Abstract
Background:
Thyroid hormones affect bone remodeling in patients with thyroid disease by acting directly or indirectly on bone cells. In view of limited information on correlation of thyroid function with bone mineral density (BMD) in euthyroid subjects, we undertook this study to evaluate the correlation between thyroid function with BMD in subjects with normal thyroid function and subclinical hypothyroidism.
Material and Methods:
A total of 1290 subjects included in this cross sectional study, were divided in Group-1 with normal thyroid function and Group-2 with subclinical hypothyroidism. Fasting blood samples were drawn for the estimation of serum 25(OH)D, intact parathyroid hormone, total and ionized calcium, inorganic phosphorus, and alkaline phosphatase. BMD at lumbar spine, femur, and forearm was measured.
Results:
BMD at all sites (radius, femur, and spine) were comparable in both groups. There was no difference in BMD when subjects were divided in tertiles of TSH in either group. In group-1, FT4 and TSH were positively associated with BMD at 33% radius whereas FT3 was negatively associated with BMD at femoral neck in multiple regression analysis after adjustment for age, sex, BMI, 25(OH)D and PTH levels. In group-2, there was no association observed between TSH and BMD at any site. Amongst all study subjects FT4 and FT3 were positively correlated with BMD at lumbar spine and radius respectively among all subjects.
Conclusion:
TSH does not affect BMD in euthyroid subjects and subjects with subclinical hypothyroidism. Thyroid hormones appear to have more pronounced positive effect on cortical than trabecular bone in euthyroid subjects.
Keywords: Bone mineral density, subclinical hypothyroidism, thyroid stimulating hormone
INTRODUCTION
Thyroid hormones affect bone remodeling in patients with thyroid disease by acting directly or indirectly on bone cells.[1–4] TSH may also affect bone health by interacting with TSH receptors expressed on osteoblasts and osteoclast precursors.[5–7] The relative contribution of thyroid hormone excess and TSH deficiency in causation of bone loss remains unresolved. In experimental animals, reduced expression of TSH receptors leads to development of osteoporosis.[5,6] Studies in subjects with exogenous subclinical hyperthyroidism did not reveal any effect on bone mineral density (BMD) in men and premenopausal women, whereas the effect in postmenopausal women is equivocal.[1,2] The association of subclinical hypothyroidism and BMD are varied.[8–11] Many authors have studied relation of thyroid functions with BMD in euthyroid postmenopausal women,[12–15] but there is limited information on correlation of thyroid function with BMD in euthyroid premenopausal women and males below 50 years of age. Hence, we undertook this cross sectional study to evaluate the correlation of thyroid function with BMD in subjects aged less than 50 years with normal thyroid function and subclinical hypothyroidism.
MATERIAL AND METHODS
This study was carried out as part of general health examination of all members of Resident Welfare Associations of 4 residential colonies of Delhi, one each from North, South, East, and West for a general health checkup on voluntary basis. Men >50 years of age, women who were either post-menopausal or > 50 years of age, and subjects with diabetes, hepatic disease, renal disease, alcoholism, family history of fracture, overt hypo-or hyper-thyroidism, or receiving any medication likely to influence bone mineral status were excluded from the study.
A total of 1290 subjects were included in the study. Body mass index (BMI) was calculated by weight in kilogram divided by square of height in meters. Fasting blood samples were drawn for the estimation of serum 25(OH)D, intact parathyroid hormone, total and ionized calcium, inorganic phosphorus, and alkaline phosphatase (ALP). The study was approved by the ethics committee of the Institute of Nuclear Medicine and Allied Sciences and all subjects gave written informed consent.
Thyroid function tests (TFT) were performed by electrochemiluminiscence assay with the following normal range FT3 (2.8-7.1 pmol/L), FT4 (12.0-22.0 pmol/L), TSH (0.27-4.20mIU/L). Anti-TPO antibody was measured by using electrochemiluminiscence kits from Roche (Germany) with normal range from 0.0-34.0 IU/L. Subjects >102.0 IU/L i.e. 3 times the upper limit of normal were considered to have significant anti-TPO antibody positivity. Biochemical estimations were carried out using automated analyzer (Hitachi 902 fully automated biochemistry analyzer; Roche, Manheim, Germany) and commercial kits (Roche, Manheim, Germany). The normal range for different biochemical parameters are as follows: serum total calcium (2.2-2.55 mmol/L), ionized calcium (1.12-1.32 mmol/L), inorganic phosphorus (0.9-1.5 mmol/L), and alkaline phosphatase were (females: <240 U/L; males: <270 U/L). The serum concentrations of 25(OH)D (reference range: 22.5-94 nmol/L) and PTH (reference range: 10-65 ng/L) were measured by RIA (Diasorin, Stillwater, MN) and electrochemiluminiscence assay (Roche diagnostics, GMDH-Manheim, Germany) respectively.
