Abstract
West Nile virus encephalomyelitis was diagnosed in 28 horses presented to the Ontario Veterinary College Veterinary Teaching Hospital between August 20 and October 15, 2002. The age range of affected horses was 5 months to 20 years (mean 6.9 years, median 6 years). Clinical signs were highly variable. Duration of hospitalization ranged from < 1 to 12 days (mean 5 days, median 5.4 days). Overall, 16 of the 28 (57%) horses were discharged and, of the 14 from which follow-up information was available, 13 (93%) were reported to be clinically normal 4 to 6 weeks following discharge, while the other horse had markedly improved. This pathogen is emerging as an important cause of neurological disease in Canada.
Introduction
West Nile virus (WNV) is an arthropod-borne Flavivirus of the Japanese encephalitis virus serocomplex (1). This serocomplex includes St. Louis encephalitis virus, Murray Valley encephalitis virus, Kunjin virus, and Japanese encephalitis virus. West Nile virus was first isolated from a woman in Uganda in 1937 (2). While found most commonly in Africa, the Middle East, and western Asia, this virus has occasionally been identified in outbreaks of disease in European countries (3,4,5,6,7). Human infection is often asymptomatic; however, meningitis, encephalitis, or meningoencephalitis may develop (8). Like some other members of the flaviviridae, the natural life cycle of WNV involves birds and mosquitoes (1,9). Birds are the reservoir hosts and may be clinically or asymptomatically infected. Transmission between birds is mainly via mosquitoes; however, other blood-sucking insects have also been implicated, and direct bird-to-bird transmission has been documented experimentally (10). Transmission of WNV to horses and humans is via mosquitoes, particularly those of the Culex genus (11,12,13). Horses, like humans, are ‘dead-end’ hosts and cannot transmit disease under normal circumstances. Cases of equine WNV encephalomyelitis have been reported in France, Egypt, Morocco, Israel, and Italy prior to 1999 (9,14,15,16,17).
Until September 1999, WNV had not been identified in the Western Hemisphere. At that time, WNV was identified as a cause of death of zoo and wild birds in New York State (18,19). Shortly thereafter, WNV-associated disease was diagnosed in humans and horses (19,20,21). By the end of 1999, 25 equine cases of WNV disease had been diagnosed in New York State, 9 (36%) of which died or were euthanized (21). Subsequently, 60 equine cases were confirmed in the United States in 2000 (21), and 738 were identified in 2001 (22). Unexpectedly, there was a dramatic increase in equine cases in North America in 2002, where, as of December 1, 14 358 cases had been reported (23). Similarly, there was a dramatic increase in human cases in the United States, with 62 cases reported in 1999, 20 in 2000, 64 in 2001, and 3737 in 2002 (22,24).
In Canada, WNV was first identified in a bird from Windsor-Essex, Ontario in August 2001 (25). Since then, WNV has frequently been identified in birds over a wide range of Canada, including Ontario, Nova Scotia, Quebec, Manitoba, and Saskatchewan (26). The first cases of equine WNV infection in Canada were reported in Manitoba in 2002.
Because 2002 was the 1st year in which cases of West Nile virus encephalomyelitis in the horse were identified in Canada, it is crucial that horses with an acute onset of neurological disease be tested for West Nile virus to better understand this emerging disease. This case series involves horses diagnosed with West Nile virus-associated neurological disease at the Ontario Veterinary College (OVC) in 2002.
