-node stations 2, 4 or 7; PET data were not available. Cervical mediastinoscopy and EBUS-TBNA were performed in all patients. The diagnostic accuracy (the combined yield of true-positive and true-negative results) of EBUS-FNA was 91%, which was significantly better than that of mediastinoscopy (78%). However, the observed difference was due to the superiority of EBUS-FNA over mediastinoscopy for subcarinal nodes and may be explained by the fact that posterior subcarinal nodes are beyond the reach of standard mediastinoscopy.54 The conclusion that EBUS-TBNA may have a superior yield to cervical mediastinoscopy requires confirmation in other studies and randomized trials.
False-negative findings in invasive mediastinal tests (EBUS-TBNA, EUS-FNA and mediastinoscopy) may result from either limitations of mediastinal access or sampling error within a lymph node. For mediastinoscopy, only restricted MLN stations can be accessed. EBUS-TBNA enables greater mediastinal (and hilar) access than mediastinoscopy but relies on needle aspiration, which can lead to sampling errors within a MLN. up to 25% of malignant MLNs contain metastatic disease in the marginal area of the lymph node only,55 which corresponds closely to the false-negative rate observed in clinical studies. Consequently, negative EBUS-TBNA and EUS-FNA results (including adequate samples) should be investigated further, by surgical staging.
EBUS-TBNA: specific groups of patients
As with EUS-FNA, EBUS-TBNA has been evaluated in specific groups of patients. A Belgian study looked at 102 patients with NSCLC and FDG-avid MLNs.56 The researchers found a prevalence of mediastinal disease of 58% in this cohort; EBUS-TBNA had a sensitivity of 95% and a negative predictive value of 91%. In 59 cases, use of EBUS-TBNA meant that mediastinoscopy need not be performed; this technique was, therefore, a highly effective initial alternative to mediastinoscopy in patients with PET-positive MLNs. Herth et al.57 examined 100 patients who had a normal mediastinum on CT scan. Current guidelines do not advocate the use of ultrasound-guided MLN aspiration in patients whose nodes are <1 cm in short-axis diameter.13 However, in this study, mediastinal metastasis was detected in 1 in 6 patients, with a sensitivity of 92% and negative predictive value of 96%. Furthermore, EBUS-TBNA detected malignancy in 9% of MLNs that were <1 cm in short-axis diameter and PET-negative, with a sensitivity of 89%.58 Currently, patients without MLN enlargement who have negative PET findings are offered curative surgery or radical radiotherapy in the absence of proven extrathoracic disease. These findings argue that EBUS-TBNA can detect MLN metastases in such patients. This technique might, therefore, have an important role in the preoperative assessment of NSCLC, and might prevent futile thoracotomies.
In the absence of randomized studies and cost-effectiveness data, and given the variable diagnostic yield associated with blind TBNA, the incorporation of EBUS-TBNA into international diagnostic and staging algorithms for NSCLC has been slow, despite its advantages over other staging modalities (Table 1). Rintoul et al.59 published their experience of their first 20 cases of NSCLC staged with this technique and achieved results in line with those of expert centers. In the US and UK, issues surrounding reimbursement60 and the lack of a specific National Health Service tariff,61 respectively, may hinder the use of EBUS-TBNA. However, the emerging data on use of EBUS-TBNA and EUS-FNA for MLN staging do reveal a significant advance for patients with NSCLC and for the multidisciplinary team charged with their care.62
Combined EUS-FNA and EBUS-TBNA
EUS and EBUS provide complementary access to the entire mediastinum with the exception of station 6 MLNs. Both techniques used in combination can access mediastinal stations beyond the scope of mediastinoscopy. Several centers have employed combined EUS-FNA and EBUS-TBNA in the same sitting for minimally invasive mediastinal staging of NSCLC.52,59,63 Initial results are encouraging. In one study of 33 patients, 31 were able to undergo both procedures sequentially under the same sedation.63 EBUS-TBNA provided additional information to that obtained by EUS-FNA and vice versa. The combined procedure had 100% sensitivity for detecting mediastinal metastases and, importantly, was well tolerated. EUS-FNA can detect extrathoracic disease whereas EBUS enables visualization of the endobronchial tree; therefore, use of both techniques in combination is a very attractive prospect. A European randomized trial to compare a combined EBUS-TBNA and EUS-FNA procedure versus mediastinoscopy is underway. Questions remain, however, over the widespread applicability of this approach. Resources and expertise in EUS-FNA and EBUS-TBNA are currently limited, particularly outside the US. A further consideration is the health-care costs of this combined procedure, particularly when compared with a radiological or PET-guided approach to mediastinal staging.
