Abstract
Blood stream infections and pneumonia caused by Pseudomonas aeruginosa is associated with high mortality, especially in an immunocompromised host. A large section of the palliative care patient population has varied forms of compromised immunity due to advanced cancer or cancer treatment, organ failures, chronic autoimmune disorders, degenerative conditions, and acquired immunodeficiency syndrome. The lung is one of the most frequently involved organs in a variety of complications in an immunocompromised host and infection is the most common complication. P. aeruginosa is one of the most common pathogens associated with bronchopulmonary infections in an immunocompromised host. Routine radiological tests like chest X-ray may often be unyielding and an early and a prompt initiation of treatment reduces mortality and morbidity risk.
Keywords: Bronchopulmonary infections, Palliative care, Pseudomonas
INTRODUCTION
Pulmonary infection in the immunocompromised host remains a major cause of morbidity and high mortality. It presents with a tremendous challenge in cancer and palliative care settings in terms of clinical assessment, diagnosis, and treatment. Infections secondary to immunodeficiency in a cancer care setting are an expected sequel following most chemotherapeutic regimens, in bone marrow transplant recipients and in patients with acute leukemia. Lungs are common site of infection in patients with cancer. The spectrum of pulmonary infection depends on the underlying immunological deficits. In neutropenic patients, Gram-negative bacterial infections such as Pseudomonas, Klebsiella, etc., predominate early, whereas fungal infections are common in persistent neutropenia. In patients with impaired cellular immunity, viral, fungal, and atypical bacterial infections are commonly seen. Streptococcus pneumoniae and Haemophilus influenzae are the primary bacterial infections encountered in patients with impaired humoral immunity.[1] Multiple simultaneous infectious processes are common. These may include dual infection with Pneumocystis jirovecii (formerly Pneumocystis carinii) and cytomegalovirus, or superimposition of another process (lung injury or drug toxicity). Sequential infection (e.g., viral infection preceding bacterial or fungal infection) is also common. Pseudomonas is the most common nosocomial pathogen attributed to infections in an immunocompromised host. In patients with cancer, the incidence of infections caused by Pseudomonas aeruginosa is 1–2.5% among all the patients presenting with fever during neutropenia and 5–12% among patients with microbiologically documented infections. The mortality rate is increased by 40% among immunocompromised patients with P. aeruginosa pneumonia.[2]
Mr. S, is a 62-year-old male, was a known case of advanced multiple myeloma (Ig A Kappa type) with extensive skeletal metastases. He received chemotherapy and radiotherapy and as his disease had progressed on treatment, he was referred to the Department of Integrative Oncology for ongoing supportive and palliative care. His bone pain was well controlled with oral morphine, steroids, and salmon calcitonin nasal sprays. He was not initiated on NSAIDs due to low platelet count.
During one of his follow-up visit he presented to the OPD with 3 days history of progressive shortness of breath, orthopnea, tightness of the chest, and extreme fatigue. On clinical examination, he was pale, dehydrated, cyanosed (SpO2 84%) and had tachypnea (RR 36/min) and tachycardia (HR 122/min). Auscultation of the chest revealed bilateral decreased air entry, bilateral polyphonic wheezing, and bi-basilar coarse crepitations. He was acutely managed with IV steroids (hydrocortisone), oxygen, IV antibiotics (Augmentin Levofloxacin), IV fluids, IV bronchodilators (Doxophyline) and nebulization (Salbutamol+Ipravent).
Chest X-ray was normal. High-resonance chest CT (HRCT) showed micro-nodules with a tree-in-bud appearance and peribronchovascular interstitial thickening [Figure 1a and 1b].
Figure 1.
