Abstract
Background & objectives:
A large number of plants have been recognized to be effective in the treatment of diabetes mellitus. Persistent hyperglycaemia is associated with decreased function of immune system and cerebral ischaemia mainly due to increased oxidative stress and inflammatory response. Andrographis paniculata is a medicinal plant widely used in folk medicine for various purposes. In this study the effect of chronic administration (7 days) of methanolic extract of A. paniculata leaves was studied in rats with experimentally induced diabetes, nootropic and immunostimulant activities were evaluated. The effect of acute administration of methanolic extract of A. paniculata leaves was also studied for cerebroprotective activity.
Methods:
Type 2 diabetes was induced in rats by streptozotocin (STZ) (65 mg/kg) + nicotinamide (150 mg/kg). Various biochemical parameters were estimated using standard methods.
Results:
A significant (P<0.05) increase in cognitive function was observed in both normal and type 2 diabetic rats. Nootropic activity in terms of per cent reduction in latency period was more in type 2 diabetic rats. A significant increase in blood lymphocyte count, splenic lymphocyte count and peritoneal macrophage count was observed in both normal and type 2 diabetic rats. Immunostimulant activity was observed more in type 2 diabetic rats. The per cent decrease in cerebral infarction was more in type 2 diabetic rats when compared to normal rats. The per cent increase in superoxide dismutase (SOD) levels was more in type 2 diabetic rats.
Interpretation & conclusions:
The antioxidant activity of the methanolic extract of A. paniculata leaves was evident by decreased tissue malondialdehyde (MDA) levels and increased SOD levels. These properties may be responsible for the observed cerebroprotective activity. The methanolic leaf extract of A. paniculata showed significant immunostimulant, cerebroprotective and nootropic activities in normal and type 2 diabetic rats.
Keywords: Andrographis paniculata, cerebroprotective, diabetes, immunostimulant, nootropic, rats, streptozotocin
Type 2 diabetes mellitus is the most common form of diabetes, which causes a growing concern all over the world, predominantly because of the consequent chronic complications1. Hyperglycaemia is associated with decreased function of immune system2, and, therefore, the patients with diabetes are more prone to infections. Decreased blood supply, along with the inadequate immune response, delays the wound healing in those affected. These patients have a 2-6 fold increased risk of thrombo embolic strokes when compared to the non diabetic and are vulnerable to stroke related mortality and morbidity. Population based studies have shown that patients with type 2 diabetes have an increased risk of cognitive impairment, dementia and neurodegeneration3.
With strict glycaemic control, diabetic complications can be delayed, but practically, it is not possible and many patients with chronic diabetes eventually develop complications. The mechanisms involved include oxidative stress, inflammatory mediators and glycation end products.
Currently used antidiabetic drugs aimed to control hyperglycaemia, are not reported to have antioxidant and anti-inflammatory properties. Hence, compounds having multiple activities like antihyperglycaemic, antioxidant and anti-inflammatory may be more useful in treating diabetes as well as complications arising out of diabetes. Many herbal drugs and extracts have been tried for this purpose. Andrographis paniculata commonly known as “King of Bitters,” is a member of the plant family, Acanthaceae. A. paniculata is reported to have antihyperglycaemic, antioxidant and other biological activities like antibacterial, anti-human immunodeficiency virus, immunostimulatory, antipyretic, antidiarrhoeal, antivenom, antihepatotoxic, anti-inflammatory, and antimalarial activities4.
Hence the present study was carried out to evaluate the effect of methanolic extract of A. paniculata leaves for its immunostimulant, cerebroprotective and nootropic activities in normal rats and those with experimentally induced type 2 diabetes.
Material & Methods
Plant material: Andrographis paniculata Nees (Acanthaceae) (10 kg) was collected in July 2006 from Mamundur forest, Mallimadugu village, Tirupati (rural), Chittor District, Andhra Pradesh, India. Leaves were dried in shade and powdered. The authentication of the plant was done by Dr K. Madhava Chetty, Department of Botany, Sri Venkateswara University, Tirupati, India, and the voucher specimen (No. 0054/AP) was deposited in the Herbarium of the Department of Botany, Andhra University, Visakhapatnam, India.
