Abstract
Introduction:
Different growth and neuro-developmental outcomes have been associated with different doses of thyroxine given to infants with congenital hypothyroidism (CH).
Materials and Methods:
We studied the longitudinal growth pattern and assessed the neurodevelopment of 45 children with CH(25 girls, 20 boys) diagnosed through the national screening program in Qatar, for 6 years or more to examine the effects of initial T4 dosage (50 μg/day) with adjustment of T4 dose to maintain serum free T4 concentrations within the upper quartile of normal range and thyroid stimulating hormone < 4 mIU/mLThe birth size of newborns with CH diagnosed through the screening program before January 2003, was recorded and their growth in weight and stature was monitored every 3 months for at least 6 years of life. The IQ of children was assessed between 3 and 6 years of age using The Wechsler Preschool and Primary Scale of Intelligence (WPPSI-III).
Results:
Birth weight, length, and head circumference of patients (3.21 ± 0.43 kg, 50.5 ± 3.21 cm and 34.1 ± 1.5 cm, respectively) did not differ than those for 10,560 normal newborns with normal thyroid function (3.19 ± 0.59 kg, 50.5 ± 2.2 cm and 34.2 ± 1.7 cm). During the first year CH children growth (25.8 ± 2.8 cm/year) was similar to those for normal infants (25.5 ± 0.75 cm/year). During the first 6 years, stature growth was normal in all children with CH versus Center for disease control and prevention (CDC) data. The mean height standard deviation score (HtSDS) of children with CH showed adjustment (± 0.5 SD) toward their mid-parental height SDS (MPHtSDS) only during the second year of life. The children's mean HtSDS was higher by an average of 0.4 SD between the 2nd and 7th year of life.
Conclusion:
These data proved that effective screening and treatment completely assures normal neurodevelopment and linear growth in patients with CH. The data showed that their HtSDS slightly exceeds their MPHtSDS during childhood.
Keywords: Congenital hypothyroidism, height standard deviation score height standard deviation score, linear growth, midparental height
INTRODUCTION
Because newborns with congenital hypothyroidism (CH) are asymptomatic at birth, screening programs developed worldwide. Data from countries with well-established newborn screening programs indicate an incidence of congenital hypothyroidism of 1 per 3000–4000.[1–6] Some of the highest incidences (1 in 1400 to 1 in 2000) have been reported from various locations in the Middle East.[7] Our average incidence rate of CH in Qatar between 1998 and 2006 was 44.13/100,000.[8]
It is known that T4 plays a crucial role in skeletal and brain growth.[1,2,4,9] Untreated CH patients are at great risk for severe growth as well as developmental retardation.[9] The key outcome measure for CH is cerebral function. However, this is difficult to measure, is influenced by factors other than CH and its treatment, and requires followup into adulthood. If infants receive excess of T4, as in severe neonatal thyrotoxicosis, early fusion of the cranial sutures may occur with frontal prominence and intellectual impairment,[1,5,6,10] whereas childhood thyrotoxicosis leads to tall stature.[11] Thus, accurate measurement of somatic growth—length, weight, and occipitofrontal circumferencein early childhood can provide a surrogate marker of T4 effect.
The aims of this study was to investigate the effect of CH on birth size and the effect of our treatment protocol (L-thyroxine = 50 μg/day followed by adjustment of the L-thyroxine dose to keep free T4 (fT4) level at the upper quartile of normal range and thyroid stimulating hormone (TSH) < 4 mIU/L ) on postnatal growth during childhood.
MATERIALS AND METHODS
All newborns delivered in Qatar were screened through the Qatar National Screening program[8] using heal prick on Guthrie paper at 36 h of age or later (up to 5 days). The samples were sent to the central laboratory of the University Children's Hospital of Heidelberg, Germany, by express mail. Confirmatory venous blood samples were sent on request to the central laboratory of Hamad Medical Center ( HMC) for measurement of fT4 and TSH. The mean age at diagnosis and the start of the treatment was 14.9 ± 6.2 days. The mean fT4 concentration of patients ranged between 2 and 10.5 pmol/L (mean = 6.2 ± 3.6 pmol/L) with TSH > 60 mIU/L ( 169 ± 55mIU/L Ten infants had goiterous hypothyroidism, 6 had ectopic thyroid, 26 had thyroid dysgenesis, and 3 had thyroid agenesis.
At or after 3 years of age, L-thyroxine was stopped for 4 weeks and the diagnosis of CH was reconfirmed in all patients.
