Abstract
Mycobacterium chelonae was identified as the cause of incidental mortality in Atlantic salmon smolts following introduction to seacages. Source of infection was not confirmed. Polymerase chain reaction was a useful method of detecting and speciating the genus Mycobacterium in infected stocks. Clinical management and public health implications of infection are discussed.
In December 1998, 150 000 cascade X Movi Atlantic salmon (Salmo salar) smolts (24 to 40 g) were transported directly to an on-growing seacage site located off the coastal waters of Vancouver Island. The smolts were evenly distributed over 4 seacages. Each cage measured 15 m2 at the surface and extended to a depth of 10 m. The smolts had originated from 2, 8-m circular fiberglass tanks at a hatchery that received single-pass, filtered, ozonated river water at 9°C. The hatchery management declined to provide the clinical history on these stocks. The environmental records for the site indicated that it had been a particularly dry summer and that, with the onset of fall, there had been significant run-off, which had made the river water turbid and rendered ozonation ineffective a few weeks prior to transport of the smolts. Between January and April 1999, the site received an additional 290 000 Atlantic salmon smolts (80 to 120 g) of similar genetic composition from a freshwater quarry; these smolts were evenly distributed over 8 similarly sized seacages.
All groups of smolts had been incubated as eggs until they became parr (8 g) at the same hatchery. None of the smolts from either delivery had been vaccinated intraperitoneally against furunculosis (Aeromonas salmonicida) or vibriosis (Vibrio salmonicida, V. ordalli). After the initial introduction of quarry-derived smolts to the seacages, the fish developed clinical signs of septicemia. Samples from the caudal kidney, cultured on plain tryptone soy agar, and incubated at room temperature for 5 d, yielded Aeromonas salmonicida. All smolts were subsequently treated with medicated feed containing florphenicol (Aquaflor; ScheringPlough Animal Health, Pointe-Claire, Quebec) at 10 mg active/kg fish for 10 d consecutively. All 440 000 smolts were transferred to a seawater grow-out site in April 1999 to avoid dangerous, seasonally recurrent, blooms of Chaetoceros convolutus and Heterosigma carterae algae that had caused mortality in previous years at this site.
From 7 to 10 d after arrival of the initial shipment of 150 000 smolts, 2 of the 4 cages had between 8 to 10 fresh mortalities per day with distinct subcutaneous and visceral granulomas with pronounced hepatosplenomegally. Due to the unusual tinctural properties noted in routine Gram-stained liver and kidney imprints, the distinct organism was considered distinct to Renibacterium salmoninarum, which causes bacterial kidney disease (BKD). Tissues from fresh mortalities and sacrificed surface catchable slow swimmers were preserved in 10% phosphate-buffered formalin (Syndel Laboratories, Vancouver, British Columbia) and forwarded to the Animal Health Centre, Abbotsford, British Columbia. Initial histopathologic examination disclosed innumerable, multisystemic, intra- and extracellular acid-fast bacilli, compatible in appearance with Mycobacterium spp. Polymerase chain reaction (PCR) assays targeting the 16S rRNA gene (1) and the superoxide dismutase gene (2) were used to further identify the suspect mycobacteria. In addition, kidney tissue was cultured at the British Columbia Centre for Disease Control, Vancouver, British Columbia, where M. chelonae was isolated and confirmed by PCR and restriction enzyme analysis (3).
Beginning in January 1999, the site was visited monthly by its veterinarian and weekly by the company's fish health personnel. All fresh mortalities and moribunds from each cage were necropsied. Spleen and kidney imprints from these fish were collected onsite, air dried, and forwarded to the Animal Health Centre for staining with an acid-fast stain (Ziehl-Neelsen). From December 1998 until April 1999, when the fish were transferred to the grow-out site, the monthly mortality rate for unclassified fresh silvers remained < 1%, which was considered to be well within acceptable production parameters. Mortality attributed to infection with Mycobacterium spp. in fresh silvers, based on necropsy lesions and acid-fast imprints, was < 0.01%/mo. Affected fish were lethargic and anorexic. External lesions consisted of numerous, superficial to deep, hemorrhagic cutaneous ulcers along the flanks and raised, subcutaneous erythematous granulomas throughout the isthmus, ventrolateral aspect of the cranial abdomen, and proximal aspect of the pectoral fins (Figure 1). Along the dorsolateral aspect of the torso, there were focally extensive regions of raised and occasionally avulsed scales. In a few fish, there was corneal opacification and lenses were occasionally collapsed and hemorrhagic. Internally, the entire coelomic cavity was often distended (fluctuant to turgid) and contained pale, tan to yellow, turbid ascitic fluid. In multiple fish, there was hepatomegaly with focally disseminate necrosis (Figure 2) and superficial capsular fibrin deposition, as well as generalized enlargement of the cranial and the caudal kidney. Generally the gastrointestinal tracts contained only small amounts of bright yellow mucoid ingesta. Cytologic examination of accompanying kidney and splenic imprints disclosed variable numbers of intralesional acid-fast bacilli. Microscopically, these lesions ranged from acute, with large numbers of bacilli, to more chronic granulomatous infiltrate, with fewer bacteria. Extracellular and intracellular organisms were observed within dermal and endomysial vasculature, splenic ellipsoids, renal sinusoids, and, more rarely, the branchial microvasculature. With progression of the disease, there was focally disseminate pyogranulomatous to granulomatous myositis, hepatitis, splenitis, and interstitial nephritis. The hematopoietic tissues were hyperplastic and the reticuloendothelial system was reactive. In more severely affected fish, virtually all of the sectioned parenchyma was obliterated.
