Abstract
Objective
To assess the evidence for the safety and effectiveness of antiemetics on gastroenteritis-induced vomiting in children and adolescents.
Design
Systematic review.
Data Sources
The Cochrane Central Register of Controlled Trials, MEDLINE and EMBASE searched from 1980 to March 2012.
Methods
Methods included comprehensive searches, data synthesis, meta-analysis and mixed treatment comparisons (MTC).
Review methods
Reference lists were checked, and missing or inconsistent data were sought from trial investigators. Randomised controlled trials comparing antiemetics in participants younger than 18 years and who were vomiting due to acute gastroenteritis. Four meta-analyses and three MTC were carried out.
Results
10 trials (1479 participants) and five treatments were included: dexamethasone, dimenhydrinate, granisetron, metoclopramide and ondansetron. There was clear evidence that ondansetron (oral or intravenous) compared with placebo increased the proportion of patients with cessation of vomiting (orally administered) (RR 1.44, 95% CI 1.29 to 1.61), reduced the immediate hospital admission rate (orally administered) (RR 0.40, 95% CI 0.19 to 0.83) and the need for intravenous rehydration therapy (orally administered) (RR 0.41, 95% CI 0.29 to 0.59). No significant difference was noted in the revisit rates, but ondansetron was associated with an increase in episodes of diarrhoea. There was no evidence for the use of dexamethasone or metoclopramide and limited evidence that dimenhydrinate or granisetron increased the cessation of vomiting. The MTC analysis suggested that ondansetron was the most likely treatment to stop the child vomiting. Nine studies were carried out in secondary care and one in primary care.
Conclusions
This systematic review used a method novel to this clinical area and found clear evidence that ondansetron was the most likely treatment to allow oral rehydration therapy to commence. Given the significance of these results, the authors urge healthcare policy makers to consider the wider use of ondansetron in secondary care. Furthermore, randomised controlled trials are needed to investigate the effectiveness of antiemetic treatment in primary care (including ambulatory care interventions).
Article summary
Article focus
To inform debate and clinical practice on the use of antiemetics for children presenting with vomiting associated with acute gastroenteritis in primary and secondary care.
Key messages
Oral or intravenous ondansetron is the most likely treatment option to stop a child from vomiting. It reduces the need for intravenous rehydration therapy and immediate hospitalisation.
There is no evidence for the use of cyclizine, dexamethasone, domperidone or metoclopramide; but limited evidence was found to support the use of dimenhydrinate or granisetron.
Ondansetron is off patent and likely to be a cost-effective treatment for acute gastroenteritis, both the National Institute for Health and Clinical Excellence and the American Academy of Pediatrics guidance should be updated to reflect the evidence available.
Strengths and limitations of this study
Ten randomised controlled trials that included 1479 participants were identified.
This is the first study to combine direct and indirect evidence to enable a comparison of all antiemetic treatments.
This review was conducted with methodological rigour and provided consistent and robust evidence to support the use of ondansetron.
Introduction
Acute gastroenteritis (AGE) is the leading cause of vomiting in children younger than 3 years and is a very common reason for children and adolescents attending emergency departments (EDs). Worldwide, there are about 2 million deaths per year from gastroenteritis in children younger than 5 years. Each year in the USA, over 1.5 million outpatient appointments result in 200 000 children younger than 5 years being admitted to hospital for treatment of dehydration due to gastroenteritis.1 In the UK during the early 1990's, over 20% of the consultations in general practice were for young children with symptoms of AGE and these resulted in 24 000 hospital admissions per year.2 However, the current burden of AGE in the UK primary care setting is unknown.
Vomiting from AGE is a distressing symptom for both children and their carers and if not swiftly arrested can lead to severe dehydration. When confronted by anxious parents, hospital doctors often face a dilemma on how to best manage these children. Both the American Academy of Pediatrics and the National Institute for Health and Clinical Excellence (NICE) do not specifically recommend the use of antiemetic treatment and highlight some of the side effects, which include diarrhoea.3 4 However, both organisations do urge the need for more robust high-level evidence of the effectiveness of these treatments.
The care pathway for children presenting to an ED with AGE is determined by the severity of their symptoms, and those with mild dehydration may be given oral rehydration therapy (ORT). Children who are able to tolerate oral rehydration are often discharged, while those who fail ORT due to persistent vomiting are given intravenous rehydration therapy (IVT) with some being admitted to hospital.4 5 The recommendation for children with AGE that are vomiting and moderate dehydration is to start IVT immediately, based on the assumption that they will be unable to tolerate ORT. Most children are likely to be discharged from hospital no later than 3 days following initial presentation; however, it is not uncommon for children who are discharged to re-present with similar symptoms shortly after discharge.
We updated this systematic review using more rigorous methods to present the evidence in line with the current UK primary and secondary care settings and identify the most likely treatment, which will allow children to tolerate ORT.6
Methods
Criteria for considering studies for inclusion in this review:
Types of studies—Randomised controlled trials (RCTs; randomised at the individual or cluster level).
