Abstract
Background:
This prospective double-blind trial was undertaken to analyze the role of perioperatively administered dexmedetomidine on the occurrence of chronic pain in cases undergoing surgery for breast cancer.
Subjects and Methods:
Eighty-six cases were randomly assigned to two groups to receive either dexmedetomidine (2 μg/ml) in group D or saline in group C, in a loading dose of 0.5 ml/kg, intravenous (IV), 30 min prior to induction, followed by a continuous infusion of 0.25 ml/kg/h IV till the completion of surgery, and then the dose tapered to 0.1 ml/kg/h for up to 24 h. The standardized questionnaires that measured chronic pain (Brief Pain Inventory, BPI; Short Form McGill Pain Questionnaire, SF-MPQ2) and quality of life (Quality of Life Scale, QOLS) were gathered after 3 months of surgery as a primary outcome. Pain (verbal numerical score, VNS), sedation scores (Ramsay scoring), and analgesic requirements were also assessed for 72 h postoperatively.
Results:
In total, 84 cases (n=42) were analyzed for acute pain and 69 (34 in group D and 35 in group C) for chronic pain. The consumption of isoflurane/fentanyl intra-operatively and paracetamol postoperatively was significantly lower in Group D. The sedation scores were non-significant between the groups. The VNS at rest and after movement was significantly lower in group D at corresponding times (except at 60 min) throughout the assessment period. The BPI and SF-MPQ2 scores were significantly lower in group D in most of the factors. The QOLS score was significantly better in group D in all items except for relationships, friends, and learning.
Conclusion:
The perioperative infusion of dexmedetomidine has a pivotal role in attenuating the incidence and severity of chronic pain and improving the quality of life in cases undergoing breast cancer surgery.
Keywords: Chronic pain, Dexmedetomidine, Postmastectomy pain, Quality of life, α2-Adrenoceptor
INTRODUCTION
The chronic pain after breast cancer surgery (postmastectomy pain syndrome) has been known to develop in 20–68% of patients.[1] It is believed to be a neuropathic pain condition associated with injury to sensory afferents during surgery and nerve entrapment, axillary hematoma, or development of a traumatic neuroma on the operated site.[1] All this contributes to chronic pain, especially in the area of mastectomy scar, axilla, shoulder, and the proximal arm. Furthermore, many patients suffer from persistent generalized pain after surgery for breast cancer. There is growing evidence that acute postoperative pain influences the development of postmastectomy pain syndrome by the chronic activation of nociceptors.[2–4] Thus, it is imperative to use medications that reduce the intensity and duration of perioperative pain/anxiety to improve the quality of life in these cases.
Dexmedetomidine, a selective α-2 adrenoceptor agonist, was initially introduced for short-term intravenous (IV) sedation of mechanically ventilated patients in intensive care units. The analgesic, sedative, and anxiolytic properties of dexmedetomidine made this drug a valuable medication for the perioperative period. Its additional advantages are the opiod-sparing effect and preservation of respiratory functions, even at higher doses. Various studies have investigated its sedative and analgesic sparing effect on acute postoperative pain after major surgical procedures.[5,6] However, the effect on chronic pain has not been the primary end-point of any trial on dexmedetomidine. Thus, the purpose of this study was to assess whether the analgesic-sparing effect of perioperatively administered dexmedetomidine has any impact on the occurrence of chronic pain in cases undergoing surgery for breast cancer.
SUBJECTS AND METHODS
After the ethical committee approval and written/informed consent, 86 ASA physical status I or II female patients, aged >18 years, and with BMI 25±20%, post breast surgery between May 2009 and Oct 2011, were included in this trial. Patients with a current history of any psychiatric disorder or presently on psychotropic, α-2 agonists or opioid medications within 28 days before scheduled surgery, any end-organ dysfunction, pregnancy, alcohol abuse, or smoking habit, and other painful or disabling medical conditions, such as arthritis, were excluded.
The study was designed in a double-blind, prospective fashion. Using computer-generated random numbers, all patients were randomly allotted to the dexmedetomidine group (group D) or the control group (group C). For group D, a volume of 1 ml of dexmedetomidine (100 μg/ml) was diluted with 49 ml of 0.9% saline to prepare a solution of 2 μg/ml (50 ml volume) concentration. For group C, a placebo was prepared by loading a 50-ml volume of the 0.9% saline solution in a syringe. Randomization was done by an anesthesiologist intended to prepare the studied drug solution. Further intervention and monitoring was done by an investigator blinded to group allocation.
