Abstract
Introduction:
Children with congenital adrenal hyperplasia (CAH) provide us an opportunity to study the clinical effects of androgen excess in humans. We studied the sequence of pubertal development in girls with congenital adrenal hyperplasia initiated on treatment at different ages, to assess the effects of androgen exposure on the Hypothalamic-Pituitary-Ovarian (HPO) axis.
Materials and Methods:
Girls more than 18 years of age, with CAH, on follow-up at this hospital were the subjects for this study. Details of history, physical findings, laboratory evaluation, and medication were noted from their case records and verified from the patients and their / parents, in addition to assessment of their present health status.
Result:
We studied 24 patients of classical CAH (SW-2, SV-22, average age – 24.5 ± 6.6 years). All had varying degrees of genital ambiguity (Prader stage 3 (n = 13), Prader stage 2 (n = 10), Prader stage 1 (n = 1). Among them were13 girls, who were started on steroids after eight years of age. Girls who received treatment from infancy and early childhood had normal pubertal development (mean age at menarche 11.4 ± 1.7 years). Hirsutism was not a problem among them. Untreated children had progressive clitoral enlargement throughout childhood, developed pubic hair at around three to six years of age, and facial hair between nine and eleven years. Plasma testosterone ranged from 3 to 6 ng / ml prior to treatment. Six of the 13 untreated CAH girls had subtle breast development starting at ages 11 – 16 years and three had spontaneous infrequent vaginal bleeding starting at ages 11 – 17. Steroid supplementation initiated pubertal changes in older girls in two-to-six months’ time.
Conclusion:
There was a delay in HPO axis maturation (as evidenced by delayed pubertal development) in the absence of treatment in girls with CAH. This could be corrected with steroid supplementation.
Keywords: Congenital adrenal hyperplasia, puberty, thelarche, hirsuitism
INTRODUCTION
Prenatal androgen exposure in nonhuman primates and sheep produces many features of polycystic ovary syndrome (PCOS).[1–4] Androgens act on the brain to induce major sex differences in neural structure and function. Female rats, prenatally exposed to androgens, demonstrate defeminization of the gonadotropin releasing hormone (GnRH) neurosecretory system, like in PCOS.[5] Raising testosterone levels with androgen infusion or implants has shown inconsistent effects on gonadotropin levels and gonadotropin response to GnRH.[6,7]
Congenital adrenal hyperplasia (CAH) is a genetic disorder where there is excess adrenal androgen production from the fetal adrenals. Girls afflicted with CAH are born with varying degree of masculinization of the external genitalia, as a result of prenatal androgen exposure. Treatment is started soon after birth or earlier, in those diagnosed antenatally. However, in our country we still see some girls with CAH coming for treatment peri pubertally or later.[8,9] These children provide an opportunity to study the effects of prenatal and continuing androgen exposure on pubertal development. Here we report our observations on pubertal development among CAH girls initiated on steroid supplementation at different ages.
MATERIALS AND METHODS
Girls with CAH who were being treated at our hospital were the subjects for this study. Patients who were 18 years or older at the time of this study (between January 2004 and December 2006) were invited to participate in this study.
Inclusion criteria
Women with classical CAH, age 18 years or older
Exclusion criteria
Non-classical CAH, CAH girls (46, XX) reared as boys, and boys with CAH were excluded.
Medical records were reviewed (in addition to assessment of the present health status) for details of perinatal events, appearance of genitalia, age at diagnosis, investigations done, initiation and duration of medication, surgery, pubertal development, menstrual patterns, and family history. Additional information / missing details were verified with the patient / parents. Patients were interviewed regarding age at onset of breast development, appearance of facial and body hair, acneform eruptions, menarche, and menstrual patterns. These details were also verified with the parents (most often, mother). Physical examination included anthropometry, pubertal status, signs of hyperandrogenism, and appearance of external genitalia.
Patients were subgrouped on the basis of age at steroid initiation. Those initiated on steroids before eight years of age and those initiated on steroids after eight years age. These two subgroups were analyzed with respect to appearance of facial hair, breast development, menarche, and menstrual patterns.
The data was analyzed using a SPSS 10.0 software. The average of various parameters studied was expressed as mean ± SD. Analysis of variance (ANOVA) and Student t-test were applied for comparison of the means of different parameters. A P Value of less than 0.05 was considered significant. The chi square test was applied for comparing the means between non-parametric variables.
RESULTS
Twenty-four patients with CAH fulfilled the inclusion criteria. The mean age at the time of study was 25.4 ± 6.2 years (18 – 29 years). They had completed 13.3 ± 8.5years (median 16 years) of follow-up in our clinic.