A new algorithm for NSCLC
Studies of EBUS-TBNA and EUS-FNA have demonstrated that the application of these techniques may reduce the need for surgical staging procedures. Some authors have suggested that EBUS-TBNA and EUS-FNA should be included in the diagnosis and staging algorithm for NSCLC only as a prelude to mediastinoscopy.64 However, these techniques enable detection of metastases in the radiologically normal mediastinum,41,42,57 and with higher accuracy than other staging investigations.58 In addition, as the data on PET matures, questions over its diagnostic accuracy (particularly in enlarged lymph nodes) are being raised.12 Minimally invasive sampling of the mediastinum has the added benefit that it might provide a tissue diagnosis and disease staging that can guide treatment in a single test. Owing to their high sensitivity and specificity as well as their ability to detect mediastinal metastases that are missed by other modalities, EBUS-TBNA or EUS-FNA may be considered a first-line test for evaluation of patients with suspected NSCLC and intrathoracic disease.
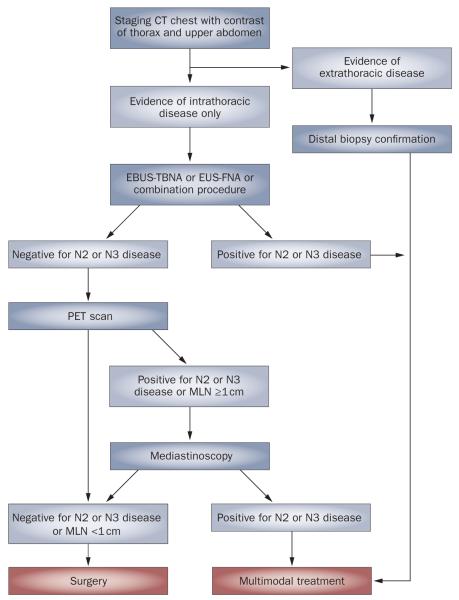
We propose a diagnostic and staging algorithm that utilizes EBUS-TBNA, EUS-FNA or the combined EBUS–EUS approach, where available, as a first diagnostic test for patients with intrathoracic disease (Figure 4). When combined EBUS–EUS cannot be used, a targeted approach to MLN sampling can be employed. This approach may be based on the identification of enlarged lymph nodes seen on CT scan or on selection of the MLNs that primary NSCLCs are most likely to spread to. Patients with enlarged lymph nodes on CT in posterior MLN stations should undergo EUS-FNA as a first test. Those with paratracheal or subcarinal lymphadenopathy whose MLNs measure ≥1 cm in short-axis diameter may benefit from first-line EBUS-TBNA.
Figure 4.
Novel diagnostic and staging algorithm for patients with suspected non-small-cell lung cancer, which incorporates endobronchial ultrasound-guided transbronchial needle aspiration, endoscopic ultrasound-guided fine-needle aspiration or the combined procedure as a first test after CT. Abbreviations: EBUS-TBNA, endobronchial ultrasound-guided transbrachial needle aspiration; EUS-FNA, endoscopic ultrasound-guided fine-needle aspiration; MLN, mediastinal lymph node.
Patients assessed as having clinical stage 1 disease on the basis of CT findings do not have any enlarged MLNs. However, as discussed above, when a cut-off of 1 cm in short-axis diameter is used to assess MLN enlargement, CT has a sensitivity as low as 51%.3 EBUS-TBNA and EUS-FNA detect MLN metastases missed by CT with a sensitivity of 61–92%,41,57 without the need for prior PET scan or subsequent mediastinoscopy. Studies have demonstrated that metastatic disease in patients with NSCLC has a predilection for certain MLN locations based on the site of the primary tumor.65-67 Table 2 summarizes the most common pattern of MLN metastases from a known or presumed primary NSCLC and the most appropriate minimally invasive technique for diagnosis of N2 disease, when the combined approach is not available. When the expected pattern of MLN metastasis is used to guide the primary investigation, its diagnostic yield can be maximized.