Premedication was omitted. In the preoperative ward, all patients were instructed on the proper use of the verbal numerical score (VNS) and Ramsay sedation score (RSS) for assessing pain and sedation. On arrival to the operative room, standard monitors were placed and baseline parameters recorded. Each patient received an initial loading dose of the studied drug solution (0.5 ml/kg IV as per group allocation) over 10 min prior to induction, followed by a continuous infusion of 0.25 ml/kg/h IV till the completion of surgery. Thereafter, the infusion rate was tapered to 0.1 ml/kg/h and continued for up to 24 h. General anesthesia was induced with lidocaine (1 mg/ kg IV), propofol (2 mg/kg IV), and fentanyl (2 μg/kg IV). Tracheal intubation was facilitated with rocuronium (0.6 mg/kg IV). Anesthesia was maintained with isoflurane (0.5–2%), and nitrous oxide/oxygen combination (60%/40%). Any rise in mean arterial pressure (MAP) of >20% from baseline was treated by administering a bolus dose of fentanyl (2 μg/kg IV) and raising the inspiratory concentration of isoflurane in steps of 0.2%. Any fall in MAP of <20% from baseline was managed by reducing the inspiratory concentration of isoflurane in steps of 0.2%. Our target was to maintain MAP within 20% limits of baseline values. The neuromuscular blockade was maintained by rocuronium (0.1 mg/kg IV), as required throughout surgery. At the end of surgery, the neuromuscular block was antagonized with neostigmine (0.05 mg/kg IV) and glycopyrrolate (0.01 mg/kg IV). The patients were extubated, and transferred to the postoperative care unit (POCU). All patients remained in the POCU for next 24 h and were thereafter shifted to the general ward. For any pain complaints (pain score ≥4), a dose of 1 g paracetamol IV was given on the first postoperative day (POD), with the shortest interval of at least 4 h between each dose. From the second POD, paracetamol tablets (500 mg) were given, as required, if the pain scores at rest rose higher than 3. On discharge from the hospital, all patients were advised to consume paracetamol tablets for any complaint of pain at home.
Acute postoperative pain (at rest and after movement) was assessed using the 11-point VNS on which 0 indicated “no pain” and 10 represented “maximal unbearable pain.” Movement consisted of 90° arm abduction of the operated side. The sedation score was assessed using the RSS (1=anxious or restless, 2=cooperative and orientated, 3=responding to commands, 4=asleep but strong response to stimulus, 5=sluggish response to stimulus, and 6=no response to stimulus). Data for pain and sedation scores were recorded at 10, 30, 60, and 120 min and at 6, 12, 24, 36, 48, and 72 h, postoperatively.
Chronic pain assessments were conducted by an appointment, using questionnaires at 3 months after surgery. Patients were also inquired about any course of postoperative adjuvant therapy (chemo/radio/anti-estrogen therapy) and the analgesic requirements. Regional pain (chronic) was defined as the persistent pain that is limited to one or two quadrants while the generalized pain (chronic) was the one that involved at least three to four quadrants of the body (quadrant classified as per American College of Rheumatology criteria for the classification of fibromyalgia) as measured at 3 months after surgery.[7] The severity of chronic pain was rated by the brief pain inventory (BPI) and the Short Form McGill Pain Questionnaire (SF-MPQ2).[8,9] BPI uses a 0 (no pain) to 10 (worst pain) numeric rating system which provides information about the intensity of pain (past 1 week) and the degree to which pain interferes with function (reactive dimension). SF-MPQ2 is a 22-item scale (6, continuous pain; 6, intermittent pain; 8, neuropathic; and 4,- affective) that uses an 11-point scale with 0 equal to “no pain” and 10 equal to “worst pain.” The psychosocial assessment was done by Quality of Life Scale (QOLS), a 16-item score that uses a 7-point rating with 1 indicating the word “terrible” and 7 signifying “delighted.”[7]
To detect a 25% difference in the measured variables among the groups with a standard deviation of 30% estimated from initial pilot observations, with 90% power and 5% alpha error (two sided), a sample size of 31 per group was required. The sample size was calculated using the power and sample size calculator of the Department of Biostatics, Vanderbilt University, USA. Taking into account a dropout rate of 25% estimated from initial pilot observations, we selected 86 cases (43 in each group) for our study.