Preparation of methanolic extract of A. paniculata leaves: Shade-dried and powdered leaves (890 g) were subjected to extraction using methanol (CH3OH) exhaustively for a minimum of eight times for every three to four days by successive cold and hot extraction processes. The extract was concentrated to dryness in vacuo. The methanolic extract of the plant leaves (15 g) was tested for immunostimulant, cerebroprotective and nootropic activities in June 2010 in the laboratories of Pharmacology Division, University College of Pharmaceutical Sciences, Andhra University, Visakhapatnam.
Chemicals used: Streptozotocin and nicotinamide were purchased from Sigma Chemicals, USA. All other chemicals (sodium carbonate, sodium hydroxide, copper sulphate, sodium potassium tartarate, bovine serum albumin, Folin - Ciocalteau's phenol, nitroblue tetrazolium, nicotinamide adenine dinucleotide hydride (NADH)) used were of analytical grade.
Animals: Wistar albino rats of either sex weighing 150-200 g procured from Mahaveer Enterprises, Hyderabad, India, were used in the study. The animals were maintained on a 12 h light - 12 h dark cycle. They were fed with standard pellet diet (Rayans Biotechnologies Pvt. Ltd., Hyderabad) and water ad libitum. Animals were fasted for 16 h prior to drug administration allowing access only to water and were deprived of both food and water during the experiment.
Induction of type I diabetes mellitus: To induce type I diabetes, albino rats of either sex were fasted over night before injecting with streptozotocin (STZ). STZ was dissolved in citrate buffer pH 4.5 at a dose of 40 mg/kg immediately before use and injected into the tail vein of rats which were lightly anaesthetized with ether.
Induction of type 2 diabetes mellitus: To induce type 2 diabetes, 65 mg/kg dose of STZ was given after the administration of 150 mg/kg dose of nicotinamide5. Blood glucose levels were estimated after 48 h for the confirmation of diabetes induction.
Dose response studies were conducted for glucose reduction in both type I and type 2 diabetes rats using 100, 200 and 400 mg per kg doses of leaf extract. The present dose i.e. 100 mg/kg (oral) was selected for chronic administration and 50 mg/kg (i.p.) was selected for acute administration.
Evaluation of effects of chronic administration (7 days): Twenty four rats were taken and divided into four groups. Group I served as vehicle treated control, Group II served as rats treated with the extract (100 mg/kg), Group III served as type 2 diabetic vehicle treated control and Group IV served as type 2 diabetic rats treated with the extract (100 mg/kg). The fasting blood samples were taken before the administration of the extract. The extract was given orally daily once for seven days. At the end of seven days again the blood samples were collected6 and analyzed. The blood glucose was estimated by glucose oxidase-peroxidase (GOD-POD) method7.
Evaluation of immunostimulant activity: Blood lymphocyte count was carried out using Leishman stain. To determine splenic lymphocyte count, spleen was dissected, macerated and washed with 10 ml balanced salt solution (BSS pH 7.2) and pellets were resuspended in 2 ml of BSS and counting was done with haemocytometer. Peritoneal macrophages were collected at different days of treatment by washing peritoneal cavity with chilled BSS. The peritoneal fluid was incubated at 37 °C for 60 min in glass petridish. Cold ethylenediaminetetraacetic acid (EDTA, 2%) was added to the petridish and flushed gently and kept at 4 °C for 30 min. Suspension was centrifuged and suspended in 1 ml of BSS. Counting was done with haemocytometer in the presence of neutral red.
Evaluation of nootropic activity: Twenty four rats were taken and divided into four groups as described earlier. Spatial memory was evaluated by using Morris water maze8 before and after the administration of the extract.