Table 2.
Patterns of mediastinal lymph-node metastasisa
| Site of primary lung tumor |
Most common site(s) of MLN metastasis |
Minimally invasive technique for MLN diagnosis |
|---|---|---|
| Right upper lobe | 4R | EBUS-TBNA |
| Right middle lobe | 4R, 7 | EBUS-TBNAb |
| Right lower lobe | 4R, 7 | EBUS-TBNAb |
| Left upper lobe | 5, 6 | EUS-FNAc |
| Left lower lobe | 5, 6 | EUS-FNAc |
According to site of primary non-small-cell lung cancer and recommended primary investigation.
Posterior station 7 nodes may also be sampled by EUS-FNA.
EUS-FNA is not able to sample station 6 (para-aortic) MLNs and left video-assisted thoracic surgery or left anterior mediastinotomy may be preferred.
Abbreviations: EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; EUS-FNA, endoscopic ultrasound-guided fine-needle aspiration; MLN, mediastinal lymph node.
Currently available data suggest that this proposed diagnostic and staging algorithm would minimize futile thoracotomies. The proposed schema, however, does raise several important issues. The false-negative rate of EBUS-TBNA and EUS-FNA demands that negative results for mediastinal aspiration should be investigated further. In the new schema, if a subsequent PET scan of the MLN is negative and it is <1 cm in short-axis diameter, surgical staging is not justified, owing to the high negative predictive value of PET in this situation.47 In the absence of extrathoracic disease thoracotomy can be offered. First-line EBUS-TBNA or EUS-FNA sampling could provide a tissue diagnosis and staging information in single investigation. If either technique detects contralateral mediastinal disease (or extrathoracic disease in the case of EUS-FNA) then surgery is clearly precluded. However, controversy still persists over the optimal management of patients with proven, single-station, N2 disease. If radical treatment is proposed with concurrent chemoradiotherapy, radical radiotherapy or neoadjuvant therapy then systemic staging with PET scan is still required. Many centers also employ PET for radiotherapy planning. A further consideration that requires evaluation is the health-care costs of the new schema.
Future directions
Large, multicenter, randomized, controlled trials of EBUS-TBNA and further randomized studies of EUS-FNA are required to address the questions of whether futile thoracotomies can be avoided and health-care costs can be saved.
Restaging of the mediastinum after neoadjuvant treatment is currently an important concern because residual MLN metastasis is the most important preoperative factor that determines survival in patients who undergo neoadjuvant treatment.68 Repeat mediastinoscopy remains a challenging procedure, owing to adhesions induced by the initial operation, and is performed by a limited number of thoracic surgeons.69 EBUS-TBNA and EUS-FNA provide a new approach that may have an important application in this area since these procedures can easily be repeated in the same patient, or following mediastinoscopy. A preliminary study of 124 patients with proven stage IIIA–N2 disease who underwent mediastinal restaging with EBUS-TBNA and then thoracotomy has been reported. EBUS-TBNA had a sensitivity of 76% in this setting.70 Another study of EUS-FNA in MLN staging of 11 patients demonstrated 86% accuracy.71 The alternative strategy of a minimally invasive initial staging procedure followed by mediastinoscopy after induction therapy warrants investigation.
With the prospect of future treatments being tailored to an individual’s cancer,72,73 EBUS-TBNA and EUS-FNA must demonstrate that the cytological samples they produce can provide sufficient information to allow molecular staging. Cytology might distinguish squamous-cell carcinoma from other NSCLCs and, therefore, preclude the use of bevacizumab. Preliminary results suggest that detection of EGFR mutations in EBUS-TBNA samples is possible.74 Overexpression of the EPCAM gene (formerly termed KS1/4), which is associated with lung cancer, has been detected by RT-PCR in EUS-FNA MLN samples.75 Confirmatory data and other translational studies are required before biomarkers detected in mediastinal aspiration samples can be used in clinical practice.
The use of PET to stage disease in patients with NSCLC may have resulted in stage migration and an apparent improvement in survival, without affecting the natural history of this disease. For example, PET results might result in re-staging of patients initially considered to have stage III disease to stage IV, which would improve survival in both groups.76 By improving the identification of patients with operable disease, EBUS-TBNA and EUS-FNA may improve long-term survival of patients with stage I and stage II disease.