Types of participants—Children and adolescents younger than 18 years and who presented with vomiting and a confirmed clinical diagnosis of gastroenteritis. Studies that included patients with surgical conditions, other systemic infections or metabolic conditions were excluded.
Types of treatment—All types of antiemetic treatment or placebo that were administered orally, intravenously or by suppository.
Types of outcome—The primary outcome was the time taken from the first administration of the treatment until cessation of vomiting. Secondary outcomes included: parental satisfaction; cessation of vomiting; number of episodes of vomiting; resumption of oral rehydration; hospitalisation during the ED stay and up to 72 h following discharge from the ED stay; the number of participants who required intravenous rehydration during the ED stay and up to 72 h following discharge from the ED stay; the number of participants who revisited and any clinically documented or patient reported adverse events.
Search methods for the selection of studies
The electronic searches to identify all published and unpublished RCTs were updated in March 2012. There were no language or date restrictions in the electronic searches. The search strategy for this review was constructed by using a combination of MESH subject headings and text words relating to the use of antiemetics for the treatment of gastroenteritis in children. Trials were identified by searching the following major electronic databases: MEDLINE (1966 to March 2012) and EMBASE (1980 to March 2012). Independent hand searching was being carried out by the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group, which included 11 journals and journal conference proceedings relevant to the scope of the review.
Selection of studies, data extraction and risk of bias assessment
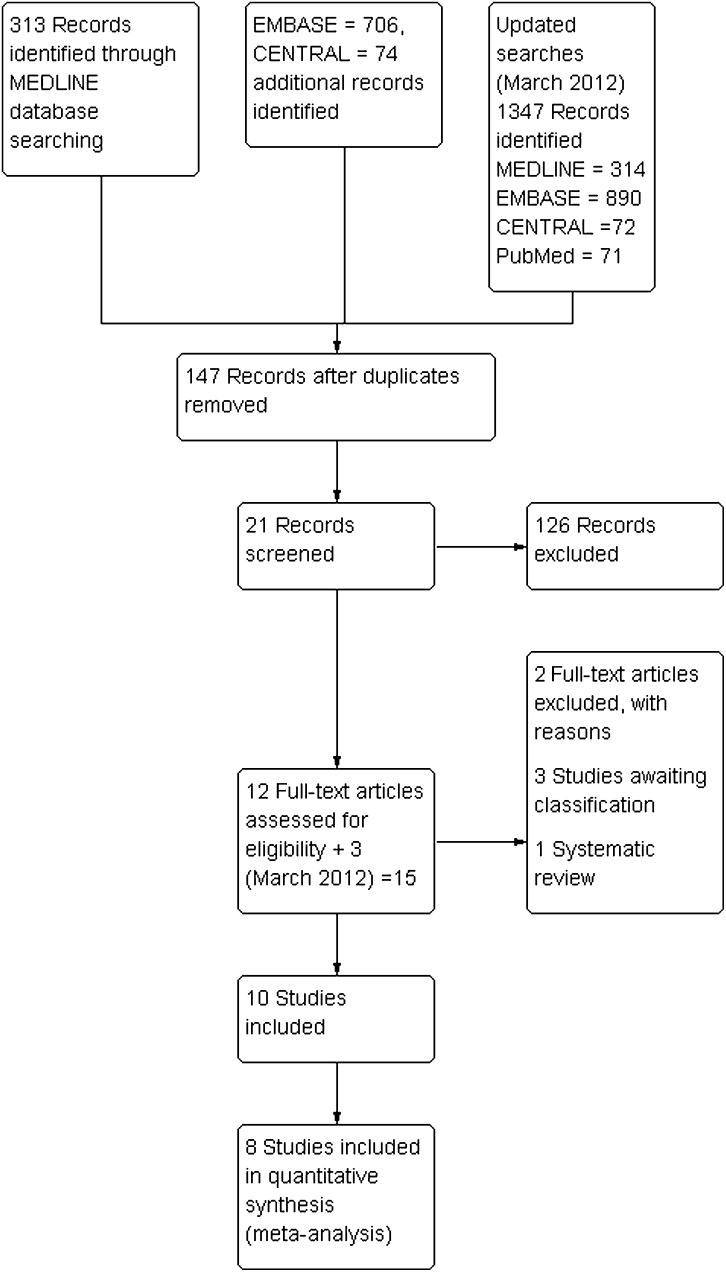
The abstracts of the studies in the searches were assessed independently by two reviewers. Full copies of all potentially relevant studies appearing to meet the inclusion criteria or had insufficient data in the title and abstract to make a clear decision were obtained. Studies not matching our inclusion criteria were excluded and the reasons for their exclusion were noted (see figure 1). Both review authors independently assessed the risk of bias using the Cochrane Collaboration's domain-based evaluation tool.7 These assessments were made for each of the included studies, and the judgements for each are documented in the full review and summarised in figure 2. Each domain was categorised as: low, unclear or high risk of bias. If all the domains in a study were judged as low risk of bias, then the overall judgement given for that study was ‘low risk’ of bias. If at least one domain was judged as high risk of bias, then the study was categorised with a ‘high risk’ of bias (plausible bias that seriously weakens confidence in the results), while the remainder were categorised as an unclear risk of bias.