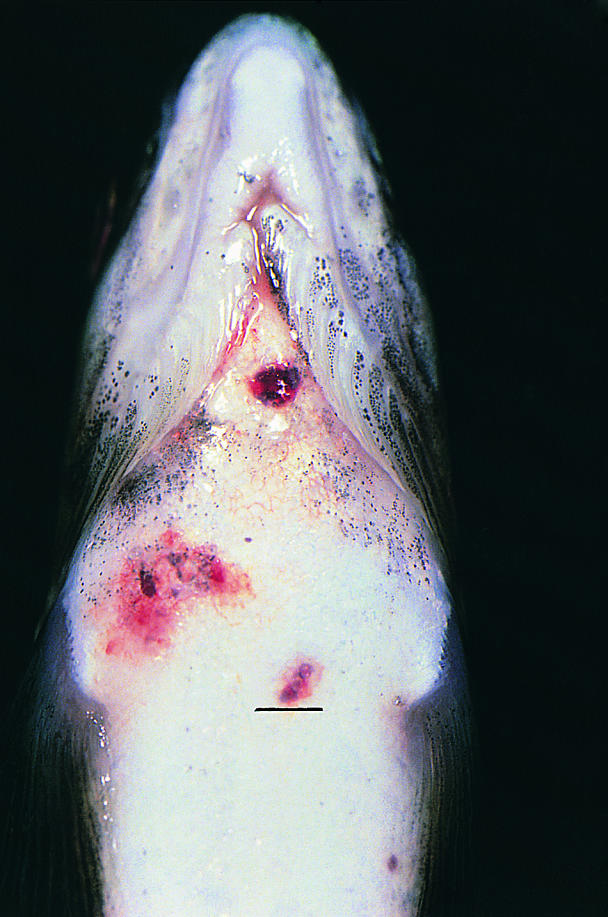
Figure 1. Atlantic salmon (150 g) with raised, subcutaneous erythemic granulomas on the ventral aspect of the cranial abdomen. Bar = 2 mm.
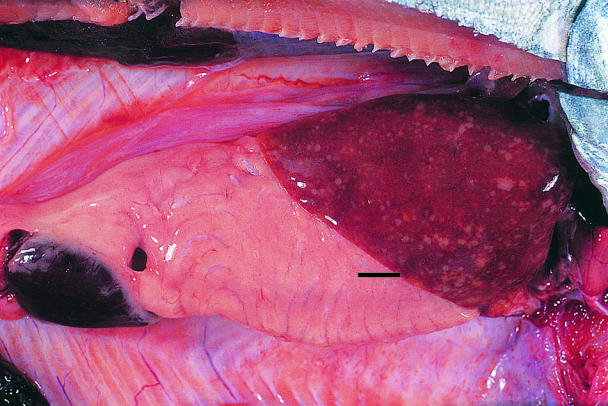
Figure 2. Atlantic salmon (140 g) with hepatomegally, splenomegally, and focally disseminate necrosis. Bar = 2 mm.
