Abstract
Context:
Hypothyroidism is a common public health problem in India. With iodine sufficiency, autoimmune thyroiditis is becoming the most important etiology of hypothyroidism. Often, thyroiditis is associated with other systemic autoimmune diseases.
Aims:
We undertook thisobservational study to find the prevalence of systemic lupus erythematosus (SLE) amongst the hypothyroid patients at our Institution.
Settings and Design:
This is probably the first study of its kind from India.
Materials and Methods:
185 patients with diagnosed hypothyroidism were included and screening for SLE was done by standard epidemiological criteria. Majority of the patients (63.8%) were young adults (20-40 years).
Statistical Analysis Used:
Two by two contingency tables were analyzed by Chi-square test or Fisher's exact test as needed. Logistic regression model was used considering the presence of SLE as a dependent variable.
Results:
Eleven (5.94%) patients were found to have SLE. However, anti nuclear factor was positive in 145 cases (78.4%). Of the patients with SLE, 8 (72.7%) were found to be anti TPO positive, but the titers of ANF and anti TPO did not correlate. Presence of discoid rash, haematological criteria and presence of antibodies like anti-dsDNA were significantly correlated with the presence of SLE in hypothyroid patients. Presence of ANF was also correlated with the grade of goiter (r=0.62; P<0.05). Also four patients with SLE had a positive family history (OR=9.37). Logistic regression model showed anti-TPO has OR=1.54 (P=0.02) for the development of SLE.
Conclusions:
Prevalence of SLE in hypothyroid patients is high compared to the general population, especially, as thyroiditis is very common in SLE.
Keywords: Anti-nuclear factor, hypothyroidism, systemic lupus erythematosus, thyroiditis
INTRODUCTION
Thyroid disorders are quite common in India.[1] In fact, among the various endocrine disorders, thyroid disorders are the next common to type 2 diabetes mellitus (T2DM) in our country.[1] Earlier thought to be confined to Himalayan and sub-Himalayan regions, thyroid disorders are now found to be widely prevalent in various parts of this country.[2] Except in the iodine deficient areas, thyroiditis is the most important cause of hypothyroidism in India.[3] Hashimoto's thyroiditis (HD) is the most important autoimmune cause of thyroiditis all over the world.[4] The patients often present with hypothyroidism and a firm goiter.[4] It is the most common cause of goitrous hypothyroidism in areas of iodine sufficiency.[4] Various rheumatologic manifestations such as arthritis, joint swelling, muscle pain and swelling are common in hypothyroidism particularly in patients of HD. On the other hand, thyroiditis is often associated with other autoimmune diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) and mixed connective tissue disease (MCTD).[5] So, patients of hypothyroidism with rheumatological symptoms may have another autoimmune disease such as SLE, RA in addition. Extensive research has shown that SLE is often associated with thyroiditis features and hypothyroidism.[6] In fact, the features of hypothyroidism or autoimmune thyroiditis can predate the occurrence of other features of SLE in some cases.[7] In these cases, early recognition of SLE can help in prevention of morbidity. Otherwise, untreated SLE can present with life-threatening complications like nephritis.
Autoimmune diseases can be divided into organ-specific and systemic illness. The systemic inflammatory autoimmune diseases are RA, SLE, dermatomyositis, polymyositis and systemic sclerosis. One of the most common organs to be affected by organ-specific autoimmune injury is the thyroid gland. The occurrence of two or more organ-specific autoimmune diseases in a single person is known as autoimmune polyglandular failure. If we exclude patients with overlap syndrome, systemic autoimmune diseases do not commonly occur in the same patient. Whether concomitant organ-specific and systemic autoimmune diseases occur more often by chance than expected is a controversial issue. In particular, a large body of conflicting data has accumulated concerning the relationship between SLE and thyroid disease. Many small studies and case reports have associated SLE with hypothyroidism, both subclinical and clinical. According to Wickham study,[8] the prevalence of subclinical hypothyroidism in females above the age of 18 was 7.5% and clinical hypothyroidism 1%. Pyne and Isenberg reported a higher prevalence of hypothyroidism in 300 patients with SLE (5.7%) than in the normal population (1%).[9] Miller and co-workers has noted a significantly higher than expected prevalence of hypothyroidism in 332 patients with SLE (6.6%).[10] Whereas hypothyroidism occurs more commonly in SLE has a large body of data, the question whether SLE occurs more commonly in hypothyroidism has not been extensively studied.
