Abstract
While several equivalent alternatives are available in the bariatric algorithm, more recently the laparoscopic sleeve gastrectomy (SG) has been gaining traction as an effective means of weight loss in patients with morbid obesity. We present the case of a 39-year-old woman with situs inversus totalis, who was taken to the operating room for laparoscopic SG. The patient had previously undergone a failed open gastric banding procedure 20 months earlier. Awareness of the inherited condition before performing the operation allows for advanced planning and preparation. Subsequent modifications to the standard trocar placement help make the procedure more technically feasible. To our knowledge, this is the first published report of a laparoscopic SG after open gastric banding in a patient with situs inversus totalis. After encountering the initial disorientation, we believe experienced laparoscopic surgeons can perform this procedure successfully and safely.
Keywords: Gastric band, laparoscopy, situs inversus totalis, sleeve gastrectomy
INTRODUCTION
Situs inversus totalis, a congenital anomaly present in approximately 0.01% of the population, results in complete mirror-image reversal of all the thoracic and abdominal organs. The laparoscopic literature has several reports of successful bariatric procedures in patients with situs inversus despite the extremely low prevalence of this condition.[1–3] While several equivalent alternatives are available in the bariatric algorithm, more recently the laparoscopic sleeve gastrectomy (SG) has been gaining traction as an effective means of weight loss in patients with morbid obesity.[4–7]
CASE REPORT
A 39-year-old woman who had previously undergone an open gastric banding procedure 20 months earlier for morbid obesity presented with increased weight gain. Preoperatively, her weight had been 108 kg. Her weight at presentation was 106 kg (body mass index 42 kg/m2), a percentage excess weight loss (EWL) of only 1.9%. Her medical history was significant for hypertension, noninsulin-dependent diabetes mellitus, hypothyroidism, obstructive sleep apnea, and situs inversus totalis. The presence of situs inversus was initially discovered during her first weight loss operation and confirmed by computed tomography of the abdomen [Figure 1]. The operating surgeon began the case laparoscopically, but upon recognizing the altered anatomy made the decision to convert to an open gastric band placement. Given the failure of this first procedure to produce meaningful weight loss, the patient was scheduled for laparoscopic removal of the gastric band with concurrent SG.
Figure 1.
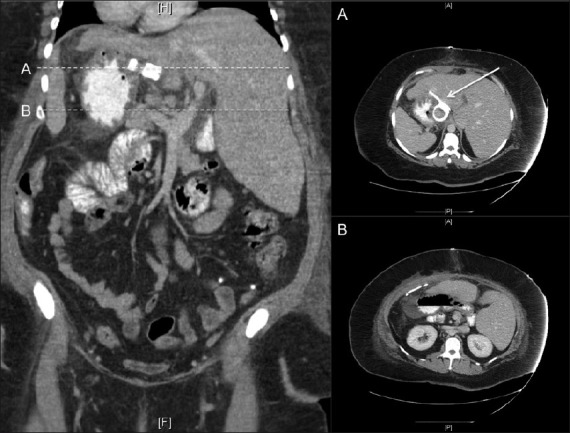
Coronal computed tomographic scan of the abdomen and pelvis with oral and intravenous contrast demonstrating the mirror-image anatomy. Panel A displays a cross-section of the band in its position on the right side of the abdomen. Panel B further demonstrates the altered anatomy with left-sided liver and right-sided stomach
Operation
The patient was positioned supine on the operating room table. Secondary to the presence of situs inversus, the Veress needle was placed in the right upper quadrant to avoid the liver. A 12-mm trocar was placed five fingerbreadths above the umbilicus, just to the right of midline. Next, a 5-mm trocar was placed in the left lower quadrant approximately two fingerbreadths below the umbilicus in the midclavicular line. A 12-mm port was then placed under direct vision in the left midclavicular line approximately five fingerbreadths above the umbilicus. A 12-mm port was placed in the right upper quadrant in the midclavicular line, and a 5-mm port was placed in the right upper quadrant one fingerbreadth below the costal margin in the midaxillary line [Figure 2]. Exploration revealed extensive adhesions as a result of her previous open procedures. At this point, the stomach was identified and noted to be severely adhered to the liver, which was, in turn, adherent to the abdominal wall. The stomach was mobilized, the band was identified, and the band buckle was cut and removed. The previous fundoplication was taken down using a 3.5-mm depth 45-mm length stapler, and the greater curvature and short gastric vessels were divided using a harmonic scalpel. After performing the dissection all the way to the angle of His and exposing the left crux of the diaphragm, a 34-French bougie was placed into the stomach with the tip of the bougie sitting in the proximal duodenum. The formation of the gastric sleeve with a 4.8-mm depth 45-mm length stapler was started from a point 5 cm proximal to the pylorus muscle and directed toward the angle of His [Figure 3]. Eight total firings were used, thus dividing the stomach completely and forming a gastric tube over the 34-French bougie. At the most proximal aspect of the stomach, several dense adhesions to the diaphragm were encountered and eventually taken down. Following the formation of the gastric tube, the staple line was oversewn with 2-0 absorbable suture using an Endo Stitch (Covidien Inc., Norwalk, CT) device in a running fashion. An upper endoscopy was then performed to ensure there was no stricture or leak. After submerging the gastric tube underwater, air was pumped into the scope, and subsequently no leak was appreciated. Before removal of the last trocar, the detached stomach was placed into a specimen retrieval bag and removed from the peritoneal cavity via the 12-mm port in the right upper quadrant. The same trocar site was slightly extended to allow for removal of the port and band. Finally, a Jackson–Pratt drain was placed by the sleeve for surveillance purposes.