Evaluation of effects of acute administration: The rats (n=30) were divided equally into 5 groups. Group I served as normal control, animals were treated with 0.2 ml saline i.p., group II served as vehicle control, animals were treated with 0.2 ml of 99 per cent dimethyl sulphoxide (DMSO) i.p., group III served as per se control, animals were treated with methanolic extract of A. paniculata 50 mg/kg i.p., group IV served as type 2 diabetic control, animals were treated with 0.2 ml of 99 per cent DMSO i.p., and group V served as type 2 diabetic, animals were treated with methanolic extract of A. paniculata leaf extract 50 mg/kg i.p. The rats were anaesthetized by giving thiopentone sodium (35 mg/kg) i.p.
Carotid artery ligation: Surgical technique for the induction of cerebral ischaemia was adapted from earlier studies9. Under anaesthesia, a midline incision in neck was given. Common carotid arteries were identified and isolated carefully from vagosympathetic nerve. Rats were made ischaemic by occluding bicommon carotid arteries9 (BCCA) with a silk thread for 30 min and reperfusion was allowed for 4 h by removing the thread. Body temperature was maintained at about 37 °C during the period with the help of a heating lamp. The rats were anaesthetized by giving thiopentone sodium (35 mg/kg) i.p.
Quantification of infarct size: Infarct size was measured by using triphenyltetrazolium chloride (TTC) stain10. At the end of the experiment, rats were sacrificed by giving high doses of anaesthesia, later decapitated and brains were isolated and thoroughly rinsed with ice chilled 0.9 per cent NaCl. The brain was weighed, and sliced to 0.1cm thick sections and incubated in 2 per cent solution of TTC prepared in pH 7.4 phosphate buffer for 60 min at 37 °C. In viable brain tissue TTC is converted by dehydrogenase enzymes to a red formazan pigment that stains tissue dark red. The infarcted brain tissue that does not take TTC stain.
The tissue malondialdehyde (MDA) levels were measured by the method of Ohkawa et al11. Superoxide dismutase (SOD) activity was determined by the method developed by Kakkar et al12.
Statistical analysis: Differences between means were tested using One-way ANOVA. Individual groups were compared using, Dunnett's multiple comparison test. P<0.05 was considered as significant.
Results