Figure 1.
PRISMA Study flow chart.
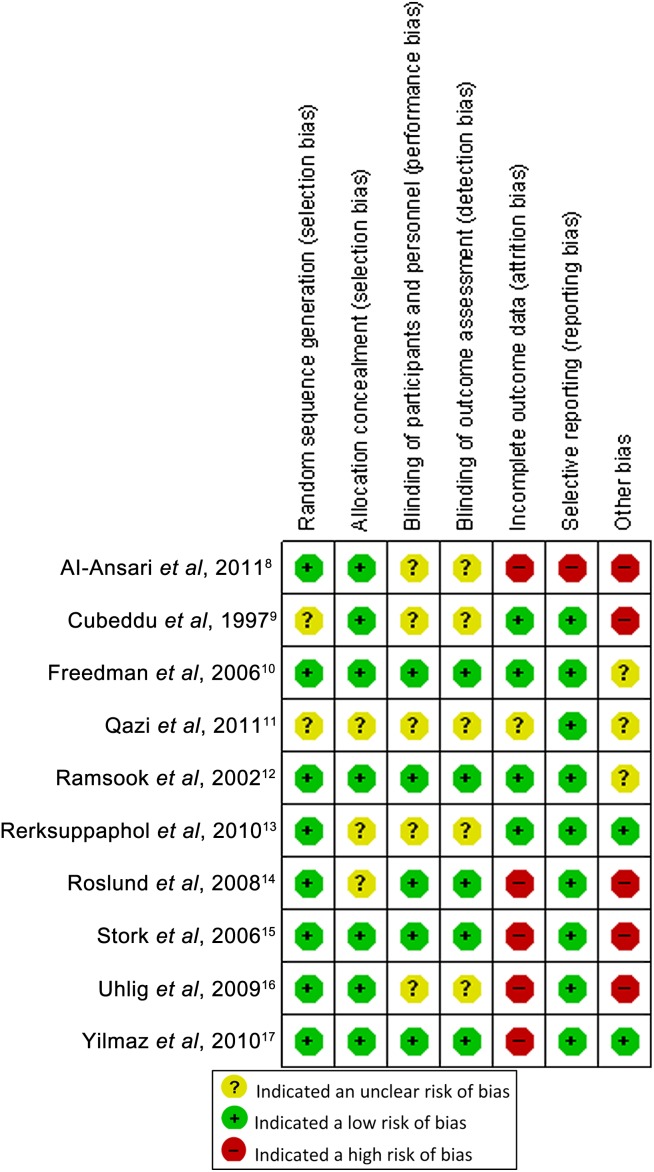
Figure 2.
The risk of bias summary for the included studies. The risk of bias summary below highlights each domain (columns) within each of the studies (rows).
Data synthesis
The continuous outcomes were presented where possible on the original scale as reported in each study. Dichotomous outcomes were presented as RR ratios with their associated 95% CIs and where possible the number needed to treat (NNT) along with the corresponding 95% CI. When a sufficient number of clinically homogeneous studies reported the same outcome (n≥3), a fixed effects meta-analysis was carried out.7 8 Clinical heterogeneity between the studies included in this review did not permit more than a limited number of treatment comparisons to be made. Statistical heterogeneity was examined by observation of the I2 statistic. If this statistic was >50%, we explored the studies to explain any differences due to underlying clinical rationale, and if the I2 was >80%, the meta-analysis was not presented.18 In cases where an I2 >50% was reported, a sensitivity analysis was carried out after excluding those studies causing the heterogeneity with the reason stated in the text. We had planned to investigate publication bias if an adequate number of studies were identified (n≥10).
In one study, it was unclear if four participants had received IVT or had been hospitalised at 72 h following discharge from the ED and therefore a sensitivity analysis was carried out to assess the potential effect of the missing data. Missing data were imputed using the best–worst and worst–best scenarios for the antiemetic treatment.7 9 We defined the best–worst scenario as the best outcome for ondansetron groups and worst for the placebo groups; worst–best scenario as the worst outcome for ondansetron groups and best for the placebo groups. An additional sensitivity analysis was carried out which compared fixed models with random-effect models to assess the potential degree of heterogeneity. The estimates of treatment effect and their corresponding 95% CI were reported and any differences discussed.
Mixed treatment comparison analysis
Standard direct evidence meta-analyses pool data across RCTs that compare an active treatment to an inactive control. However, a more useful comparison is one that considers the difference in effect estimate between active treatments. To overcome this and allow global comparisons to be made across all antiemetic medications within clinically homogeneous settings, we implemented a mixed treatment comparison (MTC) using both direct and indirect evidence. A fixed effects model was used to estimate each of the MTC within a Bayesian framework using Markov chain Monte Carlo simulation methods in WinBUGS (http:www.mrc-bsu.cam.ac.uk/bugs/).10 We summarise the MTC findings using ORs presented alongside with their 95% credible regions. Non-informative priors were fitted for normal distributions for means and uniform distributions for SDs. Care was taken to ensure that studies and comparisons were clinically homogeneous with additive treatment effects, and heterogeneity was common across the comparisons (ie, the relative effect of treatment A vs C could be estimated from the effect of A vs B and B vs C). The results of the MTC analyses were compared with the findings of the direct pair-wise meta-analyses.