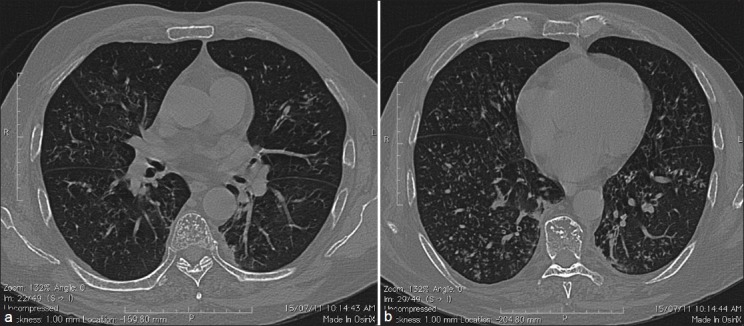
(a) HRCT of the lung showing a tree-in-bud pattern. (b) HRCT of the lung showing reticulonodular shadowing in bilateral lower zones
A bronchoalveolar lavage (BAL) was performed, and in view of his acute clinical condition, the sample was sent for the rapid identification of “pathogen spectrum causing pneumonia in an immunocompromised host” using the DNA microarray technique. Pseudomonas DNA was detected in the BAL sample. Subsequent culture and sensitivity results from the BAL sample showed a significant growth of Pseudomonas aeruginosa, which was sensitive to the antibiotics prescribed. The lavage was negative for fungal elements and parasites.
Following treatment, there was a significant improvement in dyspnea and fatigue and he was able to maintain adequate oxygen saturation in room air. He was discharged, and currently, he is on regular OPD follow-up.
ETIOPATHOGENESIS AND CLINICAL PRESENTATION
In a palliative care setting, immunocompromised states commonly ensue due to the loss of normal homeostasis. An increase in cytokines such as tumour necrosis factor, interleukins 1 and 6, interferon-γ along with altered protein, and carbohydrate and fat metabolism leads to the anorexia–cachexia syndrome.[3] Ineffective cough reflex and dysphagia increase the risk of respiratory infections by decreasing the ability of the lung to clear excessive mucus and secretions and increases the risk of aspiration.[4] Bacterial proliferation in the stomach is potentially enhanced by enteral nutrition in combination with the administration of antiulcer prophylaxis drugs, which jeopardize the physiological barrier of the gastric compartment by buffering the gastric content and thereby facilitate the colonization and proliferation of pathogenic organisms like Pseudomonas. The nasogastric tube increases the risk of the migration of these pathologic organisms from the aerodigestive tract to the respiratory tract.[5] The loss of mucosal barriers due to mucositis also increases the risk of Pseudomonas colonization and infection.
The P. aeruginosa genome is among the largest genomes in the prokaryotic world, and encodes an unusually high proportion of proteins involved in regulation, transport, and virulence functions, which explains the high versatility and adaptive capacity of this species. Around 0.3% of the total genes code for proteins involved in antimicrobial resistance. Most P. aeruginosa strains involved in infections are both invasive and toxigenic, as a result of the production of surface virulence factors (allowing bacterial attachment, colonization, and invasion) and secreted virulence factors (which damage tissues or trigger the production of cytokines), respectively.[6]
P. aeruginosa infects healthy tissues rarely, but, when defenses are compromised, it can infect virtually all tissues. Hence, it is the major nosocomial pathogen in immunocompromised and debilitated patients.[7] It has been increasingly recognized as an important source of bacterial pneumonia in HIV-infected individuals, those with neutropenia, prolonged steroid use, myelosupressive therapy, and multiple antibiotic use.[8] It causes acute pneumonia with a high mortality rate in immunocompromised patients.[9] Distal airways are usually sterile in a healthy nonsmoking individual; however, when mechanical airway defenses are altered, non potential pathogenic organisms such as Streptococcus viridans and Neisseria species frequently colonize distal airways. Colonization with non potential pathogenic organisms is seen in clinically stable populations with bronchogenic carcinoma (42%), COPD (83%), and bronchiectasis (88%). P. aeruginosa seldom colonize in a distal airway in an immunocompetent host.[10] In the EPIC study, P. aeruginosa was isolated from bronchopulmonary infection sites of patients hospitalized in 1417 intensive care units in 17 Western European countries. P. aeruginosa is a lethal pathogen with 34% of bacteraemic mortality and 50% mortality in the bacteraemic neutropenic host and 45% overall mortality in bacteraemic nosocomial pneumonia.[11] Among the anatomic sites of P. aeruginosa infection, lungs are associated with the highest mortality rate.[12]
A usual clinical presentation is of an acute illness with cough producing mucopurulent sputum, breathlessness, wheezing, along with systemic features such as fever, leucocytosis, hypoxemia, and a significant elevation in the erythrocyte sedimentation rate and C-reactive protein over previous levels.[13]
IMAGING