Prior to the movement of the salmon to the grow-out site in April 1999, mycobacteriosis had been confirmed in only the 2 pens where it had been presumptively diagnosed. Consequently, management decided to combine the population of fish in the 2 affected cages into a single, larger seacage (30 m × 45 m) at the 2nd grow-out site, so that cage density similar to that of the other pens on-site would be maintained. A sampling strategy was established to monitor the progression of the disease, to compare the growth rate of the affected population with that of the 2 cages of its cohorts, and to compare the relative sensitivity of different diagnostic modalities over the course of production at the saltwater sites. Gross pathologic, cytologic, histopathologic, and PCR studies were conducted on 60 fish arbitrarily sampled at 90 d (1st seawater site), 250 d (after transfer to 2nd seawater site), and at 550 d (at slaughter). Specialized bacterial cultures were not set up at each sampling interval, due to cost constraints and logistics. Within 4 to 5 mo of the initial introduction of the fish to seacages, the clinical signs and gross lesions had abated, and after 90 d, histopathologic examination of the liver, spleen, and kidney consistently disclosed a low grade, nonspecific lymphoplasmacytic to lymphohistiocytic infiltrate. No acid-fast bacilli were detected on cytologic examination at 90 d, 270 d, and 550 d. Polymerase chain reaction with primers for the 16S rRNA gene on pooled kidney tissue at 90 d postintroduction revealed 5 positive, 3 suspect, and 4 negative samples; at 250 d postintroduction, it revealed 4 positive and 8 negative samples; and at 550 d, all samples were negative. There was no recorded difference in the growth rate between fish in the affected pen versus those in the 2 noninfected cohorts. The superoxide dismutase gene, which was originally designed to identify cultured Mycobacterium spp. only (2), lacked the sensitivity to detect bacilli in field tissues at the later sampling periods.
Mycobacterium spp. are straight to slightly curved rods (0.4 × 1.0 to 4.0 μm), nonmotile, aerobic, acid-fast, and gram-positive (4). They can be differentiated from species of Nocardia, another acid-fast genus which are coccoid to oval, with long, slender, branching, multiseptate filaments (5). Mycobacterium spp. are similar in size to Renibacterium salmoninarum (0.5 × 1 μm), which is coryneform-like, gram-positive, and non-acid fast (6). Fish infected with Mycobacterium spp., Nocardia spp., and Renibacterium sp. appear similar on clinical presentation and necropsy. Clinical signs may include anorexia, emaciation, nervous disorders, exophthalmos, keratitis, cutaneous depigmentation, and skin ulcers (5). Specialized media and incubation temperatures are required for growth of both Mycobacterium spp. and the Renibacterium sp. (5). Clinical diagnosis and differentiation between these bacterial species is based on tinctoral, biochemical, immunological, or molecular identification of bacterial specific features in tissues or isolates (4). At present, to the best of our knowledge, there are no nonlethal means of detecting carrier fish.
Fish mycobacteriosis is a commonly recognized chronic debilitating disease affecting freshwater and brackish aquarium fish (M. fortuitum) and marine aquarium fish (M. marinum) (4,5,6,7). Asymptomatic M. chelonae infections were common (> 25%) in some freshwater, hatchery-reared, juvenile Pacific salmonid populations from California to Alaska when the practice of feeding raw carcasses and viscera (offal) of spent parent fish to their progeny was conducted (5). Clinical disease may have been prevented in the closed hatchery environment by discontinuation of this feeding practice to the juvenile Pacific salmon, as well as by concurrent treatment for bacterial gill disease with the addition of chloramine-T to the water at 10 mg/L for 24 h (5). Infection presumably persisted throughout the fresh- and saltwater phases of the life cycle. Heavy infections resulted in darkened skin and generalized emaciation, as well as miliary granulomata throughout the viscera. These infections were usually expressed clinically at smoltification, either with increased ambient water temperatures or with drought during spawning runs upriver (4,5), consistent with observations derived in this investigation.
The Fish Health Section, Pacific Biological Station (PBS), Department of Fisheries and Oceans, Nanaimo, British Columbia conducted a review for diagnosis of Mycobacterium spp. in archived records (Dorothee Keiser, PBS, personal communication, 1999). The species was not identified in each case. There was 1 case in a captive carp (Cyprinus carpio L.) population in 1973; 5 cases in Pacific salmon (Oncorhynchus spp.) in 1975; 3 cases in trout (Oncorhynchus spp.) in 1976; 1 case in Pacific salmon in 1982; 2 cases in total for grayling (Thymallus thymallus) and trout in 1989; 2 cases in whitefish (Coregonidae spp.) in 1991; 1 case in whitefish in 1992; 2 cases in whitefish in 1993; and 2 cases in whitefish in 1996. The Ministry of Agriculture, Fisheries and Food, Animal Health Centre, Abbottsford, British Columbia conducted a similar case review in 1999. Over the past 8 y, in addition to this presentation, there have been 3 presumptive cases of mycobacteriosis involving ornamental fish based on histopathologic examination and acid-fast stained bacilli (S. Raverty, unpublished data). Mycobacterium chelonae infection among farmed and laboratory-infected Atlantic salmon was reported from the Shetland Isles, Scotland in 1998 (8). Mycobacterium neoaurum was isolated from production Atlantic salmon with ocular lesions in New Brunswick in 1990 (6). Mycobacterium spp. have been isolated from cultured European sea bass (Dicentrarchus labrax), tilapia (Tilapia spp.), and striped bass (Morone saxatilis) (4,6,7).