We therefore undertook this study to find the prevalence of SLE in patients of hypothyroidism in our tertiary care center from Eastern India. Although studies showing the prevalence of thyroid disorders in SLE are many,[6,7] studies concerning the prevalence of SLE or other autoimmune disorders in hypothyroidism are scarce in available literature. We believe this is the first such study of its kind from India.
MATERIALS AND METHODS
One hundred and eighty five patients of hypothyroidism attending the endocrine outdoor of our Institution between 1st September 2010 and 31st December 2010 were included in the study. Drug- or radiation-induced hypothyroidism was excluded. Proper ethical approval and consent of the patients were taken. Patients with any active infection, history of trauma or surgery in last three months, HIV infection, with malignancy or patients on drugs known to cause positive anti nuclear factor (ANF) in blood were excluded from the study. Patients older than 70 years of age were excluded to avoid the possibility of false positive ANF results. The family histories of the patients were also noted in detail for any possible hypothyroidism or other autoimmune disorders. All patients were routinely screened for diabetes and hypertension by standard criteria. The presence of any other autoimmune features such as vitiligo was noted.
SLE was diagnosed by standard epidemiological criteria.[11] For this purpose, patients were first examined clinically, followed by laboratory tests like complete blood count, urine analysis, ANF (immunofluorescence; Hep-2 method), anti- dsDNA (immunofluorescence) and IgG/IgM lupus antibodies (ELISA). Thyroid antibodies were tested after stopping steroids if any, for six to eight weeks. For thyroid function study, serum freeT3 (FT3), freeT4 (FT4) and TSH levels were estimated by standard chemiluminescent methods.[12] Hypothyroidism was mainly screened by TSH levels, followed by confirmation with FT3 and FT4 level. Anti-TPO antibodies were measured by radioimmunoassay. Thyroid gland ultrasonography was done to look for any evidence of inflammation. The tests were done from the same laboratory by the same set of machines and ultrasonography was also done by the same operator throughout. Goiter was graded by standard criteria.[13] The data was analyzed by standard statistical methods using free software like MedCalc and EpiInfo. P value of <0.05 was considered significant. Two by two contingency tables were analyzed by Chi-square test or Fisher's exact test as needed. Logistic regression model was used considering the presence of SLE as a dependent variable.
The normal values (according to the lab kits for chemiluminescence; Architect, Abbott Diagnostics) of different tests are considered as follows: freeT3 = 1.71–3.71 pg/ml, freeT4 = 0.7–1.48 ng/dl, TSH = 0.35–4.94 μIU/ml, anti-TPO= negative, ANF (Hep-2; IF) <1:80, anti-dsDNA (immunofluorescence) <1:10.
RESULTS
We initially selected 197 patients from our outpatient department. Eight patients were excluded from the study because either they did not meet the inclusion criteria or refused to give consent to take part in the study. Also four patients were lost during follow up. Therefore, 185 patients in total completed the study. Of the 185 patients, 160 (86.5%) were females. The age distribution of the patients is shown in Table 1. It is seen that most (n=118; 63.8%) were in the 20-40 year age group. Altogether, 25(13.5%) patients were diabetic of which two was found to have type 1 diabetes. Hypertension was present in 89 patients (48.1%). Family history of hypothyroidism and SLE was present in 31(16.7%) and 14 (7.5%) patients respectively [Table 1]. Altogether 33 (17.8%) patients had associated other autoimmune diseases, rheumatoid arthritis (RA) being the most common (RA was diagnosed in 16 patients, 8.6%).
Table 1.