Chest radiography has limited sensitivity for the detection of early infection in immunocompromised patients, and findings are often nonspecific. Another confounding factor is that a patient with neutropenia who has pneumonia may have delayed or unusually subtle radiographic findings in the lungs, which reflects the depressed inflammatory response.[14] Almost half of the cases of bacterial pneumonia in immunocompromised patients have a radiological pattern other than focal consolidation. They frequently present as a bilateral pattern of alveolar or interstitial opacities, which radiologically mimics other respiratory pathologies. Early in the course of pyogenic bronchitis and bronchiolitis in an immunocompromised host, a conventional chest radiograph fails to show any abnormality. Extensive bronchiolitis may create an apparent interstitial pattern of reticulonodular opacities that represent impacted bronchioles. This pattern is typically distributed with lower lobe predominance. High-resolution chest CT is the most sensitive and specific radiographic test for detecting inflammatory changes in bronchi and bronchioles. The characteristic findings are centrilobular opacities arranged in a tree-in-bud pattern, manifested by small, Y- and V-shaped opacities in the lung periphery.[8] High-resolution CT findings were studied in 28 patients who developed bacterial pneumonia following bone marrow transplantation. Among them, 81% had nodular opacities. In three patients, the small centrilobular nodules were associated with a tree-in-bud pattern. Only 12% had pure consolidation and majority of patients had various combinations of nodules, consolidation, and ground glass attenuation.[15]
MICROBIOLOGY
Percutaneous needle aspiration using fluoroscopy, sonography, and CT is an alternative method of obtaining specimens. With the accurate targeting of a pulmonary lesion, specimens and positive results provide reliable clues for the identification of specific organisms. Several articles reported variable diagnostic yields (11.7–73%) for percutaneous needle aspiration for the identification of specific organisms.[16] A bronchoalveolar lavage has a good diagnostic yield when assessing immunocompromised patients with pulmonary infiltrates.[17]
DNA microarrays allow transcriptional profiling of the partial or entire genetic repertoires of the pathogen.[18]
TREATMENT
P. aeruginosa exhibits a remarkable capacity to resist antibiotics, either intrinsically (because of the constitutive expression of β-lactamases and efflux pumps, and low permeability of the outer membrane) or following acquisition of resistance genes, over expression of efflux pumps, decreased expression of porins, or mutations in quinolone targets. The following guidelines for the specific management of P. aeruginosa infections in immunocompromised patients and neutropenia were proposed by a joint task force of the American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA): (1) any suspicion of Pseudomonas infection should require a culture and sensitivity; (2) therapy should be initiated as soon as clinical samples have been collected. Early therapy is associated with better outcomes and will usually include an antipseudomonal β-lactam associated with either an Aminoglycoside or a Fluoroquinolone. (3) Stepping down of treatment in de-escalation or modification of the therapy is mandatory once culture and sensitivity results are available. (4) The patient's condition should be re-evaluated on a regular basis, with appropriate measurements to decide whether antibiotics should be continued.[6] In an inpatient setting, the recommendations are to use an antipseudomonal β-lactam (Piperacillin–Tazobactam, Ceftazidime, Cefepime, Imipenem, or Meropenem) plus ciprofloxacin or Levofloxacin (750 mg dose) or β-lactam plus an Aminoglycoside and Azithromycin. In penicillin-allergic patients, β-lactam was substituted with Aztreonam. In an ambulatory patient not requiring inpatient or high-dependency admission, oral Levofloxacin 750 mg daily is the recommended treatment.[19] A continuous infusion of β-lactam is considered for the treatment of resistant P. aeruginosa infections in immunocompromised patients when conventional antibiotic options are limited.[20]
The Clinical Pulmonary Infection Score (CPIS) and initiation of antibiotics according to the CPIS protocol decrease the risk of overtreatment and emergence of the resistance.[12] Multidrug resistant P. aeruginosa represents an emerging problem due to multiple bacterial resistances. The increasing resistance of P. aeruginosa to quinolones may be a limiting factor. If possible, a constant infusion of β-lactam should be used, for it provides a more intense activity against this bacteria and decreases the risk of resistance.[21]
PROGNOSIS
P. aeruginosa pneumonia is associated with high in-hospital mortality rates and prolonged lengths of stay. Injury to the alveolar epithelium allows the release of pro-inflammatory mediators into the circulation that are primarily responsible for a septic shock. P. aeruginosa pneumonia accompanied by P. aeruginosa bacteremia is associated with a particularly poor prognosis, with death occurring 3–4 days after the first signs of infection in the majority of cases. Other factors significantly associated with a poor prognosis include advanced age, serious underlying disease, septic shock, acute respiratory distress syndrome, previous surgery, use of broad-spectrum antibiotics in previous 6 months, and multidrug resistant organisms.[22–24]
CONCLUSIONS
Palliative care patients have immunocompromised states due to multifactorial causes.
