Abstract
Background & objectives:
Tuberculosis (TB) is a public health problem worldwide. Rapid and accurate diagnosis of tuberculosis is crucial to facilitate early treatment of infectious cases and to reduce its spread. The present study was aimed to evaluation of 16 kDa antigen as a serodiagnostic tool in pulmonary and extra-pulmonary tuberculosis patients in an effort to improve diagnostic algorithm for tuberculosis.
Methods:
In this study, 200 serum samples were collected from smear positive and culture confirmed pulmonary tuberculosis patients, 30 tubercular pleural effusions and 21 tubercular meningitis (TBM) patients. Serum samples from 36 healthy, age matched controls (hospital staff), along with 60 patients with non-tubercular respiratory diseases were also collected and evaluated. Humoral response (both IgG and IgA) was looked for 16 kDa antigen using indirect ELISA.
Results:
Sensitivity of detection in various categories of pulmonary TB patients ranged between 73.8 and 81.2 per cent. While in the extra-pulmonary TB samples the sensitivity was 42.8 per cent (TBM) and 63.3 per cent (tubercular pleural effusion). The test specificity in both the groups was high (94.7%). All of the non-disease controls were negative. Among non-tubercular disease controls, five patients gave a positive humoral response against 16 kDa.
Interpretation & conclusions:
Serodiagnostic tests for TB have always had drawbacks of suboptimal sensitivity and specificity. The antigen used in this study gave encouraging results in pulmonary TB only, while in extra-pulmonary TB (tubercular meningitis and tubercular pleural effusion), this has shown a limited role in terms of sensitivity. Further work is required to validate its role in serodiagnosis of TB especially extra-pulmonary TB.
Keywords: ELISA, EPTB, serodiagnosis, tuberculosis, 16 kDa
Tuberculosis (TB) is a major public health problem worldwide and was responsible for 8.8 million incident cases of TB and 1.45 million deaths in the year 20101. The failure to diagnose TB accurately and rapidly is a key challenge in curbing the epidemic2,3. Rapid and accurate diagnosis of tuberculosis is crucial to facilitate early treatment of infectious cases and thus to reduce its spread.
TB diagnosis largely depends upon clinical examination and radiographic findings, mainly confirmed by sputum smear microscopy and bacterial culture3. Many alternative methodologies have been applied in TB diagnosis, such as PCR and cell-mediated immune response reactions4. These methods require trained personnel and specific laboratory conditions, which hinder their implementation in many areas of high TB endemicity.
Extra-pulmonary TB (EPTB) remains an important diagnostic and therapeutic problem. The diagnosis of extra-pulmonary tuberculosis is challenging due to the paucibacillary nature of the specimens, inability to access the site of disease activity, the lack of adequate sample volumes and the presence of inhibitors that undermine the performance of nucleic acid amplification-based techniques. Serological tests may prove useful with the advantages of speed, technical simplicity, possible adaptation towards point-of-care formats and low cost5,6.
The availability of numerous well characterized M. tuberculosis proteins has revived interest in the serological diagnosis of tuberculosis. Several promising antigens have been reported such as 16 kDa, 45 kDa, antigen 85 complex (30 kDa), 65 kDa Hsp, 88 kDa, 38 kDa, ESAT-6, CFP-10, etc8. Antibody response to the 38 kDa in pulmonary TB has been extensively studied, and there are a few reports about the utility of the 16 kDa-based serological tests in pulmonary and extra-pulmonary TB7–9.
The aim of the present study was to explore the serodiagnostic potential of 16 kDa (HspX, Rv2031c, or α-crystalline) antigen in different groups of tuberculosis patients. 16 kDa is an immunodominant antigen that is recognized by the majority of patients with active tuberculosis10. Production of Rv2031c appears to increase as the bacteria go into the metabolically resting stage and decreases as they revert to exponential growth11, thus providing a constant source of antigen in cultures and possibly in vivo.