Materials and methods
West Nile virus encephalomyelitis was considered in horses presented to the Large Animal Clinic at the OVC with an acute onset of neurological disease. Serum samples were submitted for WNV serum neutralization and immunoglobulin M (IgM) capture enzyme-linked immunoassay (ELISA) (Cornell University, Ithaca, New York). Other diagnostic tests, such as complete blood cell count, serum biochemical profile, serology for eastern and western equine encephalitis, cerebrospinal fluid (CSF) cytological analysis, CSF Western blot assay for equine protozoal encephalomyeltis, and equine herpesvirus 1 serology, were submitted at the discretion of the attending clinician. Horses that died or were euthanized were submitted to the University of Guelph Animal Health Laboratory for postmortem examination, which included gross and histological examination of the brain and spinal cord. Immunohistochemistry (IHC) for WNV antigen was performed on selected formalin-fixed sections from the central nervous system (CNS). After the paraffin had been removed from embedded 5-μm sections were rehydrated and treated with 3% hydrogen peroxide to block endogenous tissue peroxidases, and subsequently treated with proteinase K (DAKO Cytomation, Mississauga, Ontario) for 12 min at room temperature (RT). Following 15-min incubation with universal blocker (DAKO Cytomation), sections were incubated with rabbit polyclonal anti-WNV antiserum for 30 min at RT. Goat anti-rabbit immunoglobulin conjugated to a horseradish peroxidase-labelled polymer (EnVision HRP; DAKO Cytomation) was used as the secondary antibody, with 30-min incubation at RT. Nova Red (Vector Laboratories Canada, Burlington, Ontario) was used as chromogen, and tissues were counterstained with Harris hematoxylin (Fisher Scientific, Toronto, Ontario). Frozen samples of brainstem and spinal cord were also submitted to the Manitoba Agriculture and Food Laboratory for reverse-transcriptase polymerase chain reaction (RT-PCR) detection of WNV. Diagnostic criteria for WNV infection in horses with neurological disease included an elevated WNV serum neutralization and positive IgM capture ELISA (1:100 cut-off), positive IHC or RT-PCR on nervous tissue, or both (20). Additionally, a positive IgM capture ELISA and elevated WNV serum neutralization in CSF was considered diagnostic in the presence of neurological abnormalities. All horses that did not survive were tested for rabies.
Association of different clinical signs with outcome was performed using Fisher's exact test. A P-value of < 0.05 was considered significant for all comparisons.
Results
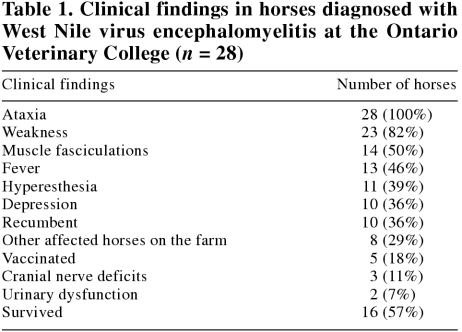
West Nile virus encephalomyelitis was diagnosed in 28 horses, ranging in age from 5 mo to 20 y (mean 6.9 y, median 6 y). Thoroughbreds and standardbreds were the most commonly represented breeds (13 and 9 cases, respectively), with a single case in each of Arabian, quarterhorse, Welsh pony, Appaloosa, Lippizaner, and mixed breed horses. Seventeen were female, while 6 were intact males and 5 were geldings. This approximates the case distribution normally encountered at this institution. The first case was admitted on August 20, 2002, and the final on October 15, 2002 (Figure 1). The geographic distribution of cases is displayed in Figure 2. Clinical signs were variable, ranging from weakness and fever to recumbency, coma, and seizure (Table 1). Additionally, a narcolepsy-like syndrome was observed in 2 horses, whereby the horses would collapse in response to any physical contact. One horse was markedly hyperesthetic and would react violently to any stimulus. Concurrent disease or evidence of immunosuppression was not reported in any case.
Figure 1. Temporal distribution of cases of West Nile virus encephalomyelitis in horses admitted to the Ontario Veterinary College (n = 28).
Figure 2. Geographic distribution of horses presented to the Ontario Veterinary College with West Nile virus-associated disease (n = 28).
Table 1.
Twenty-five of the 28 (89%) horses were diagnosed as WNV-positive on the basis of serological testing. Of these, 11 were also positive on the basis of IHC or RT-PCR. Serological testing was not performed on the other 3 horses; their diagnoses were based on IHC (n = 1), RT-PCR (n = 1), and detection of antibodies in CSF (n = 1). Cytological analysis of CSF was performed in 8 cases. An increased CSF cell count was present in 6 cases (0.021 to 0.229 × 109/L; reference range, 0 to 0.005 × 109/L); however, possible blood contamination, either iatrogenic or due to marked CNS inflammation, was reported in 3 (37.5%) of these cases, making interpretation of results somewhat difficult. Differential white blood cell counts were highly variable and included normal cell populations, marked suppurative inflammation, marked nonsuppurative inflammation, and lymphocytic pleocytosis. Mild to moderate increases in total protein were present in 3 (37.5%) samples (0.93 to 1.2 g/L; reference range, < 0.92 g/L). No other causes of neurological disease were identified in any cases diagnosed as WNV encephalomyelitis.
Supportive treatment and antiinflammatory drugs are considered to be the most important factor in management of equine WNV encephalomyelitis. Treatment was attempted in 26/28 cases; the other 2 horses were euthanized shortly after admission. Flunixin meglumine (1.1 mg/kg bodyweight (BW), IV, q12h) was administered to 25/26 (96%) cases, while 17 (65%) horses were treated with antiinflammatory doses of dexamethasone (0.05 to 0.1 mg/kg BW, IV, q24h for 3 d).