General characteristics of the patients
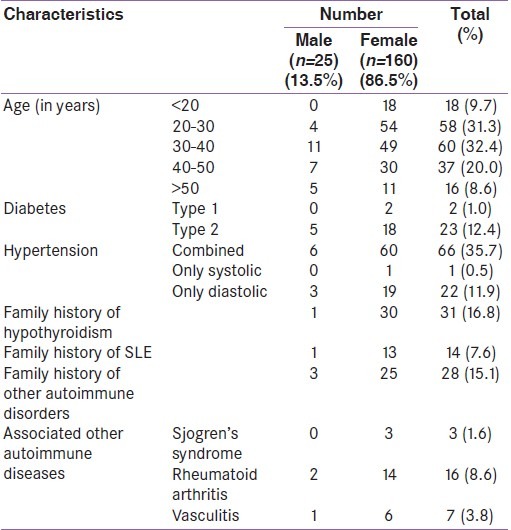
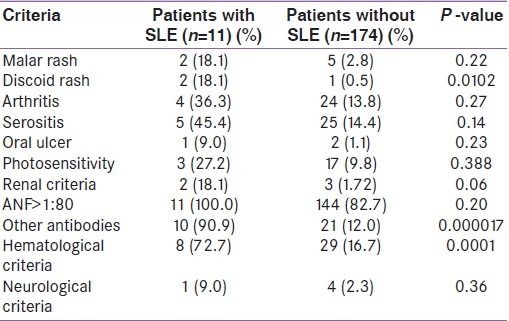
The antinuclear factor levels are shown in Table 2. It is seen that females had significantly higher levels of ANF as compared to males (36% males vs.85% females; P<0.0001). No male patient had ANF levels greater than 1:640. The most common pattern was homogenous (n=109; 75.2%). Six patients also presented with speckled pattern. To diagnose SLE, epidemiological criteria were used [Table 3]. It is seen that among the different criteria for SLE, positive ANF (>1:80) was the most common (n=145; 78.4%) followed by the presence of other antibodies such as anti dsDNA or anti- Sm antibody (n=31). Among the systemic manifestations, hematological system involvement was the most common (n=40; 21.6%). Altogether, 11 patients (5.9%) were found to fulfill the criteria (i.e.≥ 4 criteria positive out of 11 criteria) for epidemiological diagnosis of SLE. Of them, 10 (90.9%) were females. Of the patients diagnosed to have SLE, ANF was positive (>1:80) in all. The second most common manifestation in SLE patients was hematological (n= 8; 72.7%). Arthritis was present in four (36.3%) patients with SLE. As shown in Table 4, presence of discoid rash, hematological criteria and presence of antibodies like anti-dsDNA were strongly associated with the presence of SLE in hypothyroid patients.
Table 2.
Anti nuclear factor levels in the patients
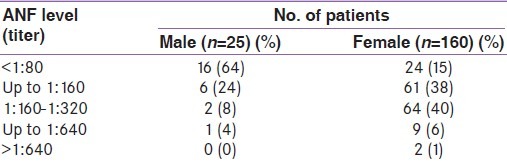
Table 3.
Different SLE criteria in the patients
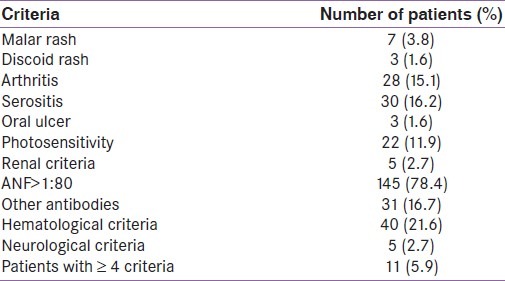
Table 4.
Different criteria in the two groups of patients
Among the 185 hypothyroid patients, anti TPO was positive in 76 (41.1%). However, among the patients diagnosed with SLE, anti- TPO was positive in 8 (72.7%; P=0.037 by Fisher's exact test). Thus the presence of anti-microsomal antibody is significantly associated with the occurrence of SLE. However, ultrasonographic evidence of inflammation in thyroid gland did not differ in the two groups (6 in SLE group vs. 72 in non- SLE group; P=0.79).