Material & Methods
A total of 347 subjects (age range= 18 to 65 yr, male= 235, female= 112) were recruited between June 2007 to July 2010 (Table I). All patients and control subjects gave informed consent prior to sampling. The cross-sectional study protocol was reviewed and approved by the ethics committee of the All India Institute of Medical Sciences (AIIMS), New Delhi. Detailed clinical history and findings were recorded in a case record form.
Table I.
Details/profile of subjects (n=347) enrolled in study
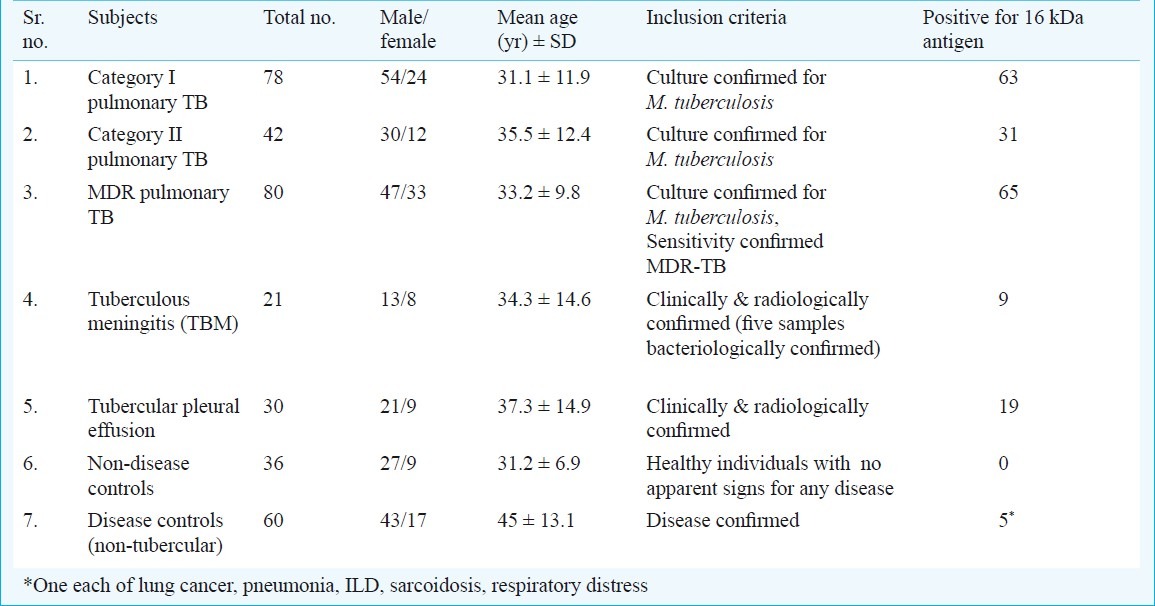
A total of 200 pulmonary TB (PTB) patients were enrolled prospectively from Comprehensive Rural Health Services Project (CRHSP), Centre for Community Medicine, Ballabgarh, Haryana (n=120) and Rajan Babu Institute for Pulmonary Medicine & Tuberculosis (RBIPMT), New Delhi (n=80); and were classified 78 category I PTB patients (new sputum smear positive), 42 category II PTB patients (sputum smear positive after default or relapse/treatment failure) and 80 multi-drug resistant PTB patients.
Sputum (5-10 ml) and blood samples (2-3 ml) were collected within two weeks of initiation of anti-tuberculosis treatment (ATT) from category I and category II patients (from CRHSP). MDR PTB patients admitted at RBIPMT were on ATT at the time of inclusion in the study. All the patients included in the study had the sputum culture positive for M. tuberculosis. After collection, samples were transported in cold chain.
Twenty one in-patients with tuberculous meningitis (TBM) were enrolled from the Department of Neurology, AIIMS, New Delhi. Sixteen patients were clinically and radiologically confirmed12 and cerebrospinal fluid (CSF) of five patients grew M. tuberculosis on culture. Blood sample (2-3 ml) was collected within two weeks of initiation of ATT.