Statistical analysis was performed using SPSS 17.0 statistical software. The continuous variables were compared using the one-way analysis of variance test. Posthoc testing was done using Bonferroni's method. Discrete variables were compared using Fisher's exact test/Chi-square test, whichever was appropriate. A P value <0.05 was considered significant.
RESULTS
Figure 1 illustrates the flow chart of the patients studied. In total, 84 cases (42 in each group) were analyzed for acute pain assessments and 69 (34 in group D and 35 in group C) for chronic pain evaluation. Patient demographics were similar among the groups [Table 1].
Figure 1.
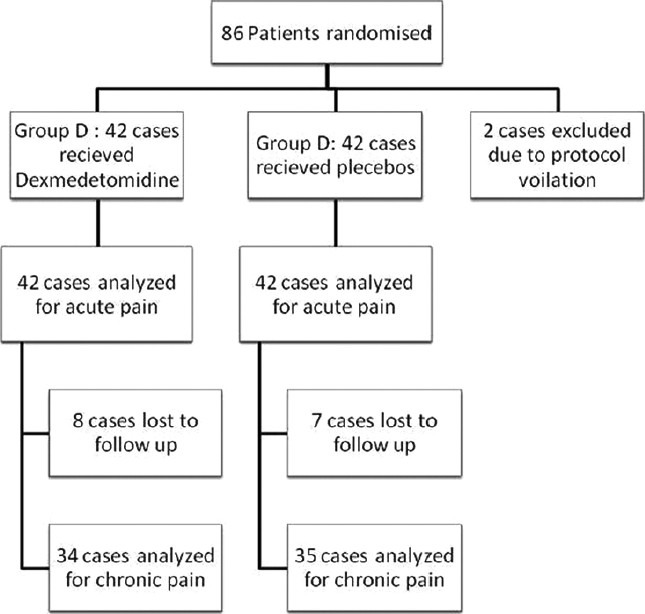
Flow chart of patients studied
Table 1.
Comparison of demographic and treatment characteristics among the groups (n=42)
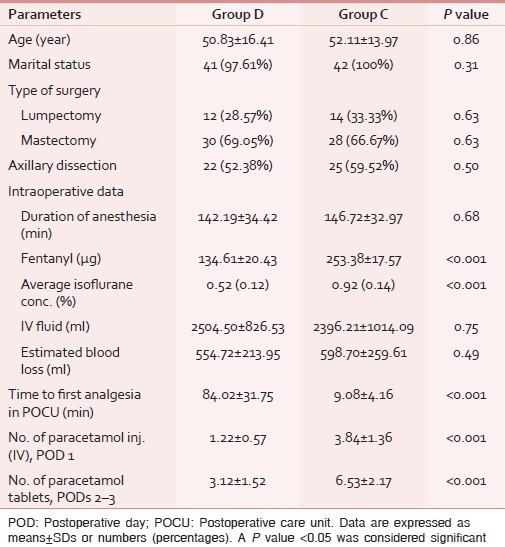
There was no significant difference in the duration of anesthesia, type of surgery, IV fluid infused, estimated blood loss, and the number of patients who received postoperative radio/chemo/anti-estrogen therapy between the groups [Tables 1 and 2]. The mean inspiratory isoflurane concentration and fentanyl required in group D were significantly lower as compared to group C (P<0.001; Table 1). There was no major complication in any of the cases; however, one patient developed an episode of bradycardia, managed promptly by a bolus dose of atropine 0.6 mg IV.