Pseudomonas bronchopulmonary infection is common in immunocompromised host and associated with high morbidity and mortality.
Clinical presents as acute progressive dyspnea mimicking respiratory failure secondary to advanced cancer involving bronchopulmonary system.
Early diagnosis and prompt initiation of treatment resolves symptoms and improves survival.
There is a need for larger prospective studies to know the extent and impact of pseudomonas infections in palliative care patients.
Footnotes
Source of Support: None.
Conflict of Interest: None.
REFERENCES
- 1.El-Mahallawy HA, Ibrahim MH, Shalaby L, Kandil A. Community respiratory viruses as a cause of lower respiratory tract infections following suppressive chemotherapy in cancer patients. J Egypt Natl Canc Inst. 2005;17:121–6. [PubMed] [Google Scholar]
- 2.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2005;171:1209–23. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevenson J, Abernethy AP, Miller C, Currow DC. Managing comorbidities in patients at the end of life. BMJ. 2004;329:909–12. doi: 10.1136/bmj.329.7471.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonneau A. Palliative Care Files Cough in the palliative care setting. Can Fam Physician. 2009;55:600–2. [PMC free article] [PubMed] [Google Scholar]
- 5.Forestier C, Guelon D, Cluytens V, Gillart T, Sirot J, De Champs C. Oral probiotic and prevention of Pseudomonas aeruginosa infections: Arandomized, double-blind, placebo-controlled pilot study in intensive care unit patients. Crit Care. 2008;12:R69. doi: 10.1186/cc6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mesaros N, Nordmann P, Plésiat P, Roussel-Delvallez M, Van Eldere J, Glupczynski Y, et al. Pseudomonas aeruginosa: Resistance and therapeutic options at the turn of the new millennium. Clin Microbiol Infect. 2007;13:560–78. doi: 10.1111/j.1469-0691.2007.01681.x. [DOI] [PubMed] [Google Scholar]
- 7.Bouza E, Garcia-Garrote F, Cercenado E, Marin M, Diaz MS. Pseudomonas aeruginosa: Asurvey of resistance in 136 hospitals in Spain. The Spanish Pseudomonas aeruginosa Study Group. Antimicrob Agents Chemother. 1999;43:981–2. doi: 10.1128/aac.43.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brecher CW, Aviram G, Boiselle PM. CT and radiography of bacterial respiratory infections in AIDS patients. AJR Am J Roentgenol. 2003;180:1203–9. doi: 10.2214/ajr.180.5.1801203. [DOI] [PubMed] [Google Scholar]
- 9.Debarbieux L, Leduc D, Maura D, Morello E, Criscuolo A, Grossi O, et al. Bacteriophages can treat and prevent Pseudomonas aeruginosa lung infections. J Infect Dis. 2010;201:1096–104. doi: 10.1086/651135. [DOI] [PubMed] [Google Scholar]
- 10.Cabello H, Torres A, Celis R, El-Ebiary M, Puig de la Bellacasa J, Xaubet A, et al. Bacterial colonization of distal airways in healthy subjects and chronic lung disease: Abronchoscopic study. Eur Respir J. 1997;10:1137–44. doi: 10.1183/09031936.97.10051137. [DOI] [PubMed] [Google Scholar]
- 11.Giamarellou H. Prescribing guidelines for severe Pseudomonas infections. J Antimicrob Chemother. 2002;49:229–33. doi: 10.1093/jac/49.2.229. [DOI] [PubMed] [Google Scholar]