Thirty patients with tubercular pleural effusion were enrolled from CRHSP, Ballabgarh, Haryana and AIIMS, New Delhi. Patients were clinically and radiologically confirmed as the microbiological confirmation could not be obtained for all patients. A total of 60 subjects, who were suffering from respiratory/lung diseases (other than tuberculosis) were included as disease controls from AIIMS, New Delhi. Lung cancer (n=21), asthma (n=9), pneumonia (n=7), chronic obstructive pulmonary disorder (n=5), interstitial lung disease (n=3), sarcoidosis (n=3), respiratory distress (n=3), allergic broncho pulmonary aspergillosis (n=3), bronchiectasis (n=2), occupational lung disease (n=1), Wegener's granulomatosis (n=1), bronchiolitis obliterans (n=1), metabolic encephalopathy (n=1) formed the spectrum. Sputum/induced sputum samples were obtained. All above disease controls were evaluated bacteriologically for M. tuberculosis using culture and AFB smear and found negative. Forty six subjects had M. bovis BCG vaccination history. The purified protein derivative (PPD) status of the patients was unknown.
Thirty six healthy individuals (with no known history of active TB) were enrolled from the staff working in Department of Microbiology, AIIMS, New Delhi, as healthy controls or non-disease controls. All these were healthy with no apparent signs of any disease. All of them had BCG vaccination history. The purified protein derivative (PPD) status of the subjects was not known.
Sample processing: Sputum samples were processed using standard NALC-NaOH method13 and smears were examined after Ziehl–Neelsen staining. Processed samples were inoculated in MGIT (Mycobacterial growth indicator tube) 960 non-radiometric automated isolation system (BD, USA). MGIT tube was supplemented with 0.5 ml of oleic acid-albumin-dextrose-catalase along with mixture of 5 antibiotics (PANTA i.e., polymyxin B, amphotericin B, nalidixic acid, trimethoprim and azlocillin) and 0.5 ml of decontaminated sample. M. tuberculosis complex and non-tuberculous mycobacteria were differentiated using p-nitrobenzoic acid (PNBA) test (as recommended in MGIT-960 protocol). Drug susceptibility testing (DST) was performed using 1 per cent proportion method using above automated culture system to reassure the MDR status (as recommended in MGIT-960 protocol). Serum samples were separated and immediately stored in -80°C till further use.
ELISA protocol: ELISA was performed with some modifications14,15 to estimate the humoral responses (IgG and IgA, in the same well) against native 16 kDa antigen. Briefly, the 96-well microtiter plates (Maxisorp Nunc, Denmark) were coated with native antigen in PBS (50 μl/well of 5 μg/ml) overnight at 4°C. Next day, plates were washed three times with PBS-T, blocked with 1 per cent BSA in PBS-T (blocking buffer) for two hours at 37°C and followed by three washings. Subsequently, 100 μl of diluted sera (1:100 in 0.1 × blocking buffer) from patients and healthy subjects was added and the plates were incubated for 90 min at 37°C. After six washes with PBS-Tween 20 (0.05% Tween 20 in PBS), a mixture of alkaline phosphatase-conjugated protein A (1:2,000) and anti-human immunoglobulin A (IgA; 1:1,000) (Sigma-Aldrich, St. Louis, USA) was added to each well and the plates were incubated for 1 h at 37°C. The wells were washed six times with PBS-T, and the bound enzyme-conjugated antibodies were detected with p - nitrophenylphosphate substrate (Sigma, USA), (1 mg/ml p - nitrophenylphosphate in 10% diethanolamine buffer containing 0.5 mM MgCl2, pH 9.8). The plates were read at 405 nm in ELISA plate reader (Microscan, ECIL, India).