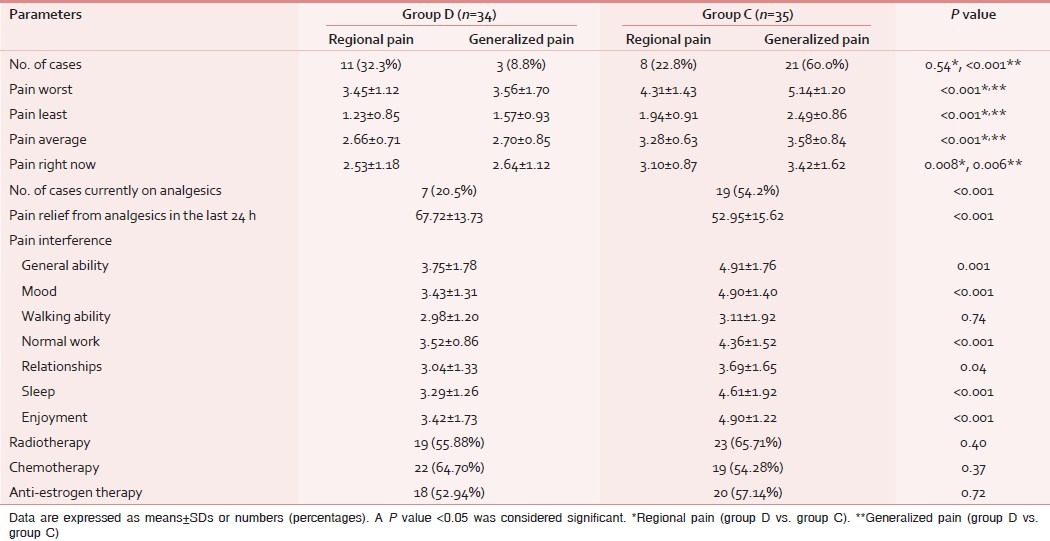
Table 2.
Comparison of the brief pain inventory score and postoperative adjuvant therapy between the groups
The time to first analgesic requirement after POCU admission was significantly longer in group D than that in group C (P<0.001). The paracetamol requirement in the POCU/ward was significantly greater in group C than group D (P<0.001; Table 1). The RSS was slightly higher in group D for the period of infusion, though it did not attain statistical significance at any data point between the groups [Figure 2]. The VNS at rest and after movement were significantly higher in group C as compared to group D at each time point (except at 60 min) assessed in the PACU/ward after surgery [Figures 3 and 4]. None of the patients developed any major postoperative complication except for nausea/vomiting in three cases (two in group D/one in group C), managed by ondansetron 8 mg IV.
Figure 2.
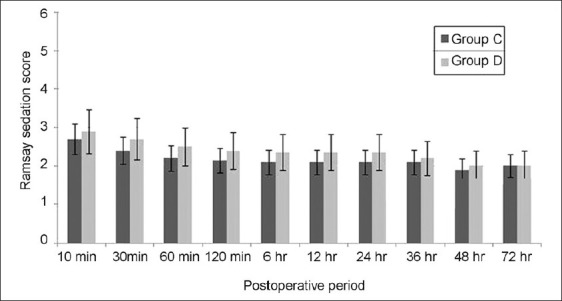
Ramsay sedation score in both the groups during the postoperative period. P<0.05 was considered significant (*P<0.05, **P<0.001). Group C=control group; group D=dexmedetomidine group
Figure 3.
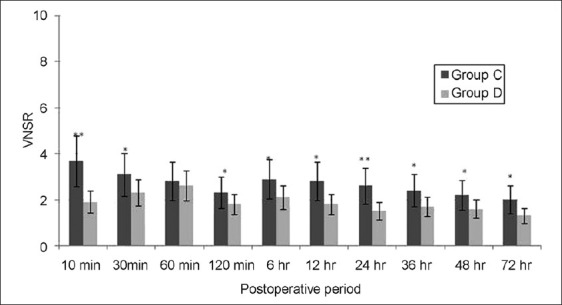
Pain scores at rest in the postoperative period. Data are expressed as means±SDs. P<0.05 was considered significant (*P<0.05, **P<0.001). Group C=control group; group D=dexmedetomidine group; VNSR=verbal numerical score at rest
Figure 4.