Bone mineral density (BMD) at anteroposterior (AP) lumbar spine (L1-L4), femur (total hip, femoral neck) and forearm (total, ultra distal and 33% radius) was measured using the Prodigy Oracle (GE Lunar Corp., Madison, WI) according to standard protocol. Quality control procedures were carried out in accordance with the manufacturer's recommendations. Instrument variation was determined regularly using a phantom supplied by the manufacturer and mean coefficient of variation was <0.5%. For in vivo measurements, mean coefficients of variation for all sites were <1%.
Subjects (1290) were divided in two groups: Group1 (1115 subjects, 86.8%) with normal thyroid function and Group 2 (175 subjects, 13.2%) with subclinical hypothyroidism defined as normal FT4 and TSH >4.2 mIU/L.
Statistical analysis was carried out using EPI INFO 3.5.3 (CDC, Atlanta, GA, USA). Data were presented as mean ± SD or number (%) unless specified. All parametric data were analysed by student's t-test. If Barlett's Chi-square test for equality of population variances was <0.05 then Kruskal-Wallis test was applied. All non parametric data were analysed by Chi-square test. Multiple regression analysis was done to ascertain association between thyroid functions and BMD at various sites. Pearson's correlation coefficient was calculated to assess the strength of relationship between thyroid function test and BMD at various sites. A P value of < 0.05 was considered statistically significant.
RESULTS
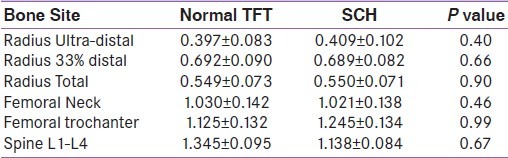
Baseline characteristics of subjects are given in Table 1. Both groups were comparable in all respect except FT4 and TSH. BMD at all sites (radius, femur and spine) were comparable in both groups [Table 2]. When both groups were divided according to TSH levels (0.3-1.6, >1.6-2.9, >2.9-4.2 and >4.2-6.2,>6.2-8.2, >8.2); there were no difference in BMD among groups. There were no statistically significant differences in BMD at all sites between groups in either of sexes analysed separately (data not shown).
Table 1.
Basic characteristics of study population
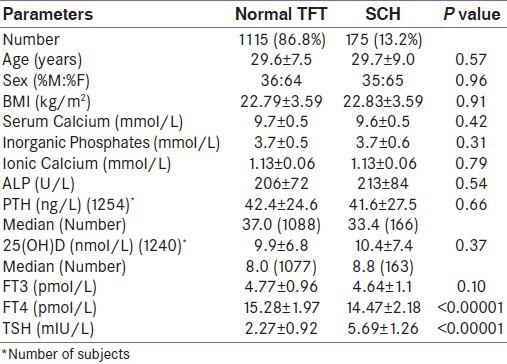
Table 2.
Bone mineral density (gm/cm2) in subjects with normal thyroid function tests and subclinical hypothyroidism
Among euthyroid subjects, FT4 were positively associated with BMD at 33% radius after adjustment for age, sex, BMI, 25(OH)D and PTH levels in multiple regression analysis The association between FT4 BMD at femoral neck did not achieve statistical significance [Table 3]. Among subjects with SCH, FT4 was negatively associated with BMD at lumbar spine in univariate (r2 =0.02, p=0.07) and multivariate analysis (r2 =0.07, p= 0.041) [Table 4]. In correlation analysis, FT4 was positively correlated with BMD at radius and femoral neck among all subjects [Table 5].
Table 3.
Multiple regression analysis of TFT and BMD in euthyroid subjects (After adjustment for age, sex, BMI, PTH and 25(OH)D levels)
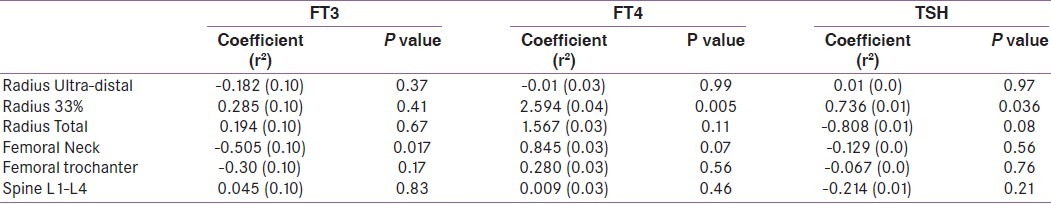
Table 4.