Results
Description of the included studies
Ten trials that assessed at least one of the outcomes were identified and included in the review.11–20 Seven of these studies compared ondansetron and placebo and out of these, four investigated oral administration. There were two three-arm studies investigating dexamethasone and metoclopramide as compared to both ondansetron and placebo. Dimenhydrinate administered as a suppository versus placebo was investigated in one study and a further compared oral granisetron versus placebo. The studies reported outcome data from presentation up until discharge and beyond, for example, one study followed participants up to 14 days after discharge. Dosing regimens varied between the studies but most of them used a weight-dependent dosage. We contacted the investigators in several of the studies to confirm trial methodology and to clarify specific outcome data. We were unsuccessful in contacting the investigators in one study that had reported incoherent data and consequently only included this study in one meta-analysis.20 Further details of the study populations or descriptions of the interventions are reported in the Cochrane review or the updated studies.6 11 14 16
Overall risk of bias of the included studies
None of the studies included in the review were considered to be at ‘low risk’ of bias. Four studies were categorised as ‘unclear risk’ of bias, while the remaining six studies were assessed as ‘high risk’ of bias because one or more of the criteria were not met.
Effects of interventions
Studies with oral ondansetron (weight-dependent dose) versus placebo
Four trials provided data on the effectiveness of oral ondansetron compared with placebo but none reported the primary outcome for this review.13 15 17 20 Data from these could be pooled, and ondansetron was found to be more effective at stopping vomiting (RR 1.44, 95% CI 1.29 to 1.61, I2=61% with an NNT of 4, 95% CI 4 to 6) (see figure 3.1.1 and repeated in analysis 1.1 of table 1). However, substantial heterogeneity was noted and attributed to one study, Yilmaz et al.20 This study appeared to report reliable data for the proportion of children with a cessation of vomiting but was responsible for the inflated treatment effect and heterogeneity across the studies.
Figure 3.
Analysis comparing oral ondansetron to placebo for the proportion of participants with cessation of vomiting.
Table 1.
Direct evidence of random-effects meta-analyses
| Analysis | Figure | Outcome | RR | 95% CI |
p Value | I2 | |
| Lower | Upper | ||||||
| Oral ondansetron versus placebo | |||||||
| 1.1 | 3.1.1 | Cessation of vomiting | 1.45 | 1.20 | 1.74 | <0.001 | 61% |
| 1.2 | 3.1.2 | Cessation of vomiting—excluding Yilmaz et al17 | 1.33 | 1.19 | 1.49 | <0.001 | 0% |
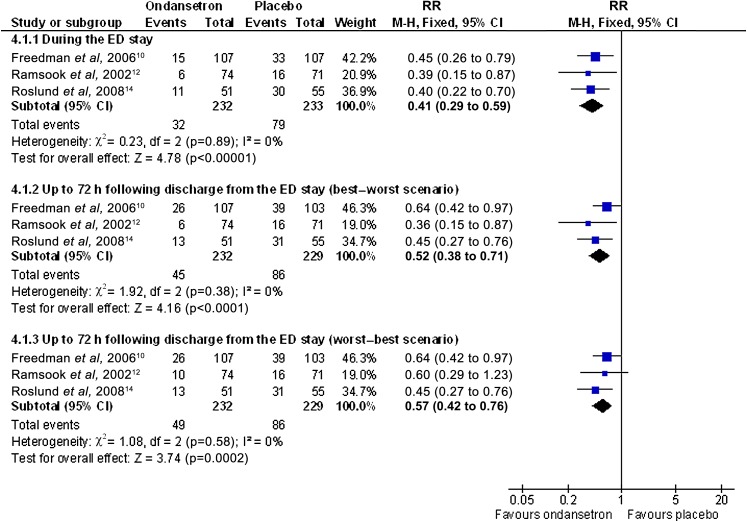
| 2.1 | 4.1.1 | Proportion with immediate IVT during the ED | 0.41 | 0.29 | 0.59 | <0.001 | 0% |
| 2.2 | 4.1.2 | Proportion with IVT up to 72 h (best–worst) | 0.57 | 0.42 | 0.76 | <0.001 | 0% |
| 2.3 | 4.1.3 | Proportion with IVT up to 72 h (worst–best) | 0.53 | 0.39 | 0.72 | <0.001 | 0% |