Conclusions
Minimally invasive MLN staging with EUS-FNA and EBUS-FNA are important advances for the staging of NSCLC. Data published over the past few years have provided evidence that these techniques are safe and highly sensitive and might reduce the number of surgical staging procedures required. The challenge now exists to determine their optimal role in the diagnostic and staging algorithm for NSCLC and to test the hypothesis that these techniques improve the accuracy of selection of surgical candidates.
Key points.
Current radiological and surgical techniques for mediastinal staging of non-small-cell lung cancer (NSCLC) do not identify all mediastinal metastases and result in futile thoracotomies
Mediastinoscopy is the current gold standard for mediastinal staging of NSCLC but cannot access the entire mediastinum and has a sensitivity of 78%
Endoscopic ultrasound allows minimally invasive sampling of posterior and left-sided mediastinal lymph nodes with a sensitivity of 84%
Endobronchial ultrasound is a new technique that allows staging of parabronchial lymph nodes with a sensitivity of 90%
Combined endoscopic and endobronchial ultrasound allows sampling of almost the entire mediastinum and may replace mediastinoscopy as the gold-standard investigation of mediastinal lymph nodes in patients with NSCLC
Review criteria.
The information for this Review was compiled by searching the PubMed and MEDLINE databases for articles published before 14 July 2008. Electronic early-release publications were also included. Only articles published in English were considered. The following search terms were used “endobronchial ultrasound”, “endoscopic ultrasound”, “lung-cancer staging”, “mediastinoscopy”, “positron emission tomography”, “PET-CT” and “mediastinal staging”. Full articles were obtained and references were checked for additional material, as appropriate. References were chosen on the basis of the highest quality clinical evidence.
Footnotes
Competing interests
The authors declared no competing interests.
Contributor Information
Neal Navani, Centre for Respiratory Research, University College London, UK..
Stephen G. Spiro, Department of Thoracic Medicine, University College London Hospital, London, UK.
Sam M. Janes, Centre for Respiratory Research, University College London, UK.
References
- 1.Herder GJ, et al. Practice, efficacy and cost of staging suspected non-small cell lung cancer: a retrospective study in two Dutch hospitals. Thorax. 2002;57:11–14. doi: 10.1136/thorax.57.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Tinteren H, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet. 2002;359:1388–1393. doi: 10.1016/s0140-6736(02)08352-6. [DOI] [PubMed] [Google Scholar]
- 3.silvestri GA, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidence-based clinical practice guidelines (2nd edn) Chest. 2007;132:178S–201S. doi: 10.1378/chest.07-1360. [DOI] [PubMed] [Google Scholar]
- 4.Kerr KM, et al. Pathological assessment of mediastinal lymph nodes in lung cancer: implications for non-invasive mediastinal staging. Thorax. 1992;47:337–341. doi: 10.1136/thx.47.5.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLoud TC, et al. Bronchogenic carcinoma: analysis of staging in the mediastinum with CT by correlative lymph node mapping and sampling. Radiology. 1992;182:319–323. doi: 10.1148/radiology.182.2.1732943. [DOI] [PubMed] [Google Scholar]
- 6.Cerfolio RJ, et al. The maximum standardized uptake values on positron emission tomography of a non-small cell lung cancer predict stage, recurrence and survival. J. Thorac. Cardiovasc. Surg. 2005;130:151–159. doi: 10.1016/j.jtcvs.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Hoekstra CJ, et al. Prognostic relevance of response evaluation using 18F-2-fluoro-2-deoxy-d-glucose positron emission tomography in patients with locally advanced non-small-cell lung cancer. J. Clin. Oncol. 2005;23:8362–8370. doi: 10.1200/JCO.2005.01.1189. [DOI] [PubMed] [Google Scholar]