Clinical management at the farm level centered on 2 major concerns: perception of the farm staff and consumers regarding the public health implications (zoonotic potential) of mycobacteriosis, and disposal of potentially contaminated mortalities. Farm staff were advised that M. chelonae is an opportunistic, ubiquitous saprophytic bacterium that is isolated from soil, treated and untreated water supplies, earthen ponds, lakes with muddy substrates, and other natural sources (4,5,6,7). This bacterium may remain viable in the environment for up to 2 y (5). Amphibians and reptiles cohabitating the water supply may release bacteria (5,7) and be a point source of contamination. It is postulated that intermittent or continuous exposure for humans occurs through ingestion, inhalation, and inoculation of traumatized skin (5,6,7). Human infection is generally acquired through recreational or employment pursuits. Examples include exposure to contaminated aquarium water, fishing paraphenalia, shrimp, or barnacles; and infection secondary to trauma inflicted by iron spikes, lawn mower blades, thorns, and soil (5,6). In the United States, mycobacteriosis is a recognized nosocomial infection associated with contaminated water (7). Humans generally present with localized superficial infections (abscessation, cellutitis) associated with direct inoculation of soft tissue, skeletal muscle, or, more rarely, deeper structures. As with M. fortuitum, there is an increasing occurence of invasive lung disease in humans (7). To prevent farm staff from acquiring infection, rubber gloves were required to be worn when handling live stock, dead fish, or contaminated fomites, and gloved hands had to be immersed in fresh betadine solution before the removal of protective wear. All dead fish were disposed of in a sealed tank. Mortality rings, buckets, dipnets, and storage tanks were immersed or sprayed with betadine and dried prior to use. The efficacy of disinfection with this compound in saltwater has not been fully resolved. However, by complying with these protocols, awareness of biosecurity was heightened among farm staff.
The public health significance of this case was discussed with representatives of the Canadian Food Inspection Agency (CFIA), fish health authorities, and epidemiologists in the United States Department of Agriculture (USDA). Although freezing does not kill Mycobacterium spp. within a stored carcass, the risk of human infection from direct exposure through skin or lung, or indirectly from consumption of infected flesh, was considered remote. There is no effective treatment for Mycobacterium spp. infection of food fish (4,5,6), and slaughter, with site disinfection of holding facilities prior to restocking, is generally recommended (4,5,6). In many cases, stocks have been destroyed due to potential human and fish infection (4,5,6,7). Nevertheless, based on the above studies, stock retention, coupled with serial sampling to assess the progression of infection within ongrowing fish may be considered a viable option, particularly in lieu of the relatively low observed mortality rates and lack of apparent zoonotic transmission to farm personnel in this investigation. CVJ
Footnotes
Address all correspondence and reprints requests to Dr. Raverty.
References
- 1.Talaat AM, Reimschussel R, Truckis M. Identification of Mycobacteria infecting fish to the species level using polymerase chain reaction and restriction enzyme analysis. Vet Microbiol 1997;58:229–237. [DOI] [PubMed]
- 2.Zolog WJ, Philippi-Schulz S. The superoxide dismutase gene, a target for detection and identification of mycobacteria by PCR. J Clin Microbiol 1994;32:2801–2812. [DOI] [PMC free article] [PubMed]
- 3.Telenti A, Marchesi F, Blaz M, Bally F, Biottger EC, Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol 1993;31:175–178. [DOI] [PMC free article] [PubMed]
- 4.Noga EJ. Fish Disease: Diagnosis and Treatment. St. Louis: Mosby-Year Book, 1996:157–159.
- 5.Inglis V, Roberts RJ, Bromage NR. Bacterial Diseases of Fish. London: Blackwell Scientific. 1993.
- 6.Roberts RJ. Fish Pathology. 3rd ed. Toronto: WB Saunders. 2001:326–327.
- 7.Belas R, Faloon P, Hannaford A. Potential applications of molecular biology to the study of fish mycobacteriosis. Annu Rev Fish Dis 1995;5:133–173.
- 8.Bruno DW, Griffiths J, Mitchell CG, et al. Pathology attributed to Mycobacterium chelonae infection among farmed and laboratory-infected Atlantic salmon (Salmo salar). Dis Aquat Org 1998; 33:101–109. [DOI] [PubMed]