- 12.Fujitani S, Sun H-Y, Yu VL, Weingarten JA. Pneumonia due to Pseudomonas aeruginosa: Part I: Epidemiology, clinical diagnosis, and source. Chest. 2011;139:909–19. doi: 10.1378/chest.10-0166. [DOI] [PubMed] [Google Scholar]
- 13.Domingo P, Ferré A, Baraldès MA, Ris J, Sánchez F. Pseudomonas aeruginosa bronchopulmonary infection in patients with AIDS, with emphasis on relapsing infection. Eur Respir J. 1998;12:107–12. doi: 10.1183/09031936.98.12010107. [DOI] [PubMed] [Google Scholar]
- 14.Oh YW, Effmann EL, Godwin JD. Pulmonary infections in immunocompromised hosts: The importance of correlating the conventional radiologic appearance with the clinical setting. [Last accessed on 2011 Oct 11];Radiology [Internet] 2000 217:647–56. doi: 10.1148/radiology.217.3.r00dc35647. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11110924 . [DOI] [PubMed] [Google Scholar]
- 15.Findings H-resolution CT, Escuissato DL, Gasparetto EL, Rocha GDM, Inoue C, Pasquini R, et al. Bone Marrow Transplantation: In 111 Patients. Bone Marrow Transplant. 2005 Sep;:608–15. doi: 10.2214/ajr.185.3.01850608. [DOI] [PubMed] [Google Scholar]
- 16.Hwang SS, Kim HH, Park SH, Jung JI, Jang HS. The value of CT-guided percutaneous needle aspiration in immunocompromised patients with suspected pulmonary infection. AJR Am J Roentgenol. 2000;175:235–8. doi: 10.2214/ajr.175.1.1750235. [DOI] [PubMed] [Google Scholar]
- 17.Xaubet A, Torres A, Marco F, Puig-De la Bellacasa J, Faus R, Agusti-Vidal A. Pulmonary infiltrates in immunocompromised patients. Diagnostic value of telescoping plugged catheter and bronchoalveolar lavage. Chest. 1989;95:130–5. doi: 10.1378/chest.95.1.130. [DOI] [PubMed] [Google Scholar]
- 18.Lory S, Ichikawa JK. Pseudomonas-epithelial cell interactions dissected with DNA microarrays. Chest. 2002;121:S36–9. doi: 10.1378/chest.121.3_suppl.36s. [DOI] [PubMed] [Google Scholar]
- 19.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moriyama B, Henning SA, Childs R, Holland SM, Anderson VL, Morris JC, et al. High-dose continuous infusion beta-lactam antibiotics for the treatment of resistant Pseudomonas aeruginosa infections in immunocompromised patients. Ann Pharmacother. 2010;44:929–35. doi: 10.1345/aph.1M717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun H-Y, Fujitani S, Quintiliani R, Yu VL. Pneumonia due to Pseudomonas aeruginosa: Part II: Antimicrobial resistance, pharmacodynamic concepts, and antibiotic therapy. Chest. 2011;139:1172–85. doi: 10.1378/chest.10-0167. [DOI] [PubMed] [Google Scholar]
- 22.Wang CY, Jerng JS, Chen KY, Lee LN, Yu CJ, Hsueh PR, et al. Pandrug-resistant Pseudomonas aeruginosa among hospitalised patients: Clinical features, risk-factors and outcomes. Clin Microbiol Infect. 2006;12:63–8. doi: 10.1111/j.1469-0691.2005.01305.x. [DOI] [PubMed] [Google Scholar]
- 23.Crabtree TD, Gleason TG, Pruett TL, Sawyer RG. Trends in nosocomial pneumonia in surgical patients as we approach the 21st century: A prospective analysis. Am Surg. 1999;65:706–9. discussion 710. [PubMed] [Google Scholar]
- 24.Kurahashi K, Kajikawa O, Sawa T, Ohara M, Gropper MA, Frank DW, et al. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. JClinInvest. 1999;104:743–50. doi: 10.1172/JCI7124. [DOI] [PMC free article] [PubMed] [Google Scholar]