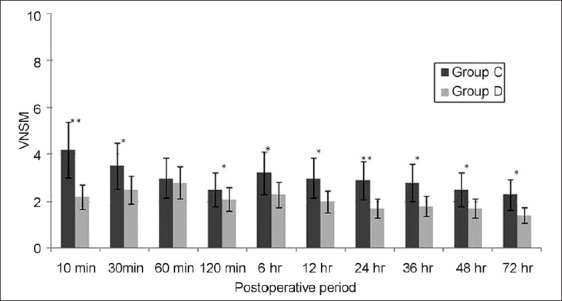
Pain scores during movement in the postoperative period. Data are expressed as means±SDs. P<0.05 was considered significant (*P<0.05, **P<0.001). Group C=control group; group D=dexmedetomidine group; VNSM=verbal numerical score during movement
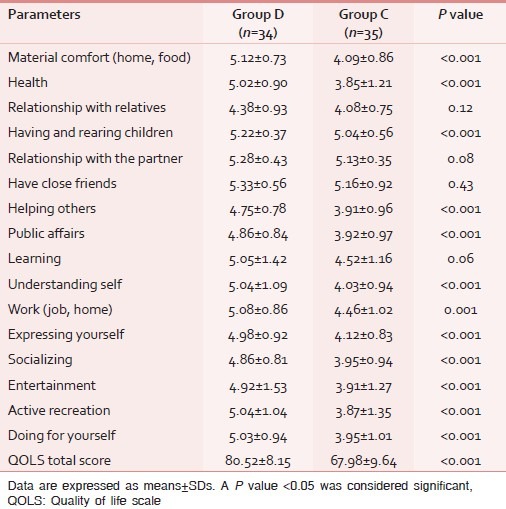
Pain intensity/interference scores as measured by BPI at 3 months after surgery were higher in group C as compared to group D with the significant differences in all parameters except for two items (relationship, walking ability; Table 2). Similar results were observed for the SF-MPQ2 score with significant differences in all except for six items (cramping, electric shock, itching, fearful punishing, and numbness; Table 3). The QOLS results show that quality of life was better in group D than group C with significant differences in all items except for relationships, friends, and learning [Table 4].
Table 3.
Comparison of the Short Form McGill Pain Questionnaire (SF-MPQ2) score among the groups
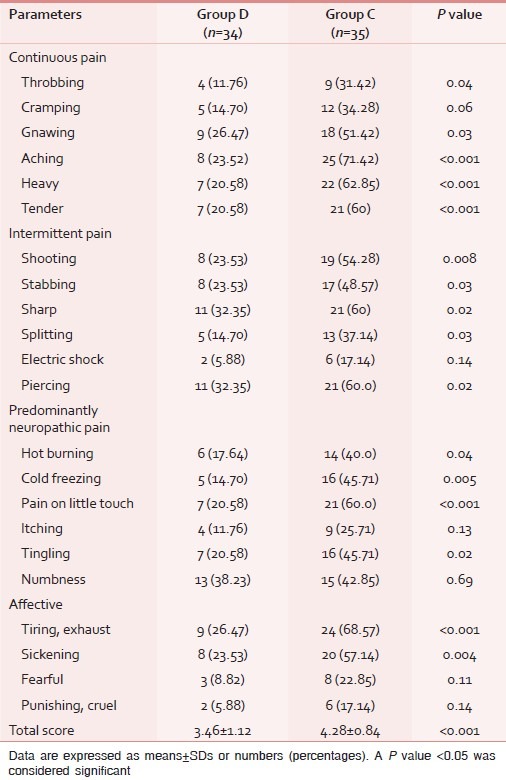
Table 4.
Comparison of quality of life scale score among the groups
DISCUSSION
Our investigations indicate that the perioperative infusion of dexmedetomidine administered as per the protocol of the study significantly reduced the pain scores and the analgesic requirement during the first 72 h of observation and also the severity of chronic pain 3 months after surgery for breast cancer. Chronic pain affected most aspects of patient's lives to a greater extent in the control group than in the dexmedetomidine group.