- 8.National Institute for Health and Clinical Excellence GC24 Lung Cancer: full guideline. 2005 [online]. http://www.nice.org.uk/Guidance/CG24/Guidance/
- 9.De Leyn P, et al. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur. J. Cardiothorac. Surg. 2007;32:1–8. doi: 10.1016/j.ejcts.2007.01.075. [DOI] [PubMed] [Google Scholar]
- 10.Fischer BM, et al. Positron emission tomography in the diagnosis and staging of lung cancer: a systematic, quantitative review. Lancet Oncol. 2002;2:659–666. doi: 10.1016/S1470-2045(01)00555-1. [DOI] [PubMed] [Google Scholar]
- 11.Gould MK, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann. Intern. Med. 2003;139:879–892. doi: 10.7326/0003-4819-139-11-200311180-00013. [DOI] [PubMed] [Google Scholar]
- 12.Al Sarraf N, et al. Lymph node staging by means of positron emission tomography is less accurate in non-small cell lung cancer patients with enlarged lymph nodes: analysis of 1,145 lymph nodes. Lung Cancer. 2008;60:62–68. doi: 10.1016/j.lungcan.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 13.Detterbeck FC, et al. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edn) Chest. 2007;132:202S–220S. doi: 10.1378/chest.07-1362. [DOI] [PubMed] [Google Scholar]
- 14.Hishida T, et al. Problems in the current diagnostic standards of clinical N1 non-small cell lung cancer. Thorax. 2008;63:526–531. doi: 10.1136/thx.2006.062760. [DOI] [PubMed] [Google Scholar]
- 15.Wang KP, et al. Bronchoscopic needle aspiration biopsy of paratracheal tumors. Am. Rev. Respir. Dis. 1978;118:17–21. doi: 10.1164/arrd.1978.118.1.17. [DOI] [PubMed] [Google Scholar]
- 16.Ernst A, et al. Interventional pulmonary procedures: guidelines from the American College of Chest Physicians. Chest. 2003;123:1693–1717. doi: 10.1378/chest.123.5.1693. [DOI] [PubMed] [Google Scholar]
- 17.Bernasconi M, et al. Combined transbronchial needle aspiration and positron emission tomography for mediastinal staging of NSCLC. Eur. Respir. J. 2006;27:889–894. doi: 10.1183/09031936.06.00125605. [DOI] [PubMed] [Google Scholar]
- 18.Holty JE, et al. Accuracy of transbronchial needle aspiration for mediastinal staging of non-small cell lung cancer: a meta-analysis. Thorax. 2005;60:949–955. doi: 10.1136/thx.2005.041525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baram D, et al. Impact of rapid on-site cytologic evaluation during transbronchial needle aspiration. Chest. 2005;128:869–875. doi: 10.1378/chest.128.2.869. [DOI] [PubMed] [Google Scholar]
- 20.Garpestad E, et al. CT fluoroscopy guidance for transbronchial needle aspiration: an experience in 35 patients. Chest. 2001;119:329–332. doi: 10.1378/chest.119.2.329. [DOI] [PubMed] [Google Scholar]
- 21.Chin R, et al. Transbronchial needle aspiration in diagnosing and staging lung cancer: how many aspirates are needed? Am. J. Respir. Crit. Care Med. 2002;166:377–381. doi: 10.1164/rccm.2106153. [DOI] [PubMed] [Google Scholar]
- 22.Hsu LH, et al. Education and experience improve the performance of transbronchial needle aspiration: a learning curve at a cancer center. Chest. 2004;125:532–540. doi: 10.1378/chest.125.2.532. [DOI] [PubMed] [Google Scholar]
- 23.Haponik EF, Shure D. Underutilization of transbronchial needle aspiration: experiences of current pulmonary fellows. Chest. 1997;112:251–253. doi: 10.1378/chest.112.1.251. [DOI] [PubMed] [Google Scholar]
- 24.Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997;111:1718–1723. doi: 10.1378/chest.111.6.1718. [DOI] [PubMed] [Google Scholar]
- 25.Coughlin M, et al. Role of mediastinoscopy in pretreatment staging of patients with primary lung cancer. Ann. Thorac. Surg. 1985;40:556–560. doi: 10.1016/s0003-4975(10)60348-7. [DOI] [PubMed] [Google Scholar]