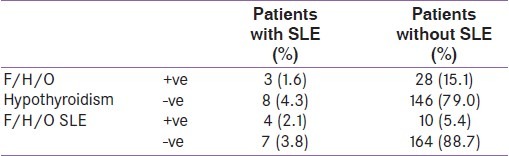
Table 5 shows the correlation of different parameters with titer of ANF. It is seen that the presence of vitiligo and higher grades of goiter are significantly associated with the presence of high titer of ANF. Lymphadenopathy was also commoner in patients with high titer of ANF (r=0.31), but the relationship was not significant (P=0.07). Table 6 shows the relation of family history with occurrence of SLE in hypothyroid patients. With positive family history of hypothyroidism, odds ratio (OR) for getting SLE was 1.95, whereas with a family history of SLE the OR of getting SLE was 9.37. By considering SLE as the dependent variable, logistic regression analysis shows that presence of anti TPO has OR of 1.54 for the development of SLE. But the total number of SLE cases are very small (n=11) to reach statistical significance in this analysis.
Table 5.
Relation of anti nuclear factor with other study parameters
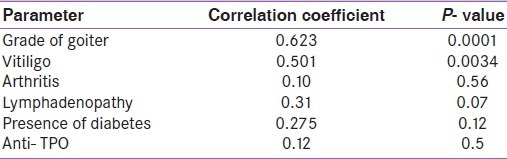
Table 6.
Relation of family history with occurrence of systemic lupus erythematosus (F/H/O: family history of)
DISCUSSION
SLE is a common autoimmune disease throughout the world. It varies from 40 to 100 per 1,00,000.[14] In India, the prevalence of SLE is lower at 3.2 per 1,00,000.[15,16] But when we consider the prevalence of SLE in selected groups like hypothyroidism, the number of patients is relatively high. In our study too, we found a prevalence of SLE 5.9% in hypothyroid patients, which is definitely high compared to general population, especially, as thyroiditis is very common in SLE.[17] Hypothyroidism is the most common presentation (as high as 36%) and the presence of thyroid disorders is often correlated with SLEDAI[14] (SLE disease activity index). The mechanism for autoimmune destruction of the thyroid probably involves both cellular immunity and humoral immunity. Lymphocytic infiltration of the thyroid gland by B cells and cytotoxic T cells is a common histologic feature of all forms of autoimmune thyroiditis.[18] Autoimmune thyroiditis is linked to HLA- DR3, 4, 5 which are also linked to SLE.[18] Also, environmental factors are implicated in thyroiditis.[19] Molecular mimicry after viral infections is said to trigger immune response against thyroid components. A study was done in UK by Pyne et al,[9] and they reported a prevalence of thyroid disorders in SLE as 5.7%. However, it was noted that the incidence of thyrotoxicosis was not increased in patients with SLE. In another study similar to us from Hungary, it was found that Hashimoto's thyroiditis (HD) was 90 times higher in population with SLE.[20] In mixed connective tissue disorder (MCTD) patients, 21% had HD. Thus, screening of thyroiditis or hypothyroid patients for these autoimmune disorders can be beneficial. In the same study, it was noted that 30% of patients with thyroid disorders had systemic autoimmune diseases.[20]
Anti nuclear antibodies can be positive in a variety of conditions. It is also found in autoimmune thyroiditis.[21] The pattern can be speckled; but the presence of ANF did not predict the presence of anti-thyroid antibodies.[21] In our study too, we did not find any correlation between ANF and anti TPO antibody (P=0.5). ANF was positive in 78.3% (n=145) of our patients, but only 11(5.9%) had SLE. All patients with SLE had been noted to be ANF positive.
However, in our study, we found significant association between ANF levels and the grade of goiter and vitiligo. Antibodies have been implicated in goiter, especially in the current era of iodine supplementation.[22] In a study from Iran, anti thyroglobulin (anti-Tg) levels have been shown to be a risk factor for goiter. In another study from Middle East, it was shown that after iodine supplementation, the level of anti- thyroid antibodies increased.[23] We did not measure anti-Tg due to cost factor. In our study, we also found 11 out of 16 (68.7%) patients with SLE had anti-TPO antibody positive which was statistically significant (P<0.05). Comparable results were also found in a study from Japan.[24] However, these antibodies in SLE are often not thyroid specific. Monoclonal anti-TPO antibodies in these cases cross-react with lactoperoxidase, which is similar to human peroxidases such as TPO, neutrophil peroxidase, uterine peroxidase or myeloperoxidase.[24] So, anti-TPO does not always correlate with the presence of thyroiditis in SLE.