Multiple regression analysis of TFT and BMD in SCH subjects (After adjustment for age, sex, BMI, PTH and 25(OH)D levels)
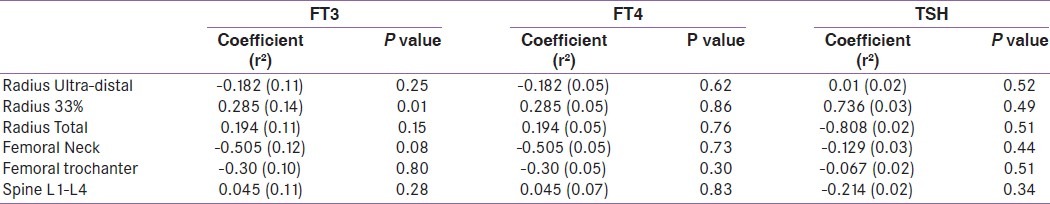
Table 5.
Correlation of thyroid function tests and bone mineral density among all subjects
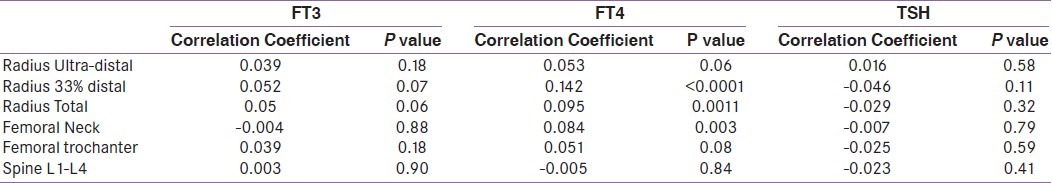
Among euthyroid subjects, FT3 was negatively associated with BMD at femoral neck after adjustment for age, sex, BMI, 25(OH)D and PTH levels in multiple regression analysis [Table 3]. Among subjects with SCH, FT3 was negatively associated with BMD at 33% radius (r2 =0.14, p=0.01) in multivariate analysis [Table 4]. In correlation analysis, FT3 showed no correlation with BMD at any site among all subjects [Table 5].
TSH was positively associated with BMD at 33% radius among euthyroid subjects in multiple regression analysis after adjustment of various factors mentioned above. TSH showed no association with BMD at any site in univariate or multiple regression analysis in subjects with SCH [Table 4]. TSH showed no correlation with BMD at any site among all subjects [Table 5].
DISCUSSION
In the present study, FT3 was noted to be negatively associated with BMD at femoral neck whereas FT4 was positively associated and correlated with BMD at radius and femoral neck; and TSH was neither associated nor correlated with BMD at any site in euthyroid subjects below 50 years of age. Griemnes et al,[13] in a large cross sectional Tromsø study among men and postmenopausal women, found no association between TSH and BMD with serum TSH being in the normal range after adjustment for age, weight, height, smoking habits, physical activity and use of hormone replacement therapy in women. In contrast, Baqi et al,[14] reported significant positive influence of TSH on BMD in postmenopausal women at lumbar spine and femoral neck independent of age, and BMI in multiple regression analysis. A negative correlation of TSH with bone mineral content has also been reported in healthy euthyroid subjects in a small study.[15]
We divided the study subjects from both groups in TSH tertiles and found no difference in BMD among tertiles in either group. Similar observation has been made in a large population based study by Svare et al.[16] However, a hospital-based study from South Korea found an increasing hip and lumbar BMD with increasing TSH from low normal to high normal TSH level.[17] Morris et al,[12] also studied the association between TSH (within normal range) and hip BMD in postmenopausal women. They reported that women with TSH values in the lower normal range (0.39-1.79 mIU/L) had higher risk for osteopenia and osteoporosis than those with TSH values in the higher normal range (1.8-4.6 mIU/L).