Data analysis: The cut-off was determined by calculating the mean optical density (OD) obtained with sera from healthy individuals plus 3 standard deviations (SD). Each assay was repeated three times. Two or three positives out of three ELISAs were considered as positive. Sensitivity and specificity were calculated by standard methods. Scatter plot and ROC (receiver operative characteristic) curves were plotted using GraphPad Prism software, version 5, USA. ROC curve describes probabilities of correct and incorrect results at different cut-off values. The area under ROC curve reflects the accuracy of a test16.
Results
The 347 subjects when grouped into different categories, 78 were in category I PTB, 42 were in category II PTB, 80 were MDR PTB, 21 were tubercular meningitis, 30 were pleural effusion, 36 were non-disease controls and 60 were disease controls (other than tuberculosis) (Table I).
Fig. 1 shows the scatter plot of antibody response in different groups of patients and controls. Each dot represents the ΔO.D. (ΔO.D.= O.D. of sample - cut-off value) for individual patient. All dots above X-axis were positive O.Ds. Fig. 2A and B shows the receiver operative characteristic (ROC) curve for PTB (category I PTB, category II PTB and MDR) and extra-pulmonary TB (tuberculous meningitis and tubercular pleural effusion), respectively with 16 kDa antigen.
Fig. 1.
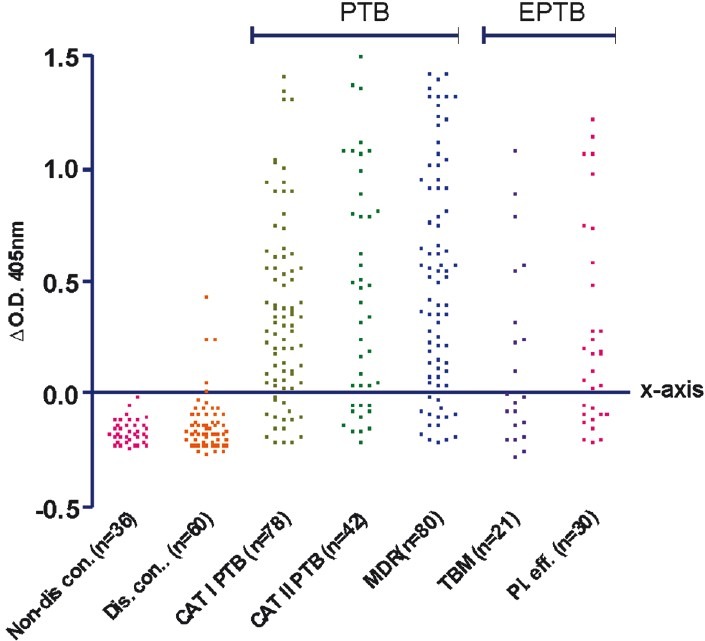
(I): Scatter plot of humoral responses in serum samples of non-disease controls, disease controls, category I PTB, category II PTB, MDR-TB, tuberculous meningitis (TBM) and tubercular pleural effusion patients. Each dot represents the ΔO.D. (ΔO.D. = O.D. of sample – cut-off value) for individual patient. All dots above X-axis are positive O.Ds. All dots below X-axis are negative O.Ds.
Fig. 2.
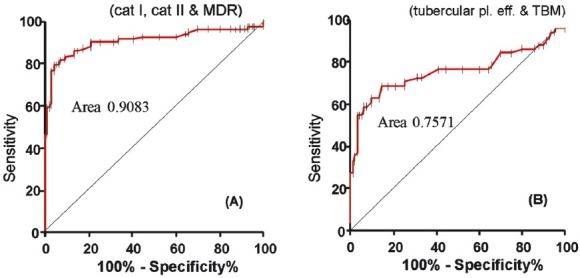
Receiver operative characteristic (ROC) curve for (A): PTB (category I PTB, category II PTB and MDR). Area under ROC curve 0.9083, 95% CI (confidence interval) 0.8739 to 0.9428, Std. error 0.01758, P<0.001. Small bars on the ROC curve showing the cut-offs. (B): EPTB (tuberculous meningitis and tubercular pleural effusion) area under ROC curve: 0.7571, 95% CI 0.6589 to 0.8554, Std. error 0.05013, P<0.001.