In our study, we found other antibodies like anti dsDNA in 16.7% cases. Of them, 10 were diagnosed to have SLE. Thus, although anti dsDNA is more likely in SLE, there were hypothyroid patients with positive dsDNA antibodies but no SLE. The role of anti dsDNA in thyroiditis has recently been known.[25] Some studies have found association of dsDNA antibodies with anti-TPO levels. We, however, did not found any association. Also, among the 185 hypothyroid patients in our study, we found arthritis in 28 cases (15.1%). But only four had SLE (14.3%, P=0.27). Rheumatologic manifestations can be a part of thyroiditis itself.[26] So, the presence of arthritis in a hypothyroid patient does not always mean an associated systemic disease. Some authors have called this a separate variety of seronegative arthritis.[26] Some others have called this a manifestation of rheumatoid arthritis.[27] Among the most important or frequent rheumatic manifestations, there are a mild non-erosive variety of arthritis, polyarthralgia, myalgia, and sicca syndrome without a true Sjögren's syndrome.[28] In our patients, 16 (8.6%) had associated rheumatoid arthritis [Table 1]. But for the purpose of SLE classification, we only considered non erosive arthritis.[14]
In this study, we found family history as a significant predictor of SLE occurrence in hypothyroid patients [Table 6]. This is also an established entity. In a study from USA, they found odds ratio of 3.3 for occurrence of SLE if family history was positive.[29] In our study, we got OR of 9.37, but that is because we had selected a particular group of patients. Another study from Finland showed that 9.41% of all SLE patients had positive family history.[30] Of our 185 hypothyroid patients, family history of hypothyroidism was positive in 31 (16.7%). In different studies, this strong relation with family history has been correlated.[31] A study from UK has found Hashimoto thyroiditis significantly linked with family history, especially in the maternal relatives.[31] In our case, the percentage of patients with positive family history is lower than that reported in other studies, as we have included all cases of hypothyroidism and not just the HD ones. We also found that the occurrence of SLE was higher in patients with family history of hypothyroidism (OR= 1.95). Priori et al, by a case control study, have found that family history of autoimmune diseases is a strong risk factor for SLE.[32] Since our study is an observational study, we can only find the odds ratio. For actual risk assessment, a large case control study is needed. However, this first observational study from India alerts us to the possibility of SLE in the presence of significant degree of goiter and vitiligo.
CONCLUSION
Our study is a small single center observational study. Still a significant proportion of our hypothyroid patients were diagnosed to have SLE. Thus, in hypothyroid cases with the appropriate clinical scenario, screening for SLE can be an important clinical tool. It can help us to identify SLE cases at an earlier stage. We know thyroid dysfunction is very common in India despite many public health measures.[33] Again, thyroiditis and consequent hypothyroidism can be the first manifestation of a variety of autoimmune diseases such as Sjogren's syndrome, Scleroderma and SLE.[7,34] The screening for SLE can perhaps help us in identifying the etiology of hypothyroidism in a substantial proportion of our cases, especially in the presence of significant goiter and vitiligo.