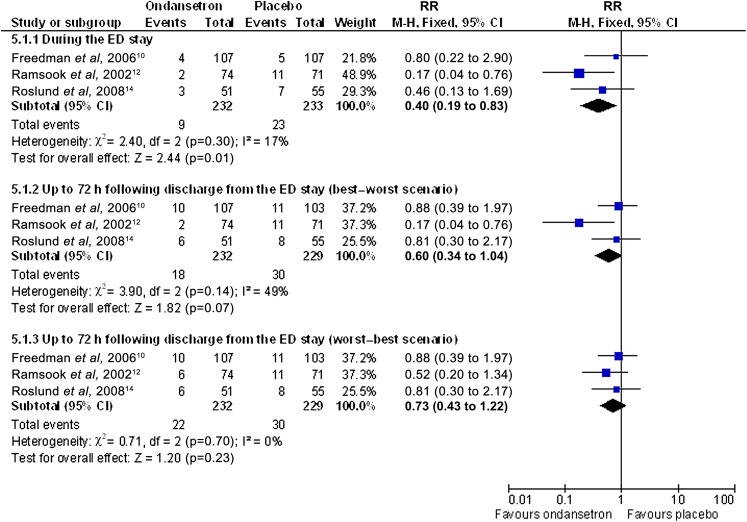
| 3.1 | 5.1.1 | Proportion admitted during the ED | 0.43 | 0.18 | 1.00 | 0.05 | 17% |
| 3.2 | 5.1.2 | Proportion admitted up to 72 h (best–worst) | 0.60 | 0.34 | 1.04 | 0.07 | 49% |
| 3.3 | 5.1.3 | Proportion admitted up to 72 h (worst–best) | 0.73 | 0.43 | 1.23 | 0.24 | 0% |
| 4.1 | 6 | Revisit rate | 1.24 | 0.49 | 3.15 | 0.66 | 28% |
| Intravenous ondansetron versus placebo | |||||||
| 5.1 | 3.1.3 | Cessation of vomiting | 2.27 | 1.05 | 4.94 | 0.04 | 76% |
ED, emergency department; IVT, intravenous rehydration therapy.
A sensitivity analysis was carried out after Yilmaz et al20 was removed and provided a RR of 1.33 (95% CI 1.19 to 1.49, I2=0%), shown in figure 3.1.2 and analysis 1.2 of table 1. The unpooled data from three of the studies indicated that the mean frequency of vomiting was lower in the ondansetron group than in the placebo group.13 15 17 Only one study provided data for the resumption of oral rehydration and reported that participants in the ondansetron group were more likely to tolerate oral hydration at 8 h (RR 1.17, 95% CI 0.99 to 1.38, p=0.06).20
In three studies, the proportion of children needing IVT during the ED was lower in the ondansetron group compared with the placebo group with a RR of 0.41 (95% CI 0.29 to 0.59, I2=0%) and an NNT of 5 (95% CI of 4 to 8) (see figure 4.1.1 and analysis 2.1).13 15 17 In one study, data were not reported for four participants at 72 h following discharge, so a best–worst and worst–best case sensitivity analysis was carried out.15 The best–worst scenario provided a RR of 0.52 (95% CI 0.38 to 0.71, I2=0%) and a worst–best scenario of RR 0.57 (95% CI 0.42 to 0.76, I2=0%) (see figure 4.1.2–4.1.3 and analyses 2.2–2.3, table 1). After combining the most extreme possibilities, the NNT was found to be between 4 and 13. These analyses indicated that ondansetron was effective at reducing the need for IVT.
Figure 4.
Analysis comparing oral ondansetron compared with placebo for the proportion of participants who require IVT.
The hospital admission rate outcome data illustrated that ondansetron reduced the immediate hospital admission rate during the ED stay (RR 0.40, 95% CI 0.19 to 0.83, I2=17%) (see figure 5.1.1 and analysis 3.1). Due to missing data in one study at 72 h following discharge, a best–worst and worst–best sensitivity analysis was carried out.15 The best–worst scenario provided a RR of 0.60 (95% CI 0.34 to 1.04, I2=49%) and a worst–best scenario of RR 0.73 (95% CI 0.43 to 1.22, p=0.23, I2=0%) (see figure 5.1.2–5.1.3 and analyses 3.2–3.3).
Figure 5.
Analysis comparing oral ondansetron compared with placebo for the proportion of participants who were admitted to hospital.
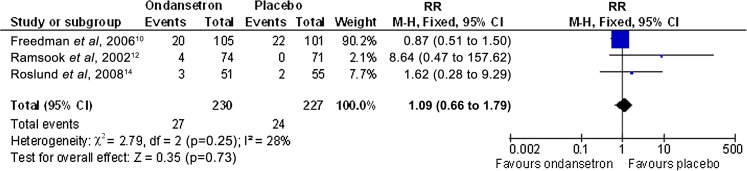
All four studies reported consistent results of no difference between the revisit rates, and pooled data from three of the studies produced a RR of 1.09 (95% CI 0.66 to 1.79, p=0.73) (see figure 6).13 15 17
Figure 6.
Analyses comparing IV administered ondansetron compared with placebo for the proportion of participants who revisit the emergency department.