Overall, 12 (43%) horses died or were euthanized. Of the horses that were euthanized, 9/12 (75%) were recumbent on admission or became recumbent within 4 h of admission. Recumbency was the only clinical finding that was associated with a negative outcome (P = 0.006). Interestingly, the presence of muscle fasciculations was associated with survival (P = 0.0006).
Duration of hospitalization ranged from < 1 to 12 d (mean 5 d, median 4.5 d). Duration of hospitalization of horses that died or were euthanized ranged from < 1 to 5 d (mean 2.5 d, median 2 d).
Followup information was available for 14 horses. Of the horses that were discharged, 13 (93%) were reported to be normal by their owner or veterinarian 4 to 6 wk following discharge, while the remaining horse was reportedly markedly improved.
Five horses had been vaccinated against WNV virus; however, all developed disease within 2 wk of receiving a single dose of vaccine.
Discussion
Following identification of the first confirmed case of WNV encephalomyelitis in Ontario, a large number of cases were identified in a short period of time. This is consistent with reports from other jurisdictions. The age distribution of affected horses differs from that encountered in human cases of WNV, as has been reported elsewhere (27). The median age of affected humans in New York State in 2000 was 63 y, suggesting that older individuals are more prone to developing disease (8). The age distribution of horses in this study was similar to that normally encountered at this college, suggesting that there is no such age predisposition in horses.
Although clinical signs were extremely variable, WNV encephalomyelitis is more easily suspected when an animal shows signs of diffuse brain involvement. This includes alteration in demeanor or behavior, which can vary from depression, seizures or unusual narcoleptic episodes, muscle fasciculations (particularly facial muscles), and vestibular ataxia. In Ontario, the leading differential diagnoses would be rabies, head trauma, other arbovirus infections, equine herpesvirus type 1 myeloencephalopathy, and occasionally botulism. Equine protozoal encephalomyelitis (EPM) can be a consideration, but it rarely causes rapidly progressive symmetrical paresis, seizures, and subtle cranial nerve deficits. The most difficult cases to identify in the early stages are those with sudden onset of weakness (paresis) and general proprioceptive deficits (spinal ataxia), suggesting a focal spinal cord lesion. Obvious paresis and general proprioceptive ataxia can be expressed with brain stem lesions, but they are usually accompanied by depression and cranial nerve deficits. West Nile virus encephalomyelitis appears to be a rapidly progressive disease in which the signs can change within hours and yet stabilize within a few days. For example, 1 horse was pyrexic for the first 24 h, then developed severe spinal ataxia. After another 12 h, the horse was profoundly depressed and then developed a narcolepsy-like syndrome where it would collapse if touched. This then resolved and a predominantly frontlimb dysmetria was present, which gradually resolved.
Rapidly progressive paresis to recumbency indicates severe spinal cord involvement, which often resulted in euthanasia for humane reasons. This may explain, in part, the positive association between the presence of muscle fasciculations and survival. Muscle fasciculations may have indicated diffuse encephalitis, which seemed to correlate with a better prognosis compared with those having primary myelitis. Alternatively, muscle fasciculations may have been missed in some of the more severe cases that were difficult to examine in safety or that were heavily sedated shortly after admission. Cytological evaluation of CSF was not useful for diagnosis of WNV infection. This is consistent with a previous report where CSF cytologic findings were normal in 3/4 WNV-positive horses (27).
The survival rate of 57% was slightly lower than that which has been reported elsewhere. A survival rate of 63% was reported in the United States (27), while survival rates were 72% in France (9) and 80% in Israel in outbreaks in 2000 (28). It must be considered, also, that the study population does not necessarily accurately reflect the severity of WNV-associated disease in the general horse population. Horses referred to this college tend to represent the more severely affected cases. Particularly encouraging was the apparent complete resolution of neurological deficits in 13/14 (93%) of the horses that survived and from which follow-up information was available. This includes 1 horse that was recumbent on presentation and indicates that initial treatment is warranted even in severe cases, although recumbency was not surprisingly associated with a negative outcome and should be considered an indicator of poor prognosis.
As of November 6, 2002, there were 239 presumptive or confirmed equine cases in Canada; 80 in Ontario, 147 in Manitoba, 10 in Saskatchewan, and 2 in Quebec (26). However, the Canadian numbers are likely a gross underestimation of the actual number of equine cases. Because WNV is not considered to be a reportable disease in Canada, there is no central reporting system in place nor is there mandatory testing or assistance with testing. There are anecdotal reports of large numbers of affected animals, particularly in the Windsor-Essex region of Ontario; however, it is unclear how many horses were actually infected with WNV.