The positive association and correlation of FT4 with BMD at radius in euthyroid subjects observed in present study was in contrast to a large population based cohort study involving healthy euthyroid postmenopausal women where FT4 was negatively correlated with BMD at hip.[18] Similarly, a negative correlation of FT4 with bone mineral content has also been reported in healthy euthyroid subjects.[15] Baqi et al,[14] however, reported no correlation between FT4 and BMD in post menopausal women. Negative association of FT3 with BMD at femoral neck in euthyroid subjects in the present study was also observed by Murphy et al,[18] in healthy postmenopausal euthyroid women. However, there are studies which did not reveal any correlation between thyroid hormones and BMD in premenopausal women[19] and cured cases of differentiated thyroid carcinoma with subnormal TSH levels on thyroxin replacement.[20] Since there was no effect of thyroid hormones noted on BMD at lumbar spine in euthyroid subjects in present study, it suggests that thyroid hormones have more pronounced effect on cortical than trabecular bone.[1,2]
There was no difference in BMD at any site between euthyroid subjects and subjects with subclinical hypothyroidism. One small study, in contrast, did report decrease in BMD at femoral neck but found no difference in BMD at lumbar spine in postmenopausal women with subclinical hypothyroidism compared to euthyroid controls.[11] However, another study[8] reported significantly higher BMD in premenopausal women with post surgical subclinical hypothyroidism. Lack of association between TSH and BMD at any site observed in subjects with SCH in the present study, was consistent with the findings of a small study in postmenopausal women at lumbar spine and total hip.[9] Bertoli et al, who measured BMD and bone mineral contents at different regional sites in 32 postmenopausal women, reported direct relationship between leg BMD and TSH.[10]
In a population based study, Svare et al,[16] demonstrated that BMD at radius was lower in subjects with TSH <0.1 mIU/L, but changed little with increasing TSH levels. We therefore hypothesize that TSH values above 0.1 mIU/L probably plays a permissive role in maintaining BMD which would also explain the lack of association of TSH with BMD in both euthyroid and SCH subjects as seen in the present study. In contrast, patients with thyrotoxicosis have a low bone mass partly because their TSH is below the required threshold for maintaining BMD. TSH receptors have been reported to be present on human osteoblasts.[7] Low concentration of TSH may be necessary for local expression of type-2 deiodinase in osteoblasts[21] which via generation of T3 locally may have a positive effect on osteoblast. This is further supported by a genetic study where TSHR knockout and haploinsufficient mice with normal thyroid hormone levels have decreased bone mass.[22] Similarly, the exogenous administration of low doses of TSH in animal experiments have positively influenced bone remodeling by inhibiting osteoclast differentiation and activating osteoblast differentiation,[5,6] thus eliciting both antiresorptive and anabolic bone effects.
The main strength of study was large sample size and population based study and limitation was being a cross sectional study, changes in BMD could not be assessed in relation to thyroid function tests over a period of time.
CONCLUSIONS
TSH does not affect BMD in euthyroid subjects and subjects with subclinical hypothyroidism. Thyroid hormones appear to have more pronounced positive effect on cortical bone than trabecular bone in euthyroid subjects. We speculate that TSH may have permissive role in bone remodeling.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76–131. doi: 10.1210/er.2006-0043. [DOI] [PubMed] [Google Scholar]
- 2.Greenspan SL, Greenspan FS. The effect of thyroid hormone on skeletal integrity. Ann Intern Med. 1999;130:750–8. doi: 10.7326/0003-4819-130-9-199905040-00016. [DOI] [PubMed] [Google Scholar]
- 3.Sato K. Graves’ disease and bone metabolism. Nippon Rinsho. 2006;64:2317–22. [PubMed] [Google Scholar]
- 4.Allain TJ, McGregor AM. Thyroid hormones and bone. J Endocrinol. 1993;139:9–18. doi: 10.1677/joe.0.1390009. [DOI] [PubMed] [Google Scholar]
- 5.Abe E, Marians RC, Yu W, Wu XB, Ando T, Li Y, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115:151–62. doi: 10.1016/s0092-8674(03)00771-2. [DOI] [PubMed] [Google Scholar]