- 26.Little AG, et al. Patterns of surgical care of lung cancer patients. Ann. Thorac. Surg. 2005;80:2051–2056. doi: 10.1016/j.athoracsur.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 27.Smulders SA, et al. Surgical mediastinal staging in daily practice. Lung Cancer. 2005;47:243–251. doi: 10.1016/j.lungcan.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 28.Silvestri GA, et al. Endoscopic ultrasound with fine-needle aspiration in the diagnosis and staging of lung cancer. Ann. Thorac. Surg. 1996;61:1441–1445. doi: 10.1016/0003-4975(95)00052-6. [DOI] [PubMed] [Google Scholar]
- 29.Wittmann J, et al. Endoscopic ultrasound-guided tissue sampling by combined fine needle aspiration and Tru-Cut needle biopsy: a prospective study. Cytopathology. 2006;17:27–33. doi: 10.1111/j.1365-2303.2006.00313.x. [DOI] [PubMed] [Google Scholar]
- 30.Tounoy KG, et al. Esophageal endoscopic ultrasound with fine-needle aspiration with an on-site cytopathologist: high accuracy for the diagnosis of mediastinal lymphadenopathy. Chest. 2005;128:3004–3009. doi: 10.1378/chest.128.4.3004. [DOI] [PubMed] [Google Scholar]
- 31.Wallace MB, et al. Randomized controlled trial of EUS-guided fine needle aspiration techniques for the detection of malignant lymphadenopathy. Gastrointest. Endosc. 2001;54:441–447. doi: 10.1067/mge.2001.117764. [DOI] [PubMed] [Google Scholar]
- 32.LeBlanc JK, et al. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest. Endosc. 2004;59:475–481. doi: 10.1016/s0016-5107(03)02863-3. [DOI] [PubMed] [Google Scholar]
- 33.Micames CG, et al. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: A systematic review and meta-analysis. Chest. 2007;131:539–548. doi: 10.1378/chest.06-1437. [DOI] [PubMed] [Google Scholar]
- 34.Tournoy KG, et al. Endoscopic ultrasound reduces surgical mediastinal staging in lung cancer: a randomized trial. Am. J. Respir. Crit. Care Med. 2008;177:531–535. doi: 10.1164/rccm.200708-1241OC. [DOI] [PubMed] [Google Scholar]
- 35.Larsen SS, et al. Endoscopic ultrasound guided biopsy performed routinely in lung cancer staging spares futile thoracotomies: preliminary results from a randomised clinical trial. Lung Cancer. 2005;49:377–385. doi: 10.1016/j.lungcan.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Van den Bruel A, et al. The evaluation of diagnostic tests: evidence on technical and diagnostic accuracy, impact on patient outcome and cost-effectiveness is needed. J. Clin. Epidemiol. 2007;60:1116–1122. doi: 10.1016/j.jclinepi.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Bossuyt PM, et al. Randomised comparisons of medical tests: sometimes invalid, not always efficient. Lancet. 2000;356:1844–1847. doi: 10.1016/S0140-6736(00)03246-3. [DOI] [PubMed] [Google Scholar]
- 38.Singh P, et al. Endoscopic ultrasound as a first test for diagnosis and staging of lung cancer: a prospective study. Am. J. Respir. Crit. Care Med. 2007;175:345–354. doi: 10.1164/rccm.200606-851OC. [DOI] [PubMed] [Google Scholar]
- 39.Annema JT, et al. Endoscopic ultrasound added to mediastinoscopy for preoperative staging of patients with lung cancer. JAMA. 2005;294:931–936. doi: 10.1001/jama.294.8.931. [DOI] [PubMed] [Google Scholar]
- 40.Eloubeidi MA, et al. Endoscopic ultrasound-guided fine-needle aspiration in patients with non-small cell lung cancer and prior negative mediastinoscopy. Ann. Thorac. Surg. 2005;80:1231–1239. doi: 10.1016/j.athoracsur.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Wallace MB, et al. Endoscopic ultrasound in lung cancer patients with a normal mediastinum on computed tomography. Ann. Thorac. Surg. 2004;77:1763–1768. doi: 10.1016/j.athoracsur.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 42.LeBlanc JK, et al. Endoscopic ultrasound in non-small cell lung cancer and negative mediastinum on computed tomography. Am. J. Respir. Crit. Care Med. 2005;171:177–182. doi: 10.1164/rccm.200405-581OC. [DOI] [PubMed] [Google Scholar]