Figure 2.
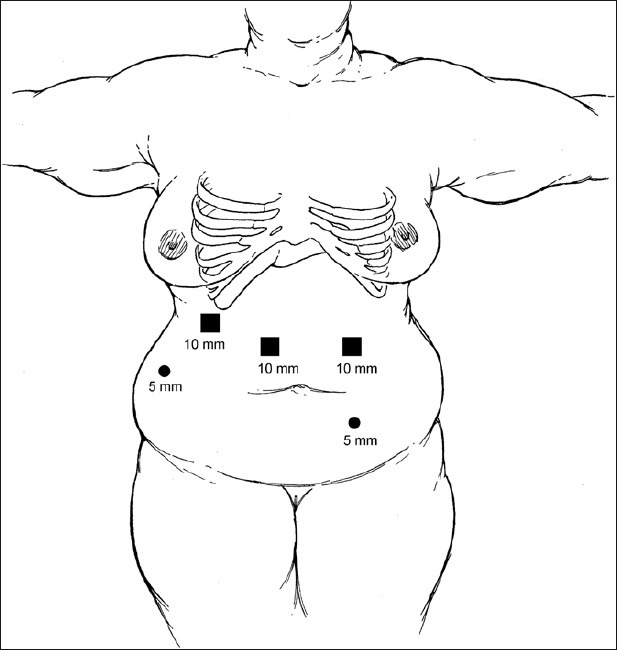
Novel Port Placement (Copyright SAGES 2010, Soper NJ, Swanstrom LL, Eubanks WS, eds. Mastery of Endoscopic and Laparoscopic Surgery)
Figure 3.
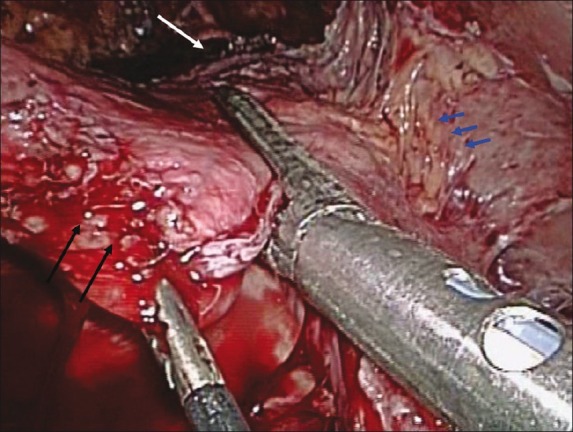
Intraoperative image (white arrow indicates the angle of His; blue arrows indicate several gastrohepatic adhesions, and black arrows indicate the greater curvature of the stomach)
Postoperative
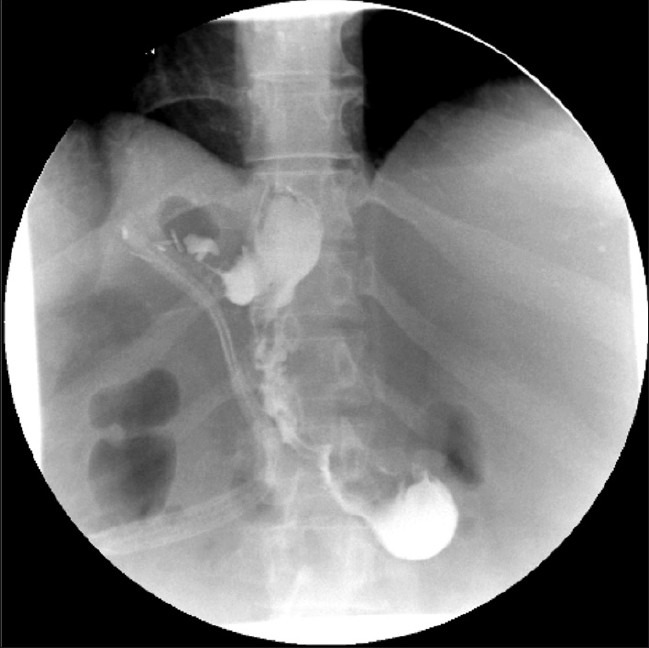
The postoperative course was complicated by a leak resulting in a 3.9 × 2.8 cm collection in the gastrosplenic ligament adjacent to the surgical suture line [Figure 4]. A Jackson–Pratt drain was left alongside the SG at the time of surgery as this is the surgeons protocol for revisional surgery. The Jackson–Pratt completely drained the collection. The patient underwent an upper endoscopy and placement of a 12-cm removable polyflex stent (Boston Scientific Inc., Natick, MA) for management of the leak [Figure 5]. Over the next few weeks her symptoms, mainly epigastric pain, persisted despite successful placement of the stent. Further imaging demonstrated interval migration of the stent into the stomach with no change in the size or location of the perigastric collection [Figure 6]. The patient was managed conservatively despite the stent movement and had eventual resolution of her symptoms. The stent was subsequently removed without any complications. Currently, the patient is 2 years out from the laparoscopic SG. She has lost approximately 60% EWL of her preoperative weight with no further complications.