Presenting complaints at the time of initial evaluation were genital ambiguity, failure to thrive, premature pubarche, hirsuitism, and primary amenorrhea or infertility. Genital ambiguity had been noticed in all the children at the time of birth, but (for different reasons) some of them sought medical help much later. Table 1 gives the profile of patients. All patients had genital ambiguity (17 – Prader stage 3, 6 – Prader stage 2, 1 – Prader stage 1). Patients one and two were initially hospitalized for salt-wasting crisis. Patients three to six sought medical help during infancy for genital ambiguity. Patients seven to eleven had genital ambiguity noted at birth, but were reared as females and sought medical attention much later, because of progressive clitoral enlargement. Patients 12 to 22 also had genital ambiguity since birth and progressive enlargement of clitoris, but they sought medical attention peripubertally. The presenting complaints were lack of breast development and primary amenorrhea. Patient 18 had clitoromegaly with posterior labial fusion. She presented with complaints of hirsuitism, genital ambiguity, and poor breast development. She had regular menstrual periods at intervals of 25 to 28 days since the age of 11 years. Patient 23 had genital ambiguity. Breast development and occasional vaginal bleeding started at age 16 years. She sought medical attention for infertility. Patient 24 presented at age 29 years for genital ambiguity, hirsuitism, and infertility. She had some breast development starting at age 16 years and menarche at age 17 years. Periods were irregular with scant bleeding occurring at intervals of six months to one year. Figure 1 gives the genital appearance of patient 24. This patient was the oldest at steroid initiation, had a phallus like clitoris, underdeveloped labia majora, and a common urogenital sinus.
Table 1.
Clinical profile of girls with started on treatment at different ages
Figure 1.
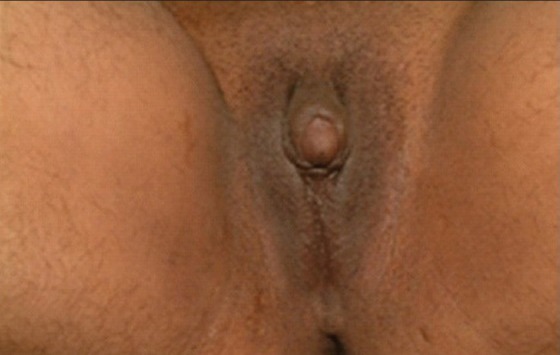
Genital appearance of patient 24
Girls who were not started on steroids during infancy experienced progressive clitoral enlargement, premature pubic hair growth, and hirsuitism. Pubic hair growth started by three years of age, and facial hair growth between the ages of 9 and 11 years. Six of the 13 untreated CAH girls had subtle breast development starting at ages 11 – 16 years and three had spontaneous infrequent vaginal bleeding starting at ages 11 – 17 years.
Hirsuitism was present in only one patient, who had started treatment earlier. She developed facial hair when she stopped steroids for six months at the age 23 years.
Treatment (steroids) was initiated during the first year of life in six patients (Patients 1 – 6), another five patients were on steroids by eight years age (Patients 7 – 11). The others had treatment initiated at different ages (age ranging from 12 to more than 20 years) (Patients 12 – 24). The standard practice in our clinic was to adjust the steroid dose to maintain plasma testosterone in a high normal range for females. Six patients were on prednisolone (dose used was 5.7 ± 1.2 mg), 17 patients were receiving dexamethasone (average dose 0.37 ± 0.15 mg), one patient had stopped steroids [patient 3 in Table 1].
Clinical course post steroid initiation
Patients initiated on steroids during infancy and up to age eight years had thelarche at 11.5 ± 1.8 years and menarche at 13.1 ± 1.5 years. Patients initiated on steroids later had delayed thelarche and menarche (15.1 ± 3.2 years and 16.0 ± 2.3 years, respectively) [Table 2].
Table 2.
Pubertal development among girls initiated on treatment early compared to those started on treatment after nine years of age

With steroid initiation, breast development and menarche were achieved within two to twelve months and two to eighteen months, respectively, in older girls / women [Figure 2]. Hirsuitism decreased after two to four years of steroid therapy in patients initiated late on steroid therapy, however, most patients required some form of treatment for facial hair.
Figure 2.