Dexmedetomidine exerts its sedative, analgesic effects via central actions in the locus ceruleus and substantia gelatinosa in the spinal cord.[10] When infused in a dose range of 0.2–0.7 μg/kg/h, dexmedetomidine exhibits linear kinetics for ≤24 h, due to which its use has been limited till the first POD in most researches including ours.[10–12] We started dexmedetomidine infusion approximately 30 min prior to surgery to attenuate the noxious stress response during induction of anesthesia and attain uniform analgesic levels throughout the intraoperative period. The same was continued in tapered doses till 24 h in the postoperative phase. A similar methodology has been utilized by many researchers assessing the efficacy of dexmedetomidine for perioperative analgesia.[5,6,13]
Several researchers have evaluated the analgesic effect of dexmedetomidine after performing the cold pressor test on human volunteers. They observed a 30% decrease in the visual analog score (VAS) at an infusion rate of 0.2 μg/kg/h.[14,15] A similar decrease in postoperative VAS scores has been observed by Sitilci et al. evaluating its role in mastoidectomy cases after an intraoperative infusion of 0.5 μg/kg/h.[6] However, Gurbet et al. observed no difference in above scores in a similar study on hysterectomy cases.[5] The possible reason could be their use of a potent postoperative rescue analgesic “morphine,” which could have significantly affected the VAS scores in the placebo group. In our study, we observed a significant reduction in the postoperative VNS of approximately 20–30% in the dexmedetomidine group for the 72-h period of observation. It could be due to the use of a weaker rescue analgesic “paracetamol” during the postoperative period as demonstrated in a previous study.[16] Human studies indicate that the systemic administration of dexmedetomidine results in a dose–response relationship for the sedation response.[15,17] Considering this, we chose a minimal therapeutic dose of dexmedetomidine (0.2 μg/kg/h) in an attempt to maintain adequate postoperative analgesia, while minimizing sedation-related side effects. In our study, IV dexmedetomidine significantly reduced the requirements of isoflurane, fentanyl, and paracetamol (postoperative) in accordance with the previous trials.[5,18] The possible mechanism could be the modulation of nociceptors at the level of spinal noradrenergic systems and the release of endogenous opiates in spinal dorsal horn neurons.[10]
SF-MPQ2 uses 22 descriptors to categorize the incidence and severity of continuous, intermittent, neuropathic, and affective components of chronic pain.[8,9] In our study, the components and severity of chronic pain in SF-MPQ2 were rated to be significantly lower in dexmedetomidine treated cases. On analyzing the individual components of SF-MPQ2, a higher percentage of cases with continuous pain (both groups) described their pain as gnawing, aching, heavy, or tender. Only a few cases described their pain as electric shocks or itching. Regarding the affective component, the cases presented mostly as tiring or sickening.
The BPI is a score that quantifies pain intensity and the degree to which pain interferes with daily routine.[8] In our study, pain intensity and interference was observed to be significantly lower in the dexmedetomidine group for most of the studied components. In the control group, the incidence of generalized pain was all higher than the dexmedetomidine group; however, such difference was not observed in regional pain. It could be due to central sensitivity in the control group as a result of persistent inputs from the peripheral nociceptors during the acute postoperative period eventually leading to vague and widespread chronic pain.[4,19] Cases in both groups described their pain as worse when they were fatigued; however, it is interesting to note that the relationship and walking ability were minimally affected in our subset of population. Similar results have been observed in the QOLS scores. The QOLS is an ideal screening tool that measures a wide range of domains such as response to moods and other psychological aspects of a patient's life.[8] In our study, the lower QOLS score in the dexmedetomidine group could be due to less psychological distress related to measures contained within the BPI and SF-MPQ2 scores. However, we must acknowledge that quality of life is also affected by many other non-evaluated factors such as family attention, mental health, social well-being, economical issues, etc., which might have affected the results.
Limitations
A few drawbacks of our study included the lower intellectual and awareness in many cases, failing which their rating, interpretation, and description of chronic pain were assumptive at times, which could have led to the misinterpretation of results. The same could have been the reason for a large number of dropouts (approximately 25% of the initial recruitment) in this study as opposed to a lower dropout rate of 5–10% in the Western world, leading to the extension of our study period. Future trials can concentrate on the role of perioperative multimodal therapy using dexmedetomidine, gabapentin, local anesthetics, etc., in different doses and regimens for attenuating chronic pain.