Figure 4.
Postoperative upper GI series demonstrating extravasation of contrast at the most proximal aspect of the staple line
Figure 5.
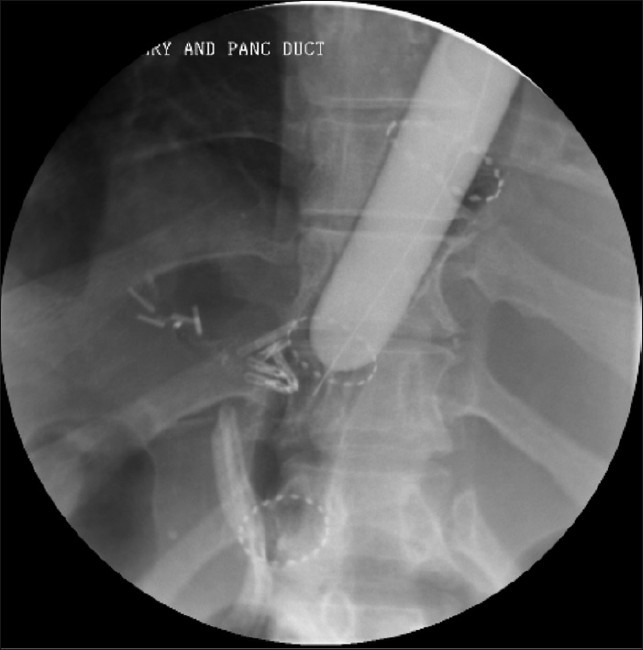
Completion radiograph following endoscopic placement of a removable stent across the anastamotic leak site
Figure 6.
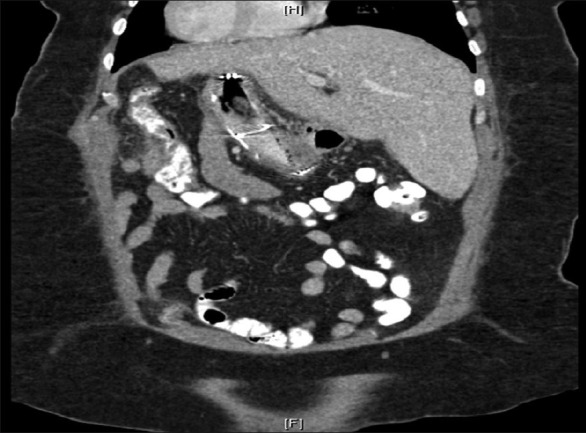
Coronal computed tomographic scan of the abdomen and pelvis with oral and intravenous contrast demonstrating complete migration of the stent into the body of the stomach
DISCUSSION
To our knowledge, this is the first published report of a laparoscopic SG after open gastric banding in a patient with situs inversus totalis. The altered anatomy presents a challenge for the surgeon. However, modifications in the standard trocar placement can help make the procedure more technically feasible. Awareness of the inherited condition before undertaking the operation allows for advanced planning and preparation. In this case, the history of prior open gastric banding made the operation especially tricky, primarily due to the numerous adhesions encountered. Revisional surgery is always difficult, but when performed in a patient with situs inversus may be even more challenging. After encountering the initial disorientation, experienced laparoscopic surgeons should have no problem adjusting to the mirror-image anatomy.