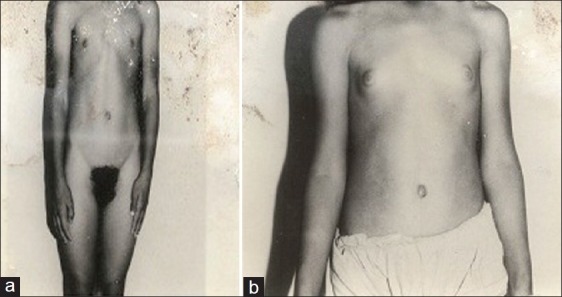
(a) Absent breast development pre-steroid initiation, (b) Breast development after four months of steroid initiation
DISCUSSION
Girls with untreated CAH provides an opportunity to observe the clinical effect of isolated androgen excess. These girls had androgen exposure starting from the prenatal period and in some it had continued, uninterrupted for one or two decades. Some of the important observations were the sequence in which the peripheral effects of androgen excess manifested. Effect of androgen excess on clitoral size started from the prenatal period and continued all through childhood. Pubic hair growth started after three years of age, while facial hair growth started only after nine years of age. Although female secondary sexual development was absent or significantly delayed in the absence of treatment, these progressed well after initiating the therapy. What could not be corrected when therapy was delayed was the genital appearance and hirsutism. These girls attained menarche and had regular menstrual cycles with steroid supplementation, irrespective of the age at which it was started.
Thus, adverse effects of androgens on different tissues, that is, genitalia, pubic hair, facial hair, and the reproductive endocrine axis, appeared at different time-points in these patients. These findings indicate a differential timed tissue response to androgens in these patients. There was a delay in pubertal development in those initiated late on steroid therapy, indicating a suppressive effect of androgens on the hypothalamo pituitary gonadal axis. Almost half the patients had a spontaneous occurrence of puberty (although not complete) even in the face of continued androgen exposure. A delay in androgen action at certain tissues (pubic hair and facial hair) and escape of the gonadal axis (from the suppressive effects of androgens) in few patients indicate a relative resistance to androgen action in these patients.
Relative resistance to androgen action has been reported earlier in children with CAH. A recent study from Netherlands observed that children with simple virilizing forms of CAH, who did not receive steroid therapy, manifested no clinical evidence of androgen excess in the form of accelerated bone age or growth velocity till 12 months of age.[10] Another study from Sweden has also showed that the effects of androgen excess in the form of growth acceleration or virilization were not observed in CAH children till the age of 18 months, after which both bone age and growth velocity increased proportionally to the degree of androgen excess.[11]
Hirsuitism and acne are the predominant presenting symptoms in 50 to 70% of the children with ovarian and adrenal tumoral hyperandrogenism, at a median age of three to four years.[12–14] There was no development of either acne or facial hair in this group of patients till the age of nine years, in spite of continuing androgen exposure.
Aceto et al. have described three untreated CAH girls, age ranging from 21 to 42 months.[15] The girl who was 42 months old had some amount of pubic hair growth, but no facial or axillary hair. The other two girls, 21 and 24 months had no virilization. Grinten et al. also observed the absence of axillary hair and hirsuitism among the children (age 18 to 86 months).[10] He attributed this to a higher threshold for androgens in the axillary region.
The present study has revealed a delayed / suboptimal pubertal development in CAH girls in the absence of steroid therapy. It is also noteworthy that with steroid supplementation there was rapid progress in development of female secondary sexual characteristics. That prompts us to speculate that the effects on the reproductive axis are due to combination of steroid deficiency and androgen excess. The clinical features of hyperandrogenism in these girls is subdued, possibly because cortisol sufficiency may be a prerequisite for optimal androgen action.
The prevalence of obesity, metabolic syndrome, and infertility are higher among CAH patients in spite of optimal treatment.[16,17] A recent population-based survey of patients with classical CAH also observed that their subjective health status and working ability were impaired, and that fertility was reduced in females.[18] These are reports from specialized centers with optimal glucocorticoid and mineralocorticoid supplementation strategies. However, fertility seems unaffected among the suboptimally treated CAH girls.[8] This study reported seven girls with classical CAH, who were not initiated on steroids till the age of nine years, who had 13 pregnancies. These observations suggest that androgen causes less harm than overtreatment with steroids. Steroid supplementation in smaller doses (aimed at normal androgen levels) than what is practised now, may have better health benefits.