- 43.Annema JT, et al. Towards a minimally invasive staging strategy in NSCLC: analysis of PET positive mediastinal lesions by EUS-FNA. Lung Cancer. 2004;44:53–60. doi: 10.1016/j.lungcan.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 44.Kramer H, et al. Oesophageal endoscopic ultrasound with fine needle aspiration improves and simplifies the staging of lung cancer. Thorax. 2004;59:596–601. doi: 10.1136/thx.2003.018028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eloubeidi MA, et al. Endoscopic ultrasound-guided fine needle aspiration of mediastinal lymph node in patients with suspected lung cancer after positron emission tomography and computed tomography scans. Ann. Thorac. Surg. 2005;79:263–268. doi: 10.1016/j.athoracsur.2004.06.089. [DOI] [PubMed] [Google Scholar]
- 46.Fritscher-Ravens A, et al. Mediastinal lymph node involvement in potentially resectable lung cancer: comparison of CT, positron emission tomography, and endoscopic ultrasonography with and without fine-needle aspiration. Chest. 2003;123:442–451. doi: 10.1378/chest.123.2.442. [DOI] [PubMed] [Google Scholar]
- 47.Cerfolio RJ, et al. Routine mediastinoscopy and esophageal ultrasound fine-needle aspiration in patients with non-small cell lung cancer who are clinically N2 negative: a prospective study. Chest. 2006;130:1791–1795. doi: 10.1378/chest.130.6.1791. [DOI] [PubMed] [Google Scholar]
- 48.Reddy NK, et al. Knowledge of indications and utilization of EUS: a survey of oncologists in the United States. J. Clin. Gastroenterol. 2008;42:892–896. doi: 10.1097/MCG.0b013e3180cab11a. [DOI] [PubMed] [Google Scholar]
- 49.Krasnik M, et al. Preliminary experience with a new method of endoscopic transbronchial real time ultrasound guided biopsy for diagnosis of mediastinal and hilar lesions. Thorax. 2003;58:1083–1086. doi: 10.1136/thorax.58.12.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shannon JJ, et al. Endobronchial ultrasound-guided needle aspiration of mediastinal adenopathy. Am. J. Respir. Crit. Care Med. 1996;153:1424–1430. doi: 10.1164/ajrccm.153.4.8616576. [DOI] [PubMed] [Google Scholar]
- 51.Herth F, et al. Conventional vs endobronchial ultrasound-guided transbronchial needle aspiration: a randomized trial. Chest. 2004;125:322–325. doi: 10.1378/chest.125.1.322. [DOI] [PubMed] [Google Scholar]
- 52.Wallace MB, et al. Minimally invasive endoscopic staging of suspected lung cancer. JAMA. 2008;299:540–546. doi: 10.1001/jama.299.5.540. [DOI] [PubMed] [Google Scholar]
- 53.Herth FJ, et al. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax. 2006;61:795–798. doi: 10.1136/thx.2005.047829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ernst A, et al. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J. Thorac. Oncol. 2008;3:577–582. doi: 10.1097/JTO.0b013e3181753b5e. [DOI] [PubMed] [Google Scholar]
- 55.Kurimoto N, et al. Targeting area in metastatic lymph nodes in lung cancer for endobronchial ultrasonography-guided transbronchial needle aspiration. J. Bronchol. 2008;15:134–138. [Google Scholar]
- 56.Bauwens O, et al. Endobronchial ultrasound and value of PET for prediction of pathological results of mediastinal hot spots in lung cancer patients. Lung Cancer. 2008;61:356–361. doi: 10.1016/j.lungcan.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 57.Herth FJ, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically normal mediastinum. Eur. Respir. J. 2006;28:910–914. doi: 10.1183/09031936.06.00124905. [DOI] [PubMed] [Google Scholar]
- 58.Herth FJ, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically and positron emission tomography-normal mediastinum in patients with lung cancer. Chest. 2008;133:887–891. doi: 10.1378/chest.07-2535. [DOI] [PubMed] [Google Scholar]
- 59.Rintoul RC, et al. Endobronchial and endoscopic ultrasound-guided real-time fine-needle aspiration for mediastinal staging. Eur. Respir. J. 2005;25:416–421. doi: 10.1183/09031936.05.00095404. [DOI] [PubMed] [Google Scholar]