Evaluation of effects of chronic administration: A significant (P<0.05) increase in cognitive function was observed in both normal and type 2 diabetic rats. A significant (P<0.05) increase in blood lymphocyte count was observed in normal and type 2 diabetic rats treated with the methanolic extract. The per cent increase was more in diabetic rats. Splenic lymphocyte count and peritoneal macrophage count were significantly increased (P<0.05) in normal and type 2 diabetic rats. Our results exhibited significant immunostimulant activity in both normal and type 2 diabetic rats. The per cent increase in blood lymphocyte, splenic lymphocyte, peritoneal macrophages was calculated and the per cent variation in cognitive function are given in Table I.
Table I.
The effect of chronic administration (7 days) of methanolic extract of A. paniculata leaves (100 mg/kg, oral) on blood glucose, immunostimulant and nootropic activities in normal and type 2 diabetic rats
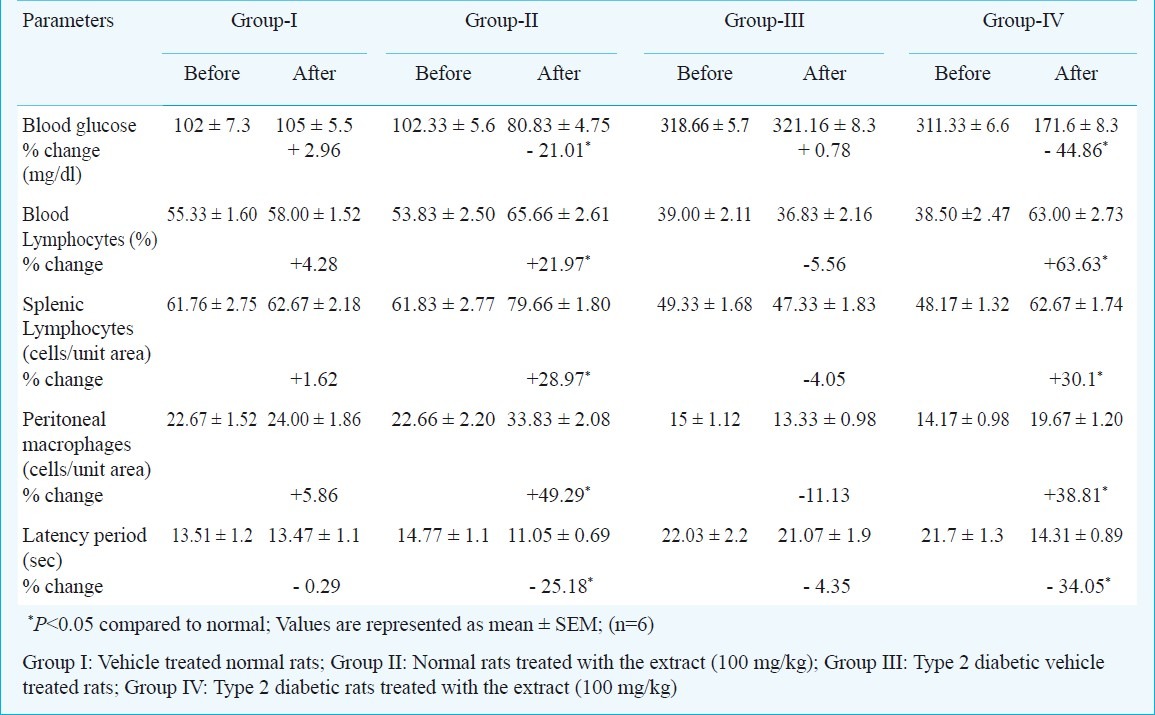
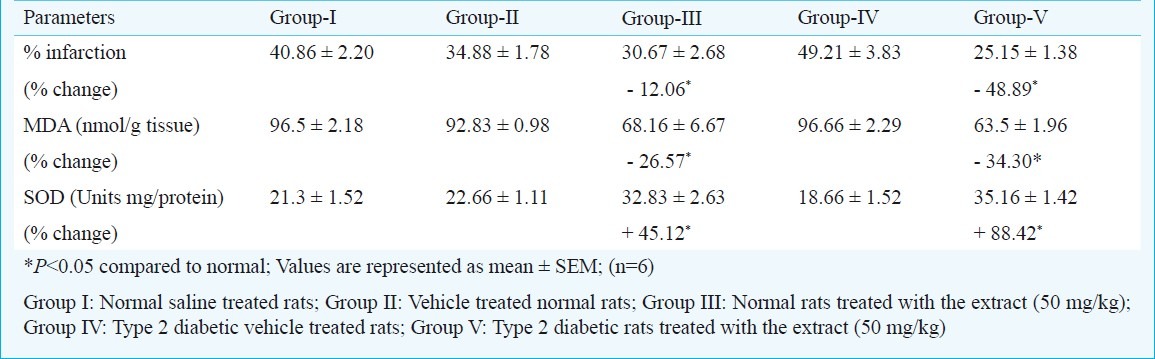
Evaluation of effects of acute administration: The per cent infarction was significantly (P<0.05) more in type 2 diabetic rats when compared to normal rats. There was a consistent association between high blood glucose levels and greater infarct size. Elevated blood glucose levels are associated with an increased hypoperfused tissue progressing to infarction13. The per cent infarction was significantly (P<0.05) reduced in the extract treated normal as well as in type 2 diabetic rats. The degree of cerebroprotective activity was more in type 2 diabetic rats. The per cent reduction in infarction, decrease in MDA and increase in SOD were calculated and are given in Table II.
Table II.
The effect of acute administration of methanolic extract of A. paniculata leaves (50 mg/kg, i.p.) on cerebroprotective activity in normal and type 2 diabetic rats
Discussion
The methanolic extract of A. paniculata leaves was found to possess significant immunostimulant activity. Many Indian medicinal plants like Withania somnifera and Mangifera indica have been reported to have immunomodulatory activities14,15. Puri et al16 reported that andrographolides of A. paniculata induced significant stimulation of antibody and delayed type hypersensitivity (DTH) response to sheep red blood cells (SRBC) in mice16. Immunostimulant property of A. paniculata was studied on human cell line cultures and it was observed that methanolic extract augmented the proliferation of human peripheral blood lymphocytes (HPBLs)17.