We measured the birth size of newborns with CH diagnosed through the screening program before January 2003, and we monitored accurately their linear growth (height, weight) while on treatment for at least 6 years of life in the Pediatric Endocrine Clinic at Hamad Medical Center. Forty-five CH children (25 girls, 20 boys) were followed up after starting an initial daily dose of T4 = 50 μg. Thyroid function, weight, length, and/or height were recorded longitudinally and the standard deviation scores calculated and compared with CDC normal growth data for children at 6, 12, 18, 24, 36, 48, 60, and 72 months of age. The midparental height (MPHt) and MPHtSDS of the parents were recorded. Adjustment of L-thyroxine dose was done every 2 months for the first year and then every 3 months thereafter to keep fT4at the upper quartile for the normal range and TSH < 4 mIU/mL. At the age of 6 years or above the bone age was determined using Greulich et al's method.[12]
The IQ of children was assessed between 3 and 6 years of age using the Wechsler Preschool and Primary Scale of Intelligence (WPPSI-III) for assessing the cognitive ability of children aged 2.5 and 7 years. The WPSSI-III provides scores that represent intellectual functioning in 4 specified cognitive domains: Verbal IQ, Performance IQ, Processing Speed, and General Language. The WPPSI- III also provides a Full Scale IQ score measuring general intellectual ability. The report is not written by a computer program but rather by an experienced Clinical Psychologist. Quotient and Composite scores have a mean of 100 and a standard deviation of 15. Subtest scaled scores have a mean of 10 and a standard deviation of 3. For Quotient and Composite score: below 70 is Extremely Low, 70–79 is borderline, 80–89 is Low Average, 90–109 is Average, 110–119 is High Average, and 120–129 is Superior.
RESULTS
In our cohort with CH, the use of 50 μg (15 μg/kg/ day) of L-thyroxine for the first 4 weeks resulted in mild hyperthyroxinemia in 12/45 of them but without significant symptoms or signs of hyperthyroidism.
Birth weight, length, and head circumference of patients (3.21 ± 0.43 kg, 50.5 ± 3.21 cm, and 34.1 ± 1.5 cm, respectively) did not differ than those for 10,560 normal newborns with normal thyroid function screened at the same period (3.19 ± 0.59 kg, 50.5 ± 2.2 cm, and 34.2 ± 1.7 cm).
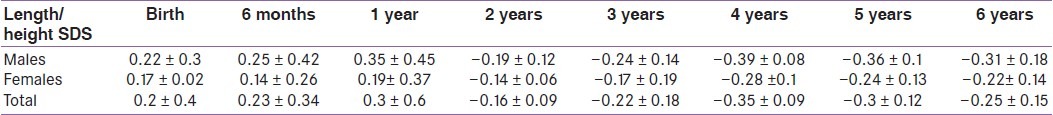
In this cohort of children with CH diagnosed by screening stature growth and weight gain were normal compared with the CDC growth data [Figures 1 and 2]. Their length/height SD Scores (L/HtSDS) were 0.2 ± 0.4, 0.23 ± 0.34, 0.3 ± 0.6, –0.16 ± 0.09, –0.22 ± 0.18, –0.35 ± 0.09, –0.3 ± 0.12, and –0.25 ± 0.15, respectively, at birth, 6 months, 1 year, 2 years, 3 years, 4 years, 5 years, and 6 years, respectively). L SDS for the first 2 years and HtSDS for the next years did not differ significantly between males and females with CH (P > 0.1 at 0, 1, 2, 3, 4, 5, and 6 years) [Table 1].
Figure 1.
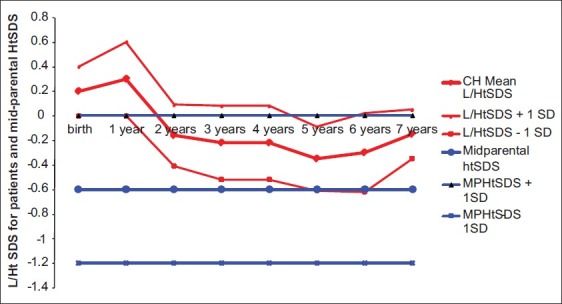
Length/height SDS for congenital hypothyroidism children versus midparental height standard deviation score
Figure 2.
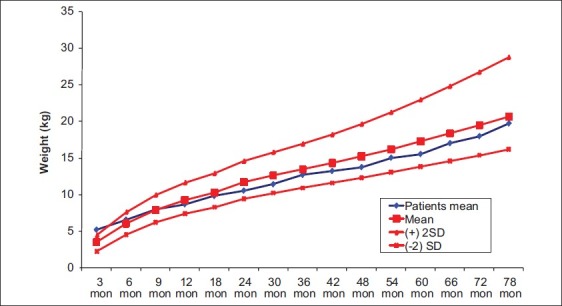
Weight gain in children with congenital hypothyroidism diagnosed through screening (vs CDC growth data)
Table 1.