In the ondansetron group, in three of the studies, there was an increase in the number of episodes of diarrhoea (p<0.05). Other side effects included a single episode of macular rash in the ondansetron group and a single episode of urticaria with placebo. However, it should be noted that none of the studies were powered to detect rare but serious adverse effects.
Studies with weight-dependent dose intravenous ondansetron (0.15–0.3 mg/kg) versus placebo
Three studies investigated intravenous administration of ondansetron versus placebo.12 16 18 Rerksuppaphol and Rerksuppaphol16 reported data for the primary outcome and indicated that there was a significant reduction in the time until cessation of vomiting in the ondansetron group compared with the placebo group (p<0.01). Data from three studies could be pooled, and ondansetron was found to be both more effective at stopping vomiting RR of 2.01 (95% CI 1.49 to 2.71, I2=76%) and clinically important with an NNT of 3 (95% CI 3 to 5) (see figure 3.1.3 and analysis 5.1). Little explanation could be found for the heterogeneity exhibited outside of the small number of studies.
In Stork et al,18 there was no statistically significant difference between the treatment for the number of vomiting episodes at 24 h and 72 h follow-up (p=0.49 and 0.46, respectively). In Cubeddu et al,12 there were fewer episodes of vomiting in the ondansetron group that were reported at 24 h (p=0.048). In Stork et al,18 the number of participants who were able to tolerate oral rehydration at 2 h after treatment were 39/45 in the ondansetron group as opposed to 29/43 in the saline group.
Cubeddu et al12 reported that at 4 h after treatment with ondansetron 11/12 compared with 8/12 patients receiving placebo were able to tolerate oral rehydration, but at 24 h, this was 10/12 and 8/12, respectively. Significantly fewer hospital admissions occurred in the ondansetron group compared with the placebo group (2 vs 9), with a RR of 0.21 (95% CI 0.05 to 0.81) in Stork et al.18
In Stork et al,18 the investigators did not report the presence of any significant side effects. In Cubbedu et al,12 more episodes of diarrhoea were reported in the ondansetron group in the first 24 h compared with the placebo group, p=0.013, but the proportions were not reported in the primary research.
Studies with intravenous metoclopramide (0.3 mg/kg) versus placebo
One study compared intravenous metoclopramide and placebo but did not report the primary outcome for this review.12 The proportion of children with cessation of vomiting in the first 24 h following treatment was 4/12 in the metoclopramide groups compared with 2/12 in the placebo groups. During the first 4 h of oral rehydration, 10/12 participants in the metoclopramide group compared with 8/12 in the placebo group were able to tolerate oral rehydration. All the patients experienced at least one episode of diarrhoea, but compared with the placebo group, there were significantly more episodes of diarrhoea in the metoclopramide group (p=0.004). Other side effects included general drowsiness, cough and tremor (metoclopramide group).
Studies with intravenous ondansetron (0.3 mg/kg) versus metoclopramide (0.3 mg/kg)
Two studies investigated intravenous ondansetron and metoclopramide, one of which reported there was not evidence of a difference in the mean time till cessation of vomiting between the groups (p=0.20).11 However, the investigators inappropriately analysed non-normally distributed data. The study also reported that 68/84 and 60/83 participants had a cessation of vomiting in the ondansetron and metoclopramide groups (p=0.21). In a second study, cessation of vomiting during the first 24 h occurred in 7/12 and 4/12 in the ondansetron and metoclopramide groups, respectively, with a RR of 2.80 (95% CI 0.53 to 14.74, p=0.21).12 The study also reported that the proportion of participants with more than four episodes of diarrhoea was 8/12 and 10/12 for the ondansetron and metoclopramide groups, respectively.
Both studies were under-powered superiority studies and could only demonstrate no evidence of a difference.
Studies with intravenous dexamethasone (1 mg/kg) versus placebo
One study investigated intravenous administration of dexamethasone (1 mg/kg) versus placebo but did not report the primary outcome for this review.18 There was no statistically significant difference between dexamethasone and placebo in the number of vomiting episodes at 24 h. At 2 h after treatment, 26/42 compared with 29/43 were able to tolerate oral rehydration (p>0.05), and at the end of the study, there was 7/42 and 9/43 hospital admissions (p>0.05) for the dexamethasone and placebo groups, respectively.
Studies with suppository dimenhydrinate versus placebo
A single study investigated the administration of dimenhydrinate and placebo by suppository19 and reported a reduction in the mean number of days that the child continued to vomit in the dimenhydrinate group of 0.34 days (95% CI −0.66 to −0.02, p=0.036). At the 18–24 h follow-up visit, 71/106 of the participants in the dimenhydrinate group compared with 46/102 in the placebo group were free of vomiting (p=0.001). There was no evidence of a difference in the parental satisfaction (p=0.65), oral rehydration (p=0.45), hospital admission rates (p=0.744) and frequency of diarrhoea (p=0.72).