A significant increase in cognitive function was observed in type 2 diabetic rats treated with the methanolic extract in the present study. There were no earlier reports on the effect of A. paniculata on cognitive function. Many population based studies have found an association between type 2 diabetes and an increased risk of developing dementia3,18–23. There are many mechanisms through which diabetes could increase risk of dementia including hyperglycaemia, insulin resistance, oxidative stress, advanced glycation end products, inflammatory cytokines, and microvascular and macrovascular disease3. A. paniculata is reported to have antidiabetic, antioxidant and anti-inflammatory properties. These properties may be responsible for the observed nootropic activity.
A. paniculata showed significant cerebroprotective activity in terms of per cent reduction in infarct size against ischaemia-reperfusion injury in both normal and diabetic rats. A significant reduction in the tissue MDA levels and increase in SOD levels were observed in normal and type 2 diabetic rats treated with A. paniculata methanolic extract. Griesmacher et al24 have reported that enhanced production of free radicals was observed in both type 1 and type 2 diabetes, significantly higher levels were observed in type 2 diabetes. Increased oxidative stress resulting from hyperglycaemia is believed to contribute to the excess cerebral damage. Free radical production is increased during hyperglycaemic stroke in rodents25. Hyperglycaemia and hyperglycaemia-induced oxidative stress may be responsible for increased cerebral infarction in diabetes. A. paniculata is reported to have antidiabetic, antioxidant and anti-inflammatory properties. In the present study also, antioxidant activity of the methanolic extract of A. paniculata leaves was evident by decreased tissue MDA levels and increased SOD levels. These properties may be responsible for the observed cerebroprotective activity.
Chan et al26 reported that andrographolide exhibited neuroprotective effects, with accompanying suppression of nuclear factor kappa B (NF-κB) and microglial activation, and reduction in the production of cytokines including tumour necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β), and pro-inflammatory factors such as prostaglandin E2 (PGE2).
In conclusion, the methanolic extract of A. paniculata leaves was found to possess significant immunostimulant, cerebroprotective and nootropic activities in normal and type 2 diabetic rats. Further studies need to be done to isolate and characterize the active constituent(s).
Acknowledgment
The first author (PR) acknowledge the Department of Science and Technology (DST), New Delhi, India, for providing financial grant under Women Scientists Scheme-A.
References
- 1.Zargar AH, Sofi FA, Laway BA, Masoodi SR, Shah NA, Dar FA. Profile of neurological problems in diabetes mellitus retrospective analysis of data from 1294 patients. Ann Saudi Med. 1997;17:20–5. doi: 10.5144/0256-4947.1997.20. [DOI] [PubMed] [Google Scholar]
- 2.Musa BOP, Onyemelukwe GC, Hambolu JO, Bakari AG, Anumah FE. Cell-mediated immunity in type 2 diabetes mellitus patients in diabetic ketoacidosis, patients with controlled type 2 diabetes mellitus and healthy control subjects. J Med Med Sci. 2010;1:290–5. [Google Scholar]
- 3.Whitmer RA. Type 2 diabetes and risk of cognitive impairment and dementia. Curr Neurol Neurosci Rep. 2007;7:373–80. doi: 10.1007/s11910-007-0058-7. [DOI] [PubMed] [Google Scholar]
- 4.Mishra SK, Sangwan NS, Sangwan RS. Andrographis paniculata (Kalmegh): A Review. Pharmacog Rev. 2007;1:283–98. [Google Scholar]
- 5.Masiello P, Broca C, Gross R, Roye M, Manteghetti M, Hillaire-Buys D, et al. Experimental NIDDM: development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes. 1998;47:224–9. doi: 10.2337/diab.47.2.224. [DOI] [PubMed] [Google Scholar]