CONCLUSION
In conclusion, perioperative analgesia with dexmedetomidine reduced the occurrence and intensity of chronic pain and its effect on the quality of life after breast cancer surgery. This provides an evidence for the extended benefit associated with preventive analgesia in the perioperative period.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Vilholm OJ, Cold S, Rasmussen L, Sindrup SH. The postmastectomy pain syndrome: An epidemiological study on the prevalence of chronic pain after surgery for breast cancer. Br J Cancer. 2008;99:604–10. doi: 10.1038/sj.bjc.6604534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carpenter JS, Andrykowski MA, Sloan P, Cunningham L, Cordova MJ, Studts JL, et al. Postmastectomy/postlumpectomy pain in breast cancer survivors. J Clin Epidemiol. 1998;51:1285–92. doi: 10.1016/s0895-4356(98)00121-8. [DOI] [PubMed] [Google Scholar]
- 3.Andrykowski MA, Curran SL, Carpenter JS, Studts JL, Cunningham L, McGrath PC, et al. Rheumatoid symptoms following breast cancer treatment: A controlled comparison. J Pain Symptom Manage. 1999;18:85–94. doi: 10.1016/s0885-3924(99)00053-6. [DOI] [PubMed] [Google Scholar]
- 4.Fassoulaki A, Melemeni A, Staikou C, Triga A, Sarantopoulos C. Acute postoperative pain predicts chronic pain and long-term analgesic requirements after breast surgery for cancer. Acta Anaesthesiol Belg. 2008;59:241–8. [PubMed] [Google Scholar]
- 5.Gurbet A, Basagan-Mogol E, Turker G, Ugun F, Kaya FN, Ozcan B. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Can J Anaesth. 2006;53:646–52. doi: 10.1007/BF03021622. [DOI] [PubMed] [Google Scholar]
- 6.Sitilci AT, Ozyuvaci E, Alkan Z, Demirgan S, Yigit O. The effect of perioperative infused dexmedetomidine on postoperative analgesic consumption in mastoidectomy operations. Agri. 2010;22:109–16. [PubMed] [Google Scholar]
- 7.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 8.Burckhardt CS, Jones KD. Effects of chronic widespread pain on the health status and quality of life of women after breast cancer surgery. Health Qual Life Outcomes. 2005;3:30. doi: 10.1186/1477-7525-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dworkin RH, Turk DC, Revicki DA, Harding G, Coyne KS, Sandner PS, et al. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2) Pain. 2009;144:35–42. doi: 10.1016/j.pain.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: A novel sedative-analgesic agent. Proc (Bayl Univ Med Cent) 2001;14:13–21. doi: 10.1080/08998280.2001.11927725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arain SR, Ruehlow RM, Uhrich TD, Ebert TJ. The efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgery. Anesth Analg. 2004;98:153–8. doi: 10.1213/01.ANE.0000093225.39866.75. [DOI] [PubMed] [Google Scholar]
- 12.Lin TF, Yeh YC, Lin FS, Wang YP, Lin CJ, Sun WZ, et al. Effect of combining dexmedetomidine and morphine for intravenous patient-controlled analgesia. Br J Anaesth. 2009;102:117–22. doi: 10.1093/bja/aen320. [DOI] [PubMed] [Google Scholar]
- 13.Jaakola ML, Salonen M, Lehtinen R, Scheinin H. The analgesic action of dexmedetomidine – a novel α2-adre-noceptor agonist – in healthy volunteers. Pain. 1991;46:281–5. doi: 10.1016/0304-3959(91)90111-A. [DOI] [PubMed] [Google Scholar]
- 14.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 15.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–94. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Fassoulaki A, Triga A, Melemeni A, Sarantopoulos C. Multimodal analgesia with gabapentin and local anesthetics prevents acute and chronic pain after breast surgery for cancer. Anesth Analg. 2005;101:1427–32. doi: 10.1213/01.ANE.0000180200.11626.8E. [DOI] [PubMed] [Google Scholar]
- 17.Jaakola ML. Dexmedetomidine premedication before intravenous regional anesthesia in minor outpatient hand surgery. J Clin Anesth. 1994;6:204–11. doi: 10.1016/0952-8180(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 18.Keniya VM, Ladi S, Naphade R. Dexmedetomidine attenuates sympathoadrenal response to tracheal intubation and reduces perioperative anaesthetic requirements. Indian J Anaesth. 2011;55:352–7. doi: 10.4103/0019-5049.84846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staud R, Cannon RC. Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia. Pain. 2003;102:87–95. doi: 10.1016/s0304-3959(02)00344-5. [DOI] [PubMed] [Google Scholar]