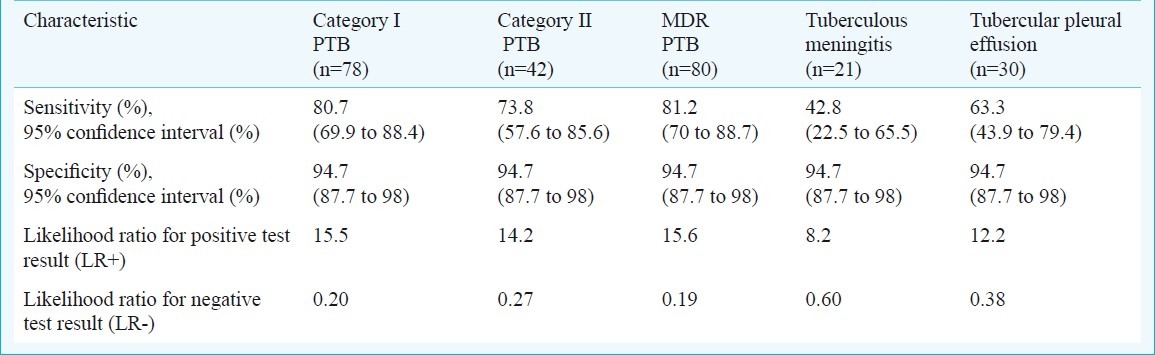
16 kDa antigen was used for detection of humoral responses (IgG and IgA) in these subjects using ELISA and sensitivity, specificity and other parameters were predicted (Table II). The diagnostic sensitivity of 16 kDa antigen with category I PTB, category II PTB and MDR PTB was found to be 80.7, 73.8 and 81.2 per cent, respectively. The sensitivity was found to be comparatively lower in category II PTB than the category I PTB and MDR PTB. While in the extra-pulmonary TB i.e. tuberculosis meningitis and tubercular pleural effusion cases, the diagnostic sensitivity was 42.8 and 63.3 per cent, respectively. Specificity was high (>94%) in PTB as well as extra-pulmonary tuberculosis groups (Table II).
Table II.
Sensitivity, specificity and likehood ratios of 16 kDa antigen ELISA for antibody detection
None of the non-disease controls was positive for antibodies to 16 kDa, however, five disease controls were found to be positive. These patients were being treated for lung cancer (1), sarcoidosis (1), interstitial lung disease (ILD 1), pneumonia (1) and respiratory distress (1).
Discussion
In the present study, we evaluated the 16 kDa antigen, in PTB and EPTB (TBM and tubercular pleural effusion) patients, and compared the antibody assay with other diagnostic modalities. In our study, none of the non-disease controls was positive for antibodies to 16 kDa. In a TB endemic country like India a majority of the population is likely to be latently infected or BCG-vaccinated; the absence of any humoral response against mycobacteria gives credence to high specificity of the antigen for detecting active disease. Geluk et al17 have shown most M. tuberculosis infected or exposed individuals responded well to HspX, whereas significantly lower responses were observed in unexposed individuals, including BCG-vaccinated individuals. Rabahi et al18 have shown higher HspX in the M. tuberculosis exposed individual and good indicator for new infection. Davidow et al19 found higher humoral response among inactive TB than the active TB.
A meta-analysis performed by Steingart et al20 has reported high specificity for detection of pulmonary tuberculosis (86 to 100%), using the commercially available “pathozyme TB complex plus” (ELISA based antibody detection, utilizes the recombinant 38 kDa and 16 kDa) in various studies21–23. Another study has shown 98 per cent specificity in a large number of Polish population24 using the above test. Raja et al25 have shown a specificity of 94 per cent using 16 kDa antigen for antibody detection in pulmonary tuberculosis patients and controls including non-tuberculous lung disease and healthy subjects.