Length/height standard deviation scores for males and females with congenital hypothyroidism
Compared with their mean MPHtSDS (–0.7±0.7 SDS, P < 0.01), the mean HtSDS of CH children showed adjustment to mid-parental height (± 0.5 SD) during the second year of life. CH children's HtSDS was higher than MPHtSDS by an average of 0.4 SD between the 2nd and 7th year of life [Figure 1]. Their mean bone age at 6 years of age = 5.8 ± 0.5 years. Growth in weight was normal in all the children [Figure 2] with no tendency to overweight. The intelligence levels of children with CH were within the normal range using WPPSI-III and ranged between 89 and 111. Only one child had IQ = 71, although it was diagnosed treated at 10 days of age. This patient had severe hypothyroidism at birth (fT4 = 2 pmol/L, TSH > 1000 uIU/mL) and experienced mild hypoxic insult during birth. Another patient had Attention Deficit Hyperactivity Disorder (ADHD), predominantly hyperactive-impulsive, with normal IQ (92). No difference in linear growth nor neurodevelopmental score was detected between infants who presented with goiter versus those with thyroid dysgenesis.
DISCUSSION
We performed a longitudinal study of a cohort of 45 children with congenital hypothyroidism (CH) detected by neonatal screening (HMC, Doha, Qatar) up to the age of 7 years, in order to study linear growth and the relationship to genetic potential expressed as midparental height. These children with CH (25 girls, 20 boys) were followed after starting an initial daily dose of T4 = 50 μg for the first 4 weeks. Adjustment of L-thyroxine dose was done every 2 months for the first year and then every 3 months thereafter to keep fT4 at the upper quartile for the normal range and TSH < 4 mIU/mL.
The mean age at diagnosis and the start of the treatment was 15.9 ± 4.3 days. Girls (n = 25) and boys (n = 20) L/ HtSDS did not show any delay in growth at birth compared with a large cohort of normal infants born at the same time. This proves that congenital hypothyroidism has no significant effect on intrauterine growth. No difference was found in linear growth between girls and boys.
Although the dose of L-thyroxine resulted in hyperthyroxinemia in 12/45 of them but there were no significant symptoms or signs of hyperthyroidism. The mean L/HtSDS of patients was normal for the whole period of 7 years compared with the CDC reference data. There was no delay of childhood component of growth reported previously in treated children with CH.[13] These data confirmed, with other longitudinal studies, the normalization of growth of children with CH compared with normal population growth standards.[14,15] However, we did not find difference in growth between males and females with CH, during the first year of life, reported previously.[16]
Comparison of L/HtSDS of children with CH to their mean MPHtSDS (–0.7 ± 0.7 SD) showed that these children showed adjustment to their MPHtSDS during the second year of life, not during the first year. Moreover, the HtSDS of children with CH has been always higher than MPHtSDS by an average of 0.4 SD between the 2nd and 7th year of life (P < 0.01). Their bone age corresponded to their chronologic age at 6 years. These data suggested that these children will end up taller than their midparental height. In support of this view, in a large Italian cohort of children with CH followedup till the end of their growth, the final height was higher than target height in both sexes.[17] Salerno et al.[13] followed up children with CH diagnosed by screening and treated for > 16 years and reported that the group of children who received higher L-thyroxine dose (> 8 μg/kg/day) at the beginning of therapy attained a final height (0.4 ± 1.0 SDS), which was within the normal range for the reference population but significantly above their target height. Grant reported that by the age of 3–4 years, the values for mean height in the children with either severe or less severe congenital hypothyroidism were equal to or greater than those for healthy children.[18]
Our results showed that treating CH infants with an initial T4 dose of 50 μg daily at the beginning of treatment with adjustment of the T4 to maintain serum fT4 concentrations within the upper quartile of normal range in children with CH maintains normal linear growth and bone maturation. The significantly higher HtSDS of these children at 6 years of life suggested that the final adult height of these children may slightly exceed their genetic potential (MPHtSDS).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Rastogi MV, LaFranchi SH. Congenital hypothyroidism. Orphanet J Rare Dis. 2010;5:17. doi: 10.1186/1750-1172-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng SM, Anand D, Weindling AM. High versus low dose of initial thyroid hormone replacement for congenital hypothyroidism. Cochrane Database Syst Rev. 2009:CD006972. doi: 10.1002/14651858.CD006972.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Vliet G. Neonatal hypothyroidism: Treatment and outcome. Thyroid. 1999;9:79–84. doi: 10.1089/thy.1999.9.79. [DOI] [PubMed] [Google Scholar]
- 4.Hrytsiuk I, Gilbert R, Logan S, Pindoria S, Brook CG. Starting dose of levothyroxine for the treatment of congenital hypothyroidism: A systematic review. Arch Pediatr Adolesc Med. 2002;156:485–91. doi: 10.1001/archpedi.156.5.485. [DOI] [PubMed] [Google Scholar]
- 5.Heyerdahl S, Oerbeck B. Congenital hypothyroidism: Developmental outcome in relation to levothyroxine treatment variables. Thyroid. 2003;13:1029–38. doi: 10.1089/105072503770867200. [DOI] [PubMed] [Google Scholar]
- 6.Pass K. Overview of newborn screening for congenital hypothyroidism. In: Houser P, Rovert J, editors. Thyroid diseases of infancy and childhood. Washington, DC: American Psychiatric Press; 1999. pp. 59–84. [Google Scholar]
- 7.Khneisser I. Newborn screening in Lebanon and Middle East. [Last accessed on 2012 Jan 4]. Available from: http://www.aphl.org/conferences/proceedings/Documents/2007_NBS_and_Genetic_Testing_Symposium/NBS_in_Lebanon.pdf .