- 60.Sheski FD, Mathur PN. Endobronchial ultrasound. Chest. 2008;133:264–270. doi: 10.1378/chest.06-1735. [DOI] [PubMed] [Google Scholar]
- 61.Callister ME, et al. Endobronchial ultrasound guided transbronchial needle aspiration of mediastinal lymph nodes for lung cancer staging: a projected cost analysis. Thorax. 2008;63:384. doi: 10.1136/thx.2007.090308. [DOI] [PubMed] [Google Scholar]
- 62.Janes SM, Spiro SG. Esophageal endoscopic ultrasound/endobronchial ultrasound-guided fine needle aspiration: a new dawn for the respiratory physician? Am. J. Respir. Crit. Care Med. 2007;175:297–299. doi: 10.1164/rccm.200609-1390ED. [DOI] [PubMed] [Google Scholar]
- 63.Vilmann P, et al. Transesophageal endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) biopsy: a combined approach in the evaluation of mediastinal lesions. Endoscopy. 2005;37:833–839. doi: 10.1055/s-2005-870276. [DOI] [PubMed] [Google Scholar]
- 64.Yasufuku K, Fujisawa T. staging and diagnosis of non-small cell lung cancer: invasive modalities. Respirology. 2007;12:173–183. doi: 10.1111/j.1440-1843.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- 65.Cerfolio RJ, et al. Distribution and likelihood of lymph node metastasis based on the lobar location of non-small-cell lung cancer. Ann. Thorac. Surg. 2006;81:1969–1973. doi: 10.1016/j.athoracsur.2005.12.067. [DOI] [PubMed] [Google Scholar]
- 66.Naruke T, et al. Lymph node sampling in lung cancer: how should it be done? Eur. J. Cardiothorac. Surg. 1999;16:S17–S24. doi: 10.1016/s1010-7940(99)00178-5. [DOI] [PubMed] [Google Scholar]
- 67.Kotoulas CS, et al. Involvement of lymphatic metastatic spread in non-small cell lung cancer according to the primary cancer location. Lung Cancer. 2004;44:183–191. doi: 10.1016/j.lungcan.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 68.Sawabata N, et al. The impact of residual multilevel N2 disease after induction therapy for non-small cell lung cancer. Lung Cancer. 2003;42:69–77. doi: 10.1016/s0169-5002(03)00245-9. [DOI] [PubMed] [Google Scholar]
- 69.De Leyn P, et al. Prospective comparative study of integrated positron emission tomography-computed tomography scan compared with remediastinoscopy in the assessment of residual mediastinal lymph node disease after induction chemotherapy for mediastinoscopy-proven stage IIIA-N2 non-small-cell lung cancer: a Leuven Lung Cancer Group Study. J. Clin. Oncol. 2006;24:3333–3339. doi: 10.1200/JCO.2006.05.6341. [DOI] [PubMed] [Google Scholar]
- 70.Herth FJ, et al. Endobronchial ultrasound with transbronchial needle aspiration for restaging the mediastinum in lung cancer. J. Clin. Oncol. 2008;26:3346–3350. doi: 10.1200/JCO.2007.14.9229. [DOI] [PubMed] [Google Scholar]
- 71.Varadarajulu S, Eloubeidi M. Can endoscopic ultrasonography-guided fine-needle aspiration predict response to chemoradiation in non-small cell lung cancer? A pilot study. Respiration. 2006;73:213–220. doi: 10.1159/000091533. [DOI] [PubMed] [Google Scholar]
- 72.Olaussen KA, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N. Engl. J. Med. 2006;355:983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 73.Lynch TJ, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 74.Nakajima T, et al. Assessment of epidermal growth factor receptor mutation by endobronchial ultrasound-guided transbronchial needle aspiration. Chest. 2007;132:597–602. doi: 10.1378/chest.07-0095. [DOI] [PubMed] [Google Scholar]
- 75.Wallace MB, et al. Accurate molecular detection of non-small cell lung cancer metastases in mediastinal lymph nodes sampled by endoscopic ultrasound-guided needle aspiration. Chest. 2005;127:430–437. doi: 10.1378/chest.127.2.430. [DOI] [PubMed] [Google Scholar]
- 76.Chee KG, et al. Positron emission tomography and improved survival in patients with lung cancer: the Will Rogers phenomenon revisited. Arch. Intern. Med. 2008;168:1541–1549. doi: 10.1001/archinte.168.14.1541. [DOI] [PubMed] [Google Scholar]