We conclude that there was a delay in maturation of the HPO axis (female secondary sexual development) in girls with untreated CAH. This could be corrected with glucocorticoid supplementation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bruns CM, Baum ST, Colman RJ, Eisner JR, Kemnitz JW, Weindruch R, et al. Insulin resistance and impaired insulin secretion in prenatally androgenized male rhesus monkeys. J Clin Endocrinol Metab. 2004;89:6218–23. doi: 10.1210/jc.2004-0918. [DOI] [PubMed] [Google Scholar]
- 2.Abbott DH, Dumesic DA, Eisner JR, Colman RJ, Kemnitz JW. Insights into the development of polycystic ovary syndrome (PCOS) from studies of prenatally androgenized female rhesus monkeys. Trends Endocrinol Metab. 1998;9:62–7. doi: 10.1016/s1043-2760(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 3.Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: A developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11:357–74. doi: 10.1093/humupd/dmi013. [DOI] [PubMed] [Google Scholar]
- 4.Abbott DH, Tarantal AF, Dumesic DA. Fetal, infant, adolescent and adult phenotypes of polycystic ovary syndrome in prenatally androgenized female rhesus monkeys. Am J Primatol. 2009;71:776–84. doi: 10.1002/ajp.20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foecking EM, Szabo M, Schwartz NB, Levine JE. Neuroendocrine consequences of prenatal androgen exposure in the female rat: Absence of luteinizing hormone surges, suppression of progesterone receptor gene expression, and acceleration of the gonadotropin-releasing hormone pulse generator. Biol Reprod. 2005;72:1475–83. doi: 10.1095/biolreprod.105.039800. [DOI] [PubMed] [Google Scholar]
- 6.Serafini P, Silva PD, Paulson RJ, Elkind-Hirsch K, Hernandez M, Lobo RA. Acute modulation of the hypothalamic-pituitary axis by intravenous testosterone in normal women. Am J Obstet Gynecol. 1986;155:1288–92. doi: 10.1016/0002-9378(86)90161-4. [DOI] [PubMed] [Google Scholar]
- 7.Dewis P, Newman M, Ratcliffe WA, Anderson DC. Does testosterone affect the normal menstrual cycle? Clin Endocrinol (Oxf) 1986;24:515–21. doi: 10.1111/j.1365-2265.1986.tb03280.x. [DOI] [PubMed] [Google Scholar]
- 8.Kulshreshtha B, Marumudi E, Khurana ML, Kriplani A, Kinra G, Gupta DK, et al. Fertility among women with classical congenital adrenal hyperplasia: Report of seven cases where treatment was started after 9 years of age. Gynecol Endocrinol. 2008;24:267–72. doi: 10.1080/09513590801945230. [DOI] [PubMed] [Google Scholar]
- 9.Ammini AC, Gupta R, Kapoor A, Karak A, Kriplani A, Gupta DK, et al. Etiology, clinical profile, gender identity and long term follow up of patients with ambiguous genitalia in India. J Pediatr Endocrinol Metab. 2002;15:423–30. doi: 10.1515/jpem.2002.15.4.423. [DOI] [PubMed] [Google Scholar]
- 10.Hedi L, Claahsen-van der G, Noordam K, Borm GF, Otten BJ. Absence of increased height velocity in the first year of life in untreated children with simple virilizing congenital adrenal hyperplasia. J Cin Endocrinol Metab. 2006;91:1205–9. doi: 10.1210/jc.2005-1701. [DOI] [PubMed] [Google Scholar]
- 11.Thilen A, Woods KA, Perry LA, Savage MO, Wedell A, Ritzen EM. Early growth is not increased in untreated moderately severe 21-hydroxylase deficiency. Acta Paediatr. 1995;84:894–8. doi: 10.1111/j.1651-2227.1995.tb13788.x. [DOI] [PubMed] [Google Scholar]
- 12.Ko JH, Lee HS, Hong J, Hwang JS. Virilizing adrenocortical carcinoma in a child with Turner syndrome and somatic TP53 gene mutation. Eur J Pediatr. 2010;169:501–4. doi: 10.1007/s00431-009-1051-8. [DOI] [PubMed] [Google Scholar]
- 13.Riberio RC, Michalkiewicz EL, Figueiredo BC, De Lacerda L, Sandrini F, Pianovsky MD, et al. Adrenocortical tumors in children. Braz J Med Biol Res. 2000;33:1225–34. doi: 10.1590/s0100-879x2000001000013. [DOI] [PubMed] [Google Scholar]
- 14.Wajchenberg BL, Albergaria Pereira MA, Medonca BB, Latronico AC, Campos Carneiro P, Alves VA, et al. Adrenocortical carcinoma: Clinical and laboratory observations. Cancer. 2000;88:711–36. [PubMed] [Google Scholar]
- 15.Aceto T, Macgillivray MH, Caprano VJ, Munschauer RV, Raiti S. Congenital virilizing hyperplasia without acceleration of growth or bone maturation. JAMA. 1966;198:1341–3. [PubMed] [Google Scholar]
- 16.Cornean RE, Hindmarsh PC, Brook CG. Obesity in 21-hydroxylase deficient patients. Arch Dis Child. 1998;78:261–3. doi: 10.1136/adc.78.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Völkl TM, Simm D, Beier C, Dörr HG. Obesity among children and adolescents with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics. 2006;117:98–105. doi: 10.1542/peds.2005-1005. [DOI] [PubMed] [Google Scholar]
- 18.Nermoen I, Husebye E, Svartberg J, Lovas K. Subjective health status in men and women with congenital adrenal hyperplasia: A population-based survey in Norway European. J Endocrinol. 2010;163:453–9. doi: 10.1530/EJE-10-0284. [DOI] [PubMed] [Google Scholar]