Several antigens have been shown to have strong immunodiagnostic potential. A couple of studies are available for the 16 kDa molecule, a polypeptide belonging to the α-crystallin family of low molecular weight heat shock proteins. The coding gene (Rv2031c) for this antigen has been found exclusively in the M. tuberculosis complex as shown by DNA hybridization studies26. It has been reported as a dominant protein, produced in the static growth phase or under oxygen deprivation and is essential for bacterial replication inside macrophages11.
In non-tubercular disease controls, five of 60 controls gave the non-specific positive humoral response. Though sputum/induced sputum were obtained from above disease control group, all were negative for AFB smear microscopy and MGIT-960 rapid culture. All the care had been employed at the time of enrollment of above disease controls to rule out history of active TB. An earlier study26 has shown that antibodies against 16 kDa do not cross-react with common environmental mycobacteria. Julian et al21 have shown some non-specificity in pneumonia population using 16 kDa antibody detection, antibodies to 16 kDa have been shown among non-TB lung diseases such as asthma, lung cancer25. The loss of specificity may be due to latent infection or to nonspecific hyperglobulinaemia, common in bronchial diseases27.
Our results have shown a significant (P<0.05) higher antibody response in PTB than EPTB for 16 kDa antigen. Many of the serological studies have been reported from patients with extensive pulmonary disease24,28. The group that would most benefit from serological tests is the one with smear negative or extra-pulmonary TB. In this group of patients, the sensitivity of different assays was reported to be significantly lower than in the confirmed cases9,24,29. The extensive extra-cellular bacterial replication in cavities and the continued shedding of antigens is possibly enhanced in the pulmonary cavitary environment. The paucibacillary and often walled off EPTB lesions30 may be different in producing lesser amount of antigen and hence may take longer to generate an antibody response. The study patients were sampled within a few days of the clinical diagnosis and hence may not have shown an adequate serological response.
The sensitivity was much higher in category I, category II PTB and MDR PTB patients. Repeated immune stimulation by mycobacterial antigens and higher antigenic load because of multiplying mycobacteria in MDR-PTB and category II PTB may explain this.
Serodiagnostic tests for tuberculosis have never been very successful due to suboptimal sensitivity and specificity. To discourage the rampant use of commercial TB serodiagnostics tests, WHO has issued a policy note discouraging the use of current serodiagnostics tests31.
The present study has limitations such as the serologic tests were performed retrospectively; using serum that had been stored at -80°C, although no serum had been thawed more than once. The total number of EPTB (TBM and tubercular pleural effusion) subjects enrolled in the study was small. The results in our study were encouraging only in PTB group; and in tuberculous meningitis and tubercular pleural effusion patients were not up to expectations. The use of additional antigen(s) combinations or epitopes need to be evaluated for serodiagnosis.
Acknowledgment
The first and third authors (AK, CP) acknowledge the research fellowships from the Council of Scientific and Industrial Research (CSIR) and Indian Council of Medical Research (ICMR), New Delhi, respectively. 16 kDa antigen was kindly provided under NIH contract HHSN266200400091C/ADB (contract NO1-AI-40091, TBVTRM).
References
- 1.World Health Organization. Global tuberculosis control, WHO report. 2010. [accessed on October 31, 2011]. Available from: http://www.who.int/tb/publications/global_report/2010/en/index.html .