We believe the postoperative leak occurred secondary to the difficult dissection around the gastric fundus, independent of the altered anatomy. These anastamotic leaks can be classified as early or late, depending on how soon postoperatively they occur. In the early period (<3 days), many advocate primary repair. Washout and drainage is also an excellent alternative in patients with severe inflammation, signs of sepsis, or hemodynamic instability.[8] A late leak presents a more difficult test. The endoscopic placement of polyester or nitinol covered stents is a fairly recent innovation, allowing patients to be maintained on oral enteral nutrition. There have been several reports in the literature supporting their use in both early and late leaks.[9–11] The accepted leak rate for SG is 1.6% at the esophagogastric junction and 0.8% at lower points along the staple line.[12]
CONCLUSION
Laparoscopic vertical SG after open gastric banding in a patient with situs inversus can be performed safely by experienced laparoscopic surgeons. While our case yielded a good result, other similar cases are needed to establish the reproducibility and long-term efficacy of the procedure.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Samaan M, Ratnasingham A, Pittathankal A, Hashemi M. Laparoscopic adjustable gastric banding for morbid obesity in a patient with situs inversus totalis. Obes Surg. 2008;18:898–901. doi: 10.1007/s11695-008-9445-7. [DOI] [PubMed] [Google Scholar]
- 2.Matar ZS. Laparoscopic adjustable gastric banding in a morbidly obese patient with situs inversus totalis. Obes Surg. 2008;18:1632–5. doi: 10.1007/s11695-008-9546-3. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed AR, O’Malley W. Laparoscopic Roux-en-Y gastric bypass in a patient with situs inversus. Obes Surg. 2006;16:1392–4. doi: 10.1381/096089206778663670. [DOI] [PubMed] [Google Scholar]
- 4.Bellanger DE, Greenway FL. Laparoscopic sleeve gastrectomy, 529 cases without a leak: Short-term results and technical considerations. Obes Surg. 2011;21:146–50. doi: 10.1007/s11695-010-0320-y. [DOI] [PubMed] [Google Scholar]
- 5.Gluck B, Movitz B, Jansma S, Gluck J, Laskowski K. Laparoscopic sleeve gastrectomy is a safe and effective bariatric procedure for the lower BMI (35.0-43.0 kg/m(2)) population. Obes Surg. 2010 doi: 10.1007/s11695-010-0332-7. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252:319–24. doi: 10.1097/SLA.0b013e3181e90b31. [DOI] [PubMed] [Google Scholar]
- 7.Akkary E, Duffy A, Bell R. Deciphering the sleeve: Technique, efficacy, and safety of sleeve gastrectomy. Obes Surg. 2008;18:1323–9. doi: 10.1007/s11695-008-9551-6. [DOI] [PubMed] [Google Scholar]
- 8.Márquez MF, Ayza MF, Lozano RB, Morales Mdel M, Díez JM, Poujoulet RB. Gastric leak after laparoscopic sleeve gastrectomy. Obes Surg. 2010;20:1306–11. doi: 10.1007/s11695-010-0219-7. [DOI] [PubMed] [Google Scholar]
- 9.Casella G, Soricelli E, Rizzello M, Trentino P, Fiocca F, Fantini A, et al. Nonsurgical treatment of staple line leaks after laparoscopic sleeve gastrectomy. Obes Surg. 2009;19:821–6. doi: 10.1007/s11695-009-9840-8. [DOI] [PubMed] [Google Scholar]
- 10.Eubanks S, Edwards CA, Fearing NM, Ramaswamy A, de la Torre RA, Thaler KJ, et al. Use of endoscopic stents to treat anastamotic complications after bariatric surgery. J Am Coll Surg. 2008;206:935–8. doi: 10.1016/j.jamcollsurg.2008.02.016. discussion 938-9. [DOI] [PubMed] [Google Scholar]
- 11.Blackmon SH, Santora R, Schwarz P, Barroso A, Dunkin BJ. Utility of removable esophageal covered self-expanding metal stents for leak and fistula management. Ann Thorac Surg. 2010;89:931–6. doi: 10.1016/j.athoracsur.2009.10.061. discussion 936-7. [DOI] [PubMed] [Google Scholar]
- 12.Deitel M, Crosby RD, Gagner M. The First International Consensus Summit for Sleeve Gastrectomy (SG), New York City, October 25-27, 2007. Obes Surg. 2008;18:487–96. doi: 10.1007/s11695-008-9471-5. [DOI] [PubMed] [Google Scholar]