- 6.Tinn KI. Orbital venous anatomy of the rat. Lab Anim Sci. 1979;29:636–8. [PubMed] [Google Scholar]
- 7.Trinder P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol. 1969;22:158–61. doi: 10.1136/jcp.22.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris R, Garrud P, Rawlins J, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 9.Iwasaki Y, Ito T, Suzuki M, Nagahori T. Blood-brain barrier damage in reperfusion following ischaemia in the hippocampus of the Mongolian gerbil brain. J Neurol Sci. 1989;90:155–65. [Google Scholar]
- 10.Jiang J, Wang W, Sun YJ. Neuroprotective effect of curcumin on focal cerebral ischaemic rats by preventing blood-brain barrier damage. Eur J Pharmacol. 2007;561:54–62. doi: 10.1016/j.ejphar.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 12.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 13.Parsons MW, Barber PA, Desmond PM, Baird TA, Darby DG, Byrnes G, et al. Acute hyperglycaemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol. 2002;52:20–8. doi: 10.1002/ana.10241. [DOI] [PubMed] [Google Scholar]
- 14.Davis L, Kuttan G. Immunomodulatory activity of Withania somnifera. J Ethnopharmacol. 2000;71:193–200. doi: 10.1016/s0378-8741(99)00206-8. [DOI] [PubMed] [Google Scholar]
- 15.Makare N, Bodhankar S, Rangari V. Immunomodulatory activity of alcoholic extract of Mangifera indica L. in mice. J Ethnopharmacol. 2001;78:133–7. doi: 10.1016/s0378-8741(01)00326-9. [DOI] [PubMed] [Google Scholar]
- 16.Puri A, Saxena R, Saxena RP, Saxena KC, Srivastava V, Tandon JS. Immunostimulant agents from Andrographis paniculata. J Nat Prod. 1993;56:995–9. doi: 10.1021/np50097a002. [DOI] [PubMed] [Google Scholar]
- 17.Kumar RA, Sridevi K, Kumar NV, Nanduri S, Rajagopal S. Anticancer and immunostimulatory compounds from Andrographis paniculata. J Ethnopharmacol. 2004;92:291–5. doi: 10.1016/j.jep.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–81. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 19.Bruce DG, Harrington N, Davis WA, Davis TM. Dementia and its associations in type 2 diabetes mellitus: the Fremantle Diabetes Study. Diabetes Res Clin Pract. 2001;53:165–72. doi: 10.1016/s0168-8227(01)00266-2. [DOI] [PubMed] [Google Scholar]
- 20.Curb JD, Rodriguez BL, Abbott RD, Petrovitch H, Ross GW, Masaki KH, et al. Longitudinal association of vascular and Alzheimer's dementias, diabetes, and glucose tolerance. Neurology. 1999;52:971–5. doi: 10.1212/wnl.52.5.971. [DOI] [PubMed] [Google Scholar]
- 21.Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O’Brien PC, et al. The risk of dementia among persons with diabetes mellitus: a population-based cohort study. Ann N Y Acad Sci. 1997;826:422–7. doi: 10.1111/j.1749-6632.1997.tb48496.x. [DOI] [PubMed] [Google Scholar]
- 22.Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer's disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol. 2001;154:635–41. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 23.Macknight C, Rockwood K, Awalt E, McDowell I. Diabetes mellitus and the risk of dementia, Alzheimer's disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dement Geriatr Cogn Disord. 2002;14:77–83. doi: 10.1159/000064928. [DOI] [PubMed] [Google Scholar]
- 24.Griesmacher A, Kindhauser M, Andert SE, Schreiner W, Toma C, Knoebl P, et al. Enhanced serum levels of thiobarbituric-acid-reactive substances in diabetes mellitus. Am J Med. 1995;98:469–75. doi: 10.1016/s0002-9343(99)80347-7. [DOI] [PubMed] [Google Scholar]
- 25.Bémeur C, Ste-Marie L, Montgomery J. Increased oxidative stress during hyperglycaemic cerebral ischaemia. Neurochem Int. 2007;50:890–904. doi: 10.1016/j.neuint.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Chan SJ, Wong WS, Wong PT, Bian JS. Neuroprotective effects of andrographolide in a rat model of permanent cerebral ischaemia. Br J Pharmacol. 2010;161:668–79. doi: 10.1111/j.1476-5381.2010.00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