Studies with oral granisetron versus placebo
A study that was included in conference proceedings investigated a comparison of oral administration of granisetron and placebo but did not consider the primary outcome for this review.14 At the 24 h follow-up, 74/80 (92.5%) of the participants in the granisetron group compared with 63/79 (79.7%) in the placebo group were free of vomiting (p=0.02). In the 48 and 72 h follow-up, no difference was reported in the proportion of children free from vomiting that remained under observation.
The study reported a reduction in the requirement for IVT during the ED stay in the granisetron (0/80) compared with placebo (9/82) group (p=0.001).
Mixed treatment comparisons
Clinical diversity restricted global MTC to separate routes of administration. Studies of both oral and intravenous administration were considered reasonably homogeneous in terms of population, setting and intervention. However, only two outcomes, cessation of vomiting and rate of intravenous administration within the ED, were found to be consistently reported in an adequate number of studies and interventions.
Cessation of vomiting following oral administration
The MTC resulted in reliable convergence after 10 000 Markov chain Monte Carlo simulations. Oral ondansetron was found to be the most likely treatment option that would stop children from vomiting (see table 2.1). The MTC comparison for placebo compared with granisetron and ondansetron provided an OR of 3.25 (95% CI 0.62 to 17.69) and 4.33 (95% CI 2.11 to 10.11), respectively, and between granisetron compared with ondansetron estimated an OR of 1.33 (95% CI 0.21 to 8.76). These results provided clear evidence that the odds of the cessation of vomiting for ondansetron compared with placebo were over four times more likely. The evidence for granisetron compared with placebo or granisetron compared with ondansetron was less clear with wide CIs caused by the small number of included studies.
Table 2.1.
Mixed treatment comparisons (MTC) and direct evidence for the oral and intravenous administered medication. Upper right quadrants indicate the number of direct comparisons available, the direct ORs and 95% CIs, lower left quadrants indicate the MTC median OR and credible regions. Beneath the table is the estimated most likely treatment to stop children from vomiting: orally administered medication
| OR of the direct evidence |
|||
| Placebo | Granisetron | Ondansetron | |
| Median OR from the MTC | |||
| Placebo | – | OR 3.13 (1.16 to 8.49)** | OR 3.88 (2.6 to 5.77)*** |
| Granisetron | 3.25 (0.62 to 17.69) | – | No data available |
| Ondansetron | 4.33 (2.11 to 10.11)*** | 1.33 (0.21 to 8.76) | – |
**p<0.05; ***p<0.01.
Estimated best treatment option: ondansetron 65%, granisetron 35% and placebo 0%.
Cessation of vomiting (outcome) following intravenous treatment administration
After 10 000 simulations, good convergence was found from the MTC analysis. Intravenous administration of ondansetron was found to be globally the most likely treatment option to stop children from vomiting (see table 2.2). The MTC comparison for placebo compared with dexamethasone, metoclopramide and ondansetron provided an OR of 0.98 (95% CI 0.08 to 9.58), 2.91 (95% CI 0.41 to 24.27) and 5.44 (95% CI 1.43 to 23.83), respectively. Only ondansetron compared with placebo showed clear evidence of a treatment effect. Comparing ondansetron with dexamethasone and metoclopramide estimated an OR of 5.55 (95% CI 0.45 to 101.7) and 1.85 (95% CI 0.30 to 11.76), respectively. The MTC analyses failed to provide statistical evidence of an effect between any active antiemetic treatments. However, the direction of effect of the MTC ORs and direct evidence indicated that there was at the least limited evidence that ondansetron was more effective than the other treatments.
Table 2.2.
Mixed treatment comparisons (MTC) and direct evidence for the oral and intravenous administered medication. Upper right quadrants indicate the number of direct comparisons available, the direct ORs and 95% CIs, lower left quadrants indicate the MTC median OR and credible regions. Beneath the table is the estimated most likely treatment to stop children from vomiting: intravenous administered medication
| OR of the direct evidence |
||||
| Placebo | Dexamethasone | Metoclopramide | Ondansetron | |
| Median OR from the MTC | ||||
| Placebo | – | 0.83 (0.36 to 1.94) | 2.50 (0.36 to 17.32) | 4.54 (2.45, 8.44)*** |
| Dexamethasone | 0.98 (0.08 to 9.58) | – | No data available | 2.55 (1.07 to 6.08)** |
| Metoclopramide | 2.95 (0.41 to 24.27) | 3.01 (0.16 to 81.22) | – | 1.78 (0.91 to 3.45)* |
| Ondansetron | 5.44 (1.43 to 23.83)*** | 5.55 (0.45 to 101.7) | 1.85 (0.30 to 11.76) | – |
*p<0.1; **p<0.05; ***p<0.01.
Estimated best treatment option: ondansetron 75%, metoclopramide 20%, dexamethasone 6% and placebo 0%.
Ondansetron (75%) was shown to be the treatment most likely to be effective compared with metoclopramide (20%), dexamethasone (6%) or placebo (0%).
Proportion of children requiring intravenous following oral treatment administration
The MTC analysis failed to successfully converge after 10 000 000 iterations, initiated from three random starting positions. This was largely due to a lack of requirement for IVT by the participants in the granisetron group.