- 2.Keeler E, Perkins MD, Small P, Hanson C, Reed S, Cunningham J, et al. Reducing the global burden of tuberculosis: the contribution of improved diagnostics. Nature. 2006;444(Suppl 1):49–57. doi: 10.1038/nature05446. [DOI] [PubMed] [Google Scholar]
- 3.Young DB, Perkins MD, Duncan K, Barry CE. Confronting the scientific obstacles to global control of tuberculosis. J Clin Invest. 2008;118:1255–65. doi: 10.1172/JCI34614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minion J, Zwerling A, Pai M. Diagnostics for tuberculosis: what new knowledge did we gain through The International Journal of Tuberculosis and Lung Disease in 2008? Int J Tuberc Lung Dis. 2009;13:691–7. [PubMed] [Google Scholar]
- 5.Menzies D. What is the current and potential role of diagnostic tests other than sputum microscopy and culture? In: Frieden T, editor. Toman's tuberculosis: case detection, treatment, and monitoring - questions and answers. 2nd ed. Switzerland: World Health Organization; 2004. pp. 87–91. [Google Scholar]
- 6.Perkins MD, Roscigno G, Zumla A. Progress towards improved tuberculosis diagnostics for developing countries. Lancet. 2006;367:942–3. doi: 10.1016/S0140-6736(06)68386-4. [DOI] [PubMed] [Google Scholar]
- 7.Silva VM, Kanaujia G, Gennaro ML, Menzies D. Factors associated with humoral response to ESAT-6, 38 kDa and 14 kDa in patients with a spectrum of tuberculosis. Int J Tuberc Lung Dis. 2003;7:478–84. [PubMed] [Google Scholar]
- 8.Steingart KR, Dendukuri N, Henry M, Schiller I, Nahid P, Hopewell PC, et al. Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis. Clin Vaccine Immunol. 2009;16:260–76. doi: 10.1128/CVI.00355-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demkow U, Zielonka TM, Strza kowski J, Micha owska-Mitczuk D, Augustynowicz-Kopeæ E, Bialas-Chromiec B, et al. Diagnostic value of IgG serum level against 38-kDa mycobacterial antigen. Pneumonol Alergol Pol. 1998;66:509–16. [PubMed] [Google Scholar]
- 10.Lee Y, Hefta SA, Brennan PJ. Characterization of the major membrane protein of virulent Mycobacterium tuberculosis. Infect Immun. 1992;60:2066–74. doi: 10.1128/iai.60.5.2066-2074.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan Y, Crane DD, Barry CE., III Stationary phase-associated protein expression in Mycobacterium tuberculosis: function of the mycobacterial α-crystallin homolog. J Bacteriol. 1996;178:4484–92. doi: 10.1128/jb.178.15.4484-4492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahuja GK, Mohan KK, Prasad K, Behari M. Diagnostic criteria for tuberculous meningitis and their validation. Tuber Lung Dis. 1994;75:149–52. doi: 10.1016/0962-8479(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 13.Kent PT, Kubica GP. Public health mycobacteriology: A guide for the level III laboratory. Atlanta (GA): Centers for Disease Control; 1985. [Google Scholar]
- 14.Singh KK, Dong Y, Hinds L, Keen MA, Belisle JT, Zolla-Pazner S, et al. Combined use of serum and urinary antibody for diagnosis of tuberculosis. J Infect Dis. 2003;188:371–7. doi: 10.1086/376532. [DOI] [PubMed] [Google Scholar]
- 15.Singh KK, Sharma N, Vargas D, Liu Z, Belisle JT, Potharaju V, et al. Peptides of a novel Mycobacterium tuberculosis-specific cell wall protein for immunodiagnosis of tuberculosis. J Infect Dis. 2009;200:571–81. doi: 10.1086/603539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altman DG, Bland JM. Statistics notes: Diagnostic tests 3: Receiver operating characteristic plots. Br Med J. 1994;309:188–9. doi: 10.1136/bmj.309.6948.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geluk A, Lin MY, van Meijgaarden KE, Leyten EM, Franken KL, Ottenhoff TH, et al. T-cell recognition of the HspX protein of Mycobacterium tuberculosis correlates with latent M. tuberculosis infection but not with M. bovis BCG vaccination. Infect Immun. 