A conditionally licensed WNV vaccine is available in the United States (West Nile virus vaccine; Fort Dodge Animal Health, Fort Dodge, Iowa, USA) and can be obtained in Canada. While in vivo efficacy data has yet to be produced, this vaccine is considered safe and has a reasonable likelihood of efficacy. There is anecdotal evidence that 2 doses of vaccine must be given 3 to 6 wk apart, and that 2 to 4 wk must pass following the 2nd dose of vaccine prior to the development of full protection. This is consistent with the cases reported here, as 5 horses developed disease after receiving a single dose of vaccine. No true vaccine failures (horses developing disease following the full course of vaccination) were identified in this group. At this point, the OVC large animal medicine service is recommending vaccination of horses in areas where WNV has been recognized or in adjacent regions, starting 6 to 8 wk prior to the anticipated emergence of mosquitoes.
Horses infected with WNV are not considered to be at risk for transmitting disease to humans or other animals, because they become minimally viremic (29). There is a risk to personnel involved in postmortem analysis or those handling CSF, and proper barrier precautions should be employed. However, all horses with an acute onset of neurological abnormalities should be suspected as having rabies and handled accordingly, because of the risk of zoonotic transmission of rabies. All cases reported here were handled as rabies suspects and full postmortem examination was not performed until rabies had been ruled out.
Recent outbreaks of WNV in horses in France have been characterized by sporadic outbreaks, followed by long silent periods (9). Unfortunately, based on the increase in cases North America over the past 3 y and continued range expansion, it appears that WNV has established itself as an endemic disease in much of North America. It is unclear what the impact of WNV will be in the Canadian horse population in future years. It is suspected that large numbers of horses have been exposed to WNV and have not developed clinical signs, while developing protective immunity. This, in combination with widespread vaccination, should eventually decrease the pool of susceptible horses. This should, however, have no impact on the disease cycle, because horses are dead-end hosts. West Nile virus will likely persist in much of Canada and continue to put susceptible horses at risk. Vaccination and mosquito control measures are warranted. Because the future impact of WNV infection in horses is unclear, it is essential that all horses displaying an acute onset of neurological disease in summer, fall, or early winter be tested for WNV. West Nile virus encephalomyelitis should be considered in all cases of acute neurological disease, particularly when fever and muscle fasciculations are present. All cases of suspected WNV-associated disease should be handled as infectious because of the possibility of rabies, a differential diagnosis that should be considered in all cases.
Footnotes
Acknowledgment
The WNV antiserum for IHC was generously provided by Dr. Hana Weingartl of the National Centre for Foreign Animal Disease, Canadian Food Inspection Agency, in Winnipeg. CVJ
Address all correspondence to Dr. J. Scott Weese; e-mail: jsweese@uoguelph.ca
Reprints will not be available from the authors.
References
- 1.Ostlund EN, Andresen JE, Andresen M. West Nile encephalitis. Vet Clin North Am Equine Pract 2000;16:427–441. [DOI] [PubMed]
- 2.Smithburn KC, Hughes TP, Burke AW. A neurotropic virus isolated from blood of a native in Uganda. Am J Trop Med 1940; 20:471.