- 6.Abe E, Sun L, Mechanick J, Iqbal J, Yamoah K, Baliram R, et al. Bone loss in thyroid disease: role of low TSH and high thyroid hormone. Ann N Y Acad Sci. 2007;1116:383–91. doi: 10.1196/annals.1402.062. [DOI] [PubMed] [Google Scholar]
- 7.Tsai JA, Janson A, Bucht E, Kindmark H, Marcus C, Stark A, et al. Weak evidence of thyrotropin receptors in primary cultures of human osteoblast-like cells. calcif Tissue Int. 2004;74:486–91. doi: 10.1007/s00223-003-0108-3. [DOI] [PubMed] [Google Scholar]
- 8.Arata N, Momotani N, Maruyama H, Saruta T, Tsukatani K, Kubo A, et al. Bone mineral density after surgical treatment for Graves’ disease. Thyroid. 1997;7:547–54. doi: 10.1089/thy.1997.7.547. [DOI] [PubMed] [Google Scholar]
- 9.Rasic-Milutinovic Z, Milicevic D, Gluvic Z, Vujovic M, Tica J, Radinovic V. Subclinical hypothyroidism and bone mineral density Endocrine Abstracts. 2008;16:75. Available from: http://www.endocrine.abstracts.org/ea/0016/ea0016p75.htm . [Google Scholar]
- 10.Bertoli A, Fusco A, Andreoli A, Magnani A, Tulli A, Lauro D, et al. Effect of subclinical hypothyroidism and obesity on whole-body and regional bone mineral content. Horm Res. 2002;57:79–84. doi: 10.1159/000057956. [DOI] [PubMed] [Google Scholar]
- 11.Lee WY, Oh KW, Rhee EJ, Jung CH, Kim SW, Yun EJ, et al. Relationship between subclinical thyroid dysfunction and femoral neck bone mineral density in women. Arch Med Res. 2006;37:511–6. doi: 10.1016/j.arcmed.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Morris MS. The association between thyroid-stimulating hormone in its reference range and bone status in postmenopausal American. Bone. 2007;40:1128–34. doi: 10.1016/j.bone.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Grimnes G, Emaus N, Joakimsen RN, Figenschau Y, Jorde R. The relationship between serum TSH and bone mineral density in men and postmenopausal women: The Tromsø study. Thyroid. 2008;18:1147–55. doi: 10.1089/thy.2008.0158. [DOI] [PubMed] [Google Scholar]
- 14.Baqi L, Payer J, Killinger Z, Susienkova K, Jackuliak P, Cierny D, et al. The level of TSH appeared favourable in maintaining bone mineral density in postmenopausal women. Endocr Regul. 2010;44:9–15. doi: 10.4149/endo_2010_01_9. [DOI] [PubMed] [Google Scholar]
- 15.Belsing TZ, Tofteng C, Langdahl BL, Charles P, Feldt-Rasmussen U. Can bone loss be reversed by antithyroid drug therapy in premenopausal women with Graves’ disease? Nutr Metab (Lond) 2010;7:72–81. doi: 10.1186/1743-7075-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svare A, Nilsen TIL, Bjøro T, Forsmo S, Schei B, Langhammer A. Hyperthyroid levels of TSH correlate with low bone mineral density: the HUNT 2 study. Eur J Endocrinol. 2009;16:779–86. doi: 10.1530/EJE-09-0139. [DOI] [PubMed] [Google Scholar]
- 17.Kim DJ, Khang YH, Koh JM, Shong YK, Kim GS. Low normal TSH levels are associated with low bone mineral density in healthy postmenopausal women. Clin Endocrinol. 2006;64:86–90. doi: 10.1111/j.1365-2265.2005.02422.x. [DOI] [PubMed] [Google Scholar]
- 18.Murphy E, Glüer CC, Reid DM, Felsenberg D, Roux C, Eastell R, et al. Thyroid function within the upper normal range is associated with reduced bone mineral density and an increased risk of nonvertebral fractures in healthy euthyroid postmenopausal women. J Clin Endocrinol Metab. 2010;95:3173–81. doi: 10.1210/jc.2009-2630. [DOI] [PubMed] [Google Scholar]
- 19.Ugur-Altun B, Altun A, Arikan E, Guldiken S, Tugrul A. Relationships existing between the serum cytokine levels and bone mineral density in women in the premenopausal period affected by Graves’ disease with subclinical hyperthyroidism. Endocr Res. 2003;29:389–98. doi: 10.1081/erc-120026945. [DOI] [PubMed] [Google Scholar]
- 20.Heemstra KA, van der Deure WM, Peeters RP, Hamdy NA, Stokkel MP, Corssmit EP, et al. Thyroid hormone independent associations between serum TSH levels and indicators of bone turnover in cured patients with differentiated thyroid carcinoma. Eur J Endocrinol. 2008;159:69–76. doi: 10.1530/EJE-08-0038. [DOI] [PubMed] [Google Scholar]
- 21.Morimura T, Tsunekawa K, Kasahara T, Seki K, Ogiwara T, Mori M, et al. Expression of type 2 iodothyronine deiodinase in human osteoblast is stimulated by thyrotropin. Endocrinology. 2005;146:2077–84. doi: 10.1210/en.2004-1432. [DOI] [PubMed] [Google Scholar]
- 22.Galliford TM, Murphy E, Williams AJ, Bassett JH, Williams GR. Effects of thyroid status on bone metabolism: A primary role for thyroid stimulating hormone or thyroid hormone? Minerva Endocrinologica. 2005;30:237–46. [PubMed] [Google Scholar]