2007;75:2914–21. doi: 10.1128/IAI.01990-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabahi MF, Junqueira-Kipnis AP, Dos Reis MC, Oelemann W, Conde MB. Humoral response to HspX and GlcB to previous and recent infection by Mycobacterium tuberculosis. BMC Infect Dis. 2007;7:148. doi: 10.1186/1471-2334-7-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davidow A, Kanaujia GV, Shi L, Kaviar J, Guo XD, Sung N, et al. Antibody profiles characteristic of Mycobacterium tuberculosis infection state. Infect Immun. 2005;73:6846–51. doi: 10.1128/IAI.73.10.6846-6851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steingart KR, Henry M, Laal S, Hopewell PC, Ramsay A, Menzies D, et al. Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: a systematic review. PLoS Med. 2007;4:e202. doi: 10.1371/journal.pmed.0040202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Julián E, Matas L, Alcaide J, Luquin M. Comparison of antibody responses to a potential combination of specific glycolipids and proteins for test sensitivity improvement in tuberculosis serodiagnosis. Clin Diagn Lab Immunol. 2004;11:70–6. doi: 10.1128/CDLI.11.1.70-76.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butt T, Malik HS, Abbassi SA, Ahmad RN, Mahmood A, Karamat KA, et al. Genus and species-specific IgG and IgM antibodies for pulmonary tuberculosis. J Coll Physicians Surg Pak. 2004;14:105–7. [PubMed] [Google Scholar]
- 23.Imaz MS, Comini MA, Zerbini E, Sequeira MD, Latini O, Claus JD, et al. Evaluation of commercial enzyme-linked immunosorbent assay kits for detection of tuberculosis in Argentinean population. J Clin Microbiol. 2004;42:884–7. doi: 10.1128/JCM.42.2.884-887.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demkow U, Ziółkowski J, Filewska M, Białas-Chromiec B, Zielonka T, Michalowska-Mitczuk D, et al. Diagnostic value of different serological tests for tuberculosis in Poland. J Physiol Pharmacol. 2004;55(Suppl 3):57–66. [PubMed] [Google Scholar]
- 25.Raja A, Uma Devi KR, Ramalingam B, Brennan PJ. Immunoglobulin G, A, and M responses in serum and circulating immunocomplexes elicited by the 16-kilodalton antigen of Mycobacterium tuberculosis. Clin Diagn Lab Immunol. 2002;9:308–12. doi: 10.1128/CDLI.9.2.308-312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manca C, Lyashchenkoo K, Wiker HG, Usai D, Colangeli R, Gennaro M. Molecular cloning, purification and serological characterization of MPT63, a novel antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1997;65:16–23. doi: 10.1128/iai.65.1.16-23.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bothamley GH, Rudd RM. Clinical evaluation of a serological assay using a monoclonal antibody (TB72) to the 38kDa antigen of Mycobacterium tuberculosis. Eur Respir J. 1994;7:240–6. doi: 10.1183/09031936.94.07020240. [DOI] [PubMed] [Google Scholar]
- 28.Julian E, Matas L, Hernandez A, Alcaide J, Luquin M. Evaluation of a new serodiagnostic TB test based on immunoglobulin A detection against Kp-90 antigen. Int J Tuberc Lung Dis. 2000;4:1082–5. [PubMed] [Google Scholar]
- 29.Daniel TM, Debanne SM. Serodiagnosis of TB and other mycobacterial diseases by enzyme linked immunosorbent assay. Am Rev Respir Dis. 1987;135:1137–51. doi: 10.1164/arrd.1987.135.5.1137. [DOI] [PubMed] [Google Scholar]
- 30.Chaisson RE, Jean N. Tuberculosis. In: Warrell DA, Cox TM, Firth JD, editors. Oxford textbook of medicine. Vol. 1. United Kingdom: Oxford University Press; 2003. pp. 556–71. [Google Scholar]
- 31.Strategic and Technical Advisory Group for Tuberculosis (STAG-TB), WHO/HTM/TB/2010.18. Report of Tenth Meeting. Geneva, Switzerland: WHO Headquarters; 2010. Sep 27-29, [accessed on June 31, 2011]. Available from: http://www.who.int/tb/advisory_bodies/stag_tb_report_2010.pdf . [Google Scholar]