ACKNOWLEDGEMENT
To Dr. Amit K Banerjee for allowing us to do the study in the department and also helping with calculations.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kochupillai N. Clinical endocrinology in India. Curr Sci. 2000;79:1061–7. [Google Scholar]
- 2.Das S, Bhansali A, Datta P, Aggarwal A, Bansal MP, Garg D, et al. Persistence of goitre in the post-iodization phase: Micronutrient deficiency or thyroid autoimmunity? Indian J Med Res. 2011;133:103–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Desai MP. Disorders of thyroid gland in India. Indian J Pediatr. 1997;64:11–20. doi: 10.1007/BF02795771. [DOI] [PubMed] [Google Scholar]
- 4.Brent GA, Larsen PR, Davies TF. Hypothyroidism and Thyroiditis. In: Kronenberg HM, Melmed S, Polonsky KS, Larsen PR, editors. Williams Textbook of Endocrinology. 11th ed. Philadelphia: Saunders; 2008. [Google Scholar]
- 5.Latif S, Jamal A, Memon I, Yasmeen S, Tresa V, Shaikh S. Multiple autoimmune syndrome: Hashimoto's thyroiditis, coeliac disease and systemic lupus erythematosus (SLE) J Pak Med Assoc. 2010;60:863–5. [PubMed] [Google Scholar]
- 6.Weetman AP, Walport MJ. The association of autoimmune thyroiditis with systemic lupus erythematosus. Rheumatology. 1987;26:359–61. doi: 10.1093/rheumatology/26.5.359. [DOI] [PubMed] [Google Scholar]
- 7.Dhir R, Ahluwalia AI, Sridhar J, Mani H, Pruthi HS, Shah KM. Autoimmune thyroiditis perdating the presentation of systemic lupus erythematosus: Two cases and a review of literature. Indian J Dermatol Venereol Leprol. 2002;68:292–4. [PubMed] [Google Scholar]
- 8.Tunbridge WM, Evered DC, Hall R, et al. The spectrum of thyroid disease in the community: The Wickham survey. Clin Endocrinol. 1997;7:41–93. doi: 10.1111/j.1365-2265.1977.tb01340.x. [DOI] [PubMed] [Google Scholar]
- 9.Pyne D, Isenberg DA. Autoimmune thyroid disease in systemic lupus erythematosus. Ann Rheum Dis. 2002;61:70–2. doi: 10.1136/ard.61.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller FW, Moore GF, Weintraub BD, Steinberg AD. Prevalence of thyroid disease and abnormal thyroid function test results in patients with systemic lupus erythematosus. Arthritis Rheum. 1987;30:1124–31. doi: 10.1002/art.1780301006. [DOI] [PubMed] [Google Scholar]
- 11.Edworthy SM. Clinical manifestations of systemic lupus erythematosus. In: Ruddy S, Harris ED, Sledge CB, Kelley WN, editors. Kelley's Textbook of rheumatology. 6th ed. Philadelphia: Saunders; 2001. pp. 1105–19. [Google Scholar]
- 12.Becker DV, Bigos ST, Gaitan E, Morris JC, Rallison ML, Spencer CA, et al. Optimal use of blood tests for assessment of thyroid function. JAMA. 1993;269:2736–42. [PubMed] [Google Scholar]
- 13.Hazarika NC, Mahanta J. Environmental iodine deficiency and goiter prevalence in a block area of the North Eastern Region: A retrospective analysis. J Hum Ecol. 2004;15:113–7. [Google Scholar]
- 14.Bartels CM. Systemic lupus erythematosus. Medscape reference. 2011. [cited on 2011 Jun 4]. Available from: http://emedicine.medscape.com/article/332244.overview#a0199 .
- 15.Malaviya AN, Singh RR, Singh YN, Kapoor SK, Kumar A. Prevalence of systemic lupus erythematosus in India. Lupus. 1993;2:115–8. doi: 10.1177/096120339300200209. [DOI] [PubMed] [Google Scholar]
- 16.Mahajan A, Jasrotia DS, Manhas AS, Jamwal SS. Prevalence of major rheumatic disorders in Jammu. JK Sci. 2003;5:63–6. [Google Scholar]
- 17.Kumar K, Kole AK, Karmakar PS, Ghosh A. The spectrum of thyroid disorders in systemic lupus erythematosus. Rheumatol Int. 2010 Jul 25; doi: 10.1007/s00296-010-1556-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Pearce EN, Farwell AP, Braverman LE. Current concepts: Thyroiditis. N Engl J Med. 2003;348:2646–55. doi: 10.1056/NEJMra021194. [DOI] [PubMed] [Google Scholar]