- 3.Le Guenno B, Bougermouh A, Azzam T, Bouakaz R. West Nile: a deadly virus? Lancet 1996;348:1315. [DOI] [PubMed]
- 4.Hubalek Z, Savage HM, Halouzka J, Juricova Z, Sanogo YO, Lusk S. West Nile virus investigations in South Moravia, Czechland. Viral Immunol 2000;13:427–433. [DOI] [PubMed]
- 5.Lvov DK, Butenko AM, Gromashevsky VL, et al. Isolation of two strains of West Nile virus during an outbreak in Southern Russia. Emerg Infect Dis 1999;6:373–376. [DOI] [PMC free article] [PubMed]
- 6.Weinberger M, Pitlik SD, Gandacu D, et al. West Nile fever outbreak, Israel, 2000: Epidemiologic aspects. Emerg Infect Dis 2001;7:686–691. [DOI] [PMC free article] [PubMed]
- 7.Tsai TF, Popovici F, Cernescu C, Campbell GI, Nedeku NI. West Nile encephalitis epidemic in Southeastern Romania. Lancet 1998;352:767–771. [DOI] [PubMed]
- 8.Weiss D, Carr D, Kellachan J, et al. Clinical findings of West Nile virus infection in hospitalized patients, New York and New Jersey, 2000. Emerg Infect Dis 2001;7:654–658. [DOI] [PMC free article] [PubMed]
- 9.Durand B, Chevalier V, Pouillot R, et al. West Nile virus outbreak in horses, Southern France, 2000: results of a serosurvey. Emerg Infect Dis 2002;8:777–782. [DOI] [PMC free article] [PubMed]
- 10.Komar N, Langevin S, Hinten S, et al. Experimental infection of North American Birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis 2003;9:311–322. [DOI] [PMC free article] [PubMed]
- 11.Murgue B, Murri S, Zientara S, Durand B, Durand J-P, Zeller H. West Nile outbreak in horses in southern France, 2000: the return after 35 years. Emerg Infect Dis 2001;7:692–696. [DOI] [PMC free article] [PubMed]
- 12.Nasci RS, Savage HM, White DJ, et al. West Nile virus in overwintering Culex mosquitoes, New York city, 2000. Energ Infect Dis 2001;7:1–3. [DOI] [PMC free article] [PubMed]
- 13.Kulasekera VL, Kramer L, Nasci RS, et al. West Nile virus infection in mosquitoes, birds, horses and humans, Staten Island, New York, 2000. Emerg Infect Dis 2001;7:722–725. [DOI] [PMC free article] [PubMed]
- 14.Malkinson M, Banet C, Weisman Y, et al. Introduction of West Nile virus in the Middle East by migrating white storks. Emerg Infect Dis 2002;8:391–396. [DOI] [PMC free article] [PubMed]
- 15.Cantile C, DiGuardo G, Eleni C, Arispic M. Clinical and neuropatholoogical features of West Nile virus equine encephalomyelitis in Italy. Equine Vet J 2000;32:31–35. [DOI] [PubMed]
- 16.Tber A. West Nile fever in horses in Morocco. Bull Off Int Epizoot 1996;11:867–869.
- 17.Schmidt JR, El Mansoury HK. Natural and experimental infection of Egyptian equines with West Nile virus. Ann Trop Med Parasitol 1963;57:415–527. [DOI] [PubMed]
- 18.Anderson JF, Andreadis G, Vossbrinck CR, et al. Isolation of West Nile virus from mosquitoes, crows and a Cooper's hawk in Connecticut. Science 1999;286:2331. [DOI] [PubMed]
- 19.Lanciotti RS, Roehrig JT, Deubel V, et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the Northeastern United State. Science 1999;286:2333. [DOI] [PubMed]
- 20.Trock SC, Meade BJ, Glaser AL, et al. West Nile virus outbreak among horses in New York State, 1999 and 2000. Emerg Infect Dis 2001;7:745–747. [DOI] [PMC free article] [PubMed]
- 21.Ostlund EN, Crom RL, Pedersen DD, Johnson DJ, Williams WO, Schmitt BJ. Equine West Nile encephalitis, United States. Emerg Infect Dis 2001;7:665–669. [DOI] [PMC free article] [PubMed]
- 22.Anonymous. West Nile virus: Historical summary. 2002; http://www.cfe/cornell.edu/erap/WNV/Summary.cfm#year
- 23.Anonymous. Update on the current status of West Nile Virus. 2002; http://www.aphis.usda.gov/oa/wnv/wnvstats.html
- 24.Centers for Disease Control and Prevention. 2002; http://www.cdc.gov/od/media/wnvcount/html
- 25.Anonymous. West Nile virus confirmed Windsor area. 2001; http://www.wechealthunit.org/pages/hottopics/westnileresult.html
- 26.Health Canada. West Nile virus: Canada. 2002; http://www.hc-sc.gc.ca/pphb-dgspsp/wnv-vwn/pdf_sr-rs/2002/situation_report/110102_db.pdf
- 27.Snook CS, Hyman SS, Del Piero F, et al. West Nile virus encepahlomyelitis in eight horses. J Am Vet Med Assoc 2001;218:1576–1579. [DOI] [PubMed]
- 28.Steinman A, Banet C, Sutton GA, Yadin H, Hadar S, Brill A. Clinical signs of West Nile virus encephalomyelitis in horses during the outbreak in Israel in 2000. Vet Rec 2002;151:47–49. [DOI] [PubMed]
- 29.Bunning ML, Bowen RA, Cropp B, et al. Experimental infection of horses with West Nile virus. Emerg Infect Dis 2002;8:380–386. [DOI] [PMC free article] [PubMed]