- 8.Lindner M, Abdoh G, Fang-Hoffmann J, Shabeck N, Al-Sayrafi M, Al-Janahi M, et al. Implementation of extended neonatal screening and a metabolic unit in the State of Qatar: Developing and optimizing strategies in cooperation with the Neonatal Screening Center in Heidelberg. J Inherit Metab Dis. 2007;30:522–9. doi: 10.1007/s10545-007-0553-7. [DOI] [PubMed] [Google Scholar]
- 9.Soliman AT, Omar M, El Awwa A, Rizk MM, El Alaily RK, Bedair EM. Linear growth, growth-hormone secretion and IGF-I generation in children with neglected hypothyroidism before and after thyroxine replacement. J Trop Pediatr. 2008;54:347–9. doi: 10.1093/tropej/fmn030. [DOI] [PubMed] [Google Scholar]
- 10.Johnsonbaugh RE, Bryan RN, Hierlwimmer R, Georges LP. Premature craniosynostosis: A common complication of juvenile thyrotoxicosis. J Pediatr. 1978;93:188–91. doi: 10.1016/s0022-3476(78)80493-4. [DOI] [PubMed] [Google Scholar]
- 11.Schlesinger S, MacGillivray MH, Munschauer RW. Acceleration of growth and bone maturation in childhood thyrotoxicosis. J Pediatr. 1973;83:233–6. doi: 10.1016/s0022-3476(73)80481-0. [DOI] [PubMed] [Google Scholar]
- 12.Greulich WW, Pyle SI, Waterhouse AM. A radiographic standard of reference for the growing hand and wrist. Chicago: Case Western Reserve University; 1971. [Google Scholar]
- 13.Heyerdahl S, Ilicki A, Karlberg J, Kase BF, Larsson A. Linear growth in early treated children with congenital hypothyroidism. Acta Paediatr. 1997;86:479–83. doi: 10.1111/j.1651-2227.1997.tb08917.x. [DOI] [PubMed] [Google Scholar]
- 14.Salerno M, Micillo M, Di Maio S, Capalbo D, Ferri P, Lettiero T, et al. Longitudinal growth, sexual maturation and final height in patients with congenital hypothyroidism detected by neonatal screening. Eur J Endocrinol. 2001;145:377–83. doi: 10.1530/eje.0.1450377. [DOI] [PubMed] [Google Scholar]
- 15.Adachi M, Asakura Y, Tachibana K. Final height and pubertal growth in Japanese patients with congenital hypothyroidism detected by neonatal screening. Acta Paediatr. 2003;92:698–703. doi: 10.1080/08035250310002759. [DOI] [PubMed] [Google Scholar]
- 16.Morin A, Guimarey L, Apezteguía M, Ansaldi M, Santucci Z. Linear growth in children with congenital hypothyroidism detected by neonatal screening and treated early: A longitudinal study. J Pediatr Endocrinol Metab. 2002;15:973–7. doi: 10.1515/jpem.2002.15.7.973. [DOI] [PubMed] [Google Scholar]
- 17.Delvecchio M, Salerno M, Acquafredda A, Zecchino C, Fico F, Manca F, et al. Factors predicting final height in early treated congenital hypothyroid patients. Clin Endocrinol (Oxf) 2006;65:693–7. doi: 10.1111/j.1365-2265.2006.02651.x. [DOI] [PubMed] [Google Scholar]
- 18.Grant BD. Growth in early treated congenital hypothyroidism. Arch Dis Child. 1994;70:464–8. doi: 10.1136/adc.70.6.464. [DOI] [PMC free article] [PubMed] [Google Scholar]