- 19.Guarneri F, Benvenga S. Environmental factors and genetic background that interact to cause autoimmune thyroid disease. Curr Opin Endocrinol Diabetes Obes. 2007;14:398–409. doi: 10.1097/MED.0b013e3282ef1c48. [DOI] [PubMed] [Google Scholar]
- 20.Biro E, Szekanecz Z, Czirzak L, Danko L, Kiss E, Szabo NA, et al. Association of systemic and thyroid autoimmune diseases. Clin Rheumatol. 2006;25:240–5. doi: 10.1007/s10067-005-1165-y. [DOI] [PubMed] [Google Scholar]
- 21.Torok KS, Arkachaisri T. Autoimmune thyroiditis in antinuclear antibody positive children without rheumatologic disease. Pediatr Rheumatol Online J. 2010;8:15. doi: 10.1186/1546-0096-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashemipour M, Amini M, Aminorroaya A, Dastjerdi MS, Rezvanian H, Kachoei A, et al. High prevalence of goiter in an iodine replete area: Do thyroid auto-antibodies play a role? Asia Pac J Clin Nutr. 2007;16:403–10. [PubMed] [Google Scholar]
- 23.Markou KB, Georgopoulos NA, Makri M, Vlasopoulou B, Anastasiou E, Vagenakis GA, et al. Improvement of iodine deficiency after iodine supplementation in schoolchildren of Azerbaijan was accompanied by hypo and hyperthyrotropinemia and increased title of thyroid autoantibodies. J Endocrinol Invest. 2003;26:43–8. [PubMed] [Google Scholar]
- 24.Kohno Y, Naito N, Saito K, Hoshioka A, Niimi H, Nakajima H, et al. Anti-thyroid peroxidase antibody activity in sera of patients with systemic lupus erythematosus. Clin Exp Immunol. 1989;75:217–21. [PMC free article] [PubMed] [Google Scholar]
- 25.Vagapova G, Sattarova L. The role of anti-DNA antibodies in pathogenesis of Hashimoto's thyroiditis. Endocr Abstracts. 2009;20:72. [Google Scholar]
- 26.LeRiche NG, Bell DA. Hashimoto's thyroiditis and polyarthritis: A possible subset of seronegative polyarthritis. Ann Rheum Dis. 1984;43:594–8. doi: 10.1136/ard.43.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pongratz R, Buchinger W, Semlitsch G, Meister E, Nadler K, Rainer F. Increased occurrence of autoimmune thyroiditis in patients with chronic rheumatoid arthritis. Acta Med Austriaca. 2000;27:58–60. [PubMed] [Google Scholar]
- 28.Punzi L, Betterli C. Chronic autoimmune thyroiditis and rheumatic manifestations. Joint Bone Spine. 2004;71:275–83. doi: 10.1016/j.jbspin.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Cooper GS, Dooley MA, Treadwell EL, St Clair EW, Gilkeson GS. Risk factors for development of systemic lupus erythematosus: Allergies, infections, and family history. J Clin Epidemiol. 2002;55:982–9. doi: 10.1016/s0895-4356(02)00429-8. [DOI] [PubMed] [Google Scholar]
- 30.Koskenmies S, Widen E, Kere J, Julkunen H. Familial systemic lupus erythematosus in Finland. J Rheumatol. 2001;28:758–60. [PubMed] [Google Scholar]
- 31.Manji N, Carr-Smith JD, Boelaert K, Allahabadia A, Armitage M, Chatterjee VK, et al. Influences of age, gender, smoking, and family history on autoimmune thyroid disease phenotype. J Clin Endocrinol Metab. 2006;91:4873–80. doi: 10.1210/jc.2006-1402. [DOI] [PubMed] [Google Scholar]
- 32.Priori R, Medda E, Conti F, Cassara EA, Danieli MG, Gerli R, et al. Familial autoimmunity as a risk factor for systemic lupus erythematosus and vice versa: A case-control study. Lupus. 2003;12:735–40. doi: 10.1191/0961203303lu457oa. [DOI] [PubMed] [Google Scholar]
- 33.Usha Menon V, Sundaram KR, Unnikrishnan AG, Jayakumar RV, Nair V, Kumar H. High prevalence of undetected thyroid disorders in an iodine sufficient adult south Indian population. J Indian Med Assoc. 2009;107:72–7. [PubMed] [Google Scholar]
- 34.Picco P, Gattorno M, Buoncompagni A, Piaggio G, Stalla F, Pistoia V, et al. Autoimmune thyroiditis developing into systemic lupus erythematosus in a 15-year-old girl. Clin Exp Rheumatol. 1996;14:348–9. [PubMed] [Google Scholar]