Discussion
This review included 10 trials, which provided some evidence regarding the clinical effectiveness and safety of antiemetics prescribed for children vomiting due to AGE. It was disappointing to see that the primary outcome ‘time till the cessation of vomiting’ and secondary outcome ‘parental satisfaction’ were assessed in only three studies. The majority of studies focused on clinician centred, rather than patient and parent-preferred outcomes including: number of vomiting events, incidence of intravenous rehydration and hospitalisation. Pooling of data in a meta-analysis was only feasible for the comparison of ondansetron compared with placebo and was only possible for four outcomes.
Both the American Academy of Pediatrics and NICE guidelines indicate that there is a consensus of opinion that antiemetics are not needed for the management of vomiting due to gastroenteritis in children.3–5 Current practice suggests that attitudes of anxious parents may subconsciously influence the attending physician to treat with IVT.21 In a recent survey in the USA and Canada, practicing emergency physicians were questioned on their use of antiemetics for AGE in children, and 90/90 and 107/136 of clinicians responded that ondansetron was frequently prescribed.22 A similar study in Italy revealed that almost all secondary and primary care physicians were willing to prescribe ondansetron to children for this indication. In the UK, neither ondansetron nor any alternative serotonin 5-HT3 receptor antagonist is licensed for AGE in either children or adults. Anecdotally, cyclizine is commonly used for AGE in primary care in the UK, and metoclopramide and domperidone are listed in the British National Formulary for Children; however, this is in contrary of the evidence from this review. We argue that ORT in conjunction with oral ondansetron should be more widely used in the UK. NICE guidance states that IVT should be given if there is evidence of clinical deterioration and red flag signs or symptoms or if a child vomits during ORT (at presentation). NICE guidelines that were published in 2009 consider that the availability of more evidence in support of the effectiveness of ondansetron may reduce the need for IVT and hospitalisation. We urge that future updates to this guidance should consider the clinical acceptance of oral ondansetron to reduce the number of children given IVT. The benefits of this are not solely financial but would result in a reduction of the proportion of children having an invasive intravenous intervention, fewer children continuing to vomit and lower bed occupancy rates. We estimate that if no treatment is given, approximately 63% (95% CI 59% to 69%) of children will stop vomiting; however, if oral ondansetron is administered, this would increase to 81% to 89% (based on data reported in this review13 15 17). The guidelines warn that clinicians should be aware of certain potential, but unspecified, adverse effects associated with antiemetics, yet these studies, while reporting some side effects, appeared to indicate that other than an increase in the number of episodes of diarrhoea, the drugs were well tolerated.
Other considerations in this indication are which antiemetic to use. The global MTC suggests that a child is far more likely to stop vomiting following a course of ondansetron compared with any alternative antiemetic.
Quality of the evidence
Although study design in the included studies appeared to have been adequate overall, our study-level assessments of the risk of bias for a number of the domains in several of these studies revealed some of the limitations in their implementation. While these inconsistencies are more likely to be as a result of systematic errors, they emphasize the challenges faced in the screening and follow-up of emergency paediatric participants. We actively encourage that investigators in future studies should try to achieve a clearer diagnosis of AGE before randomisation and ensure closer observation of children recruited to research studies.
However, while recognising these limitations, we consider that the body of evidence summarised in this review is sufficient to allow certain conclusions to be drawn about the effectiveness of the interventions and to provide recommendations for improving the methodology in future trials.
The studies within this review identified five antiemetic treatments (dexamethasone, granisetron, metoclopramide, ondansetron and dimenhydrinate), but because there were different routes of administration, care should be taken when comparing between the review results. The dosages implemented were weight or age dependent and varied considerably between the studies. Results for specific outcomes were consistent across the studies, and where pooling of data were feasible, there was little evidence for statistical heterogeneity. In one study, we were unable to clearly determine if four of the participants had either been admitted to hospital or had received intravenous rehydration therapy.15 In view of the uncertain status of these participants, a best–worst and worst–best scenario sensitivity analysis was conducted and it was found that the hospital admission outcome was sensitive to the missing data, and after taking into consideration the degree of heterogeneity (I2 statistic) in the two scenarios, the worst–best scenario would be more typical, and therefore, it would appear less likely that ondansetron does reduce the hospital admission rate in the longer term.
Agreements and disagreements with other studies or reviews
The latest version of this review is a synthesis of studies from previous versions and includes additional trials, all of which complement and add to the evidence base of two recent non-Cochrane reviews. This review also contributes further to the body of evidence supporting the effectiveness of antiemetics for vomiting related to AGE in children.23 24 One recent review was reported as a reliable source of evidence on the use of antiemetics for vomiting related to AGE in the child and adolescent.25 However, we have highlighted a number of issues regarding the validity of the decision by the review authors to pool some of these data in view of the apparent clinical diversity between the selected studies and most specifically in the distinct differences in their routes of administration of the interventions. Additionally, where possible, we have contacted the primary research authors and implemented our analyses by treatment allocated compared with the complete-case analysis used in the other reviews. The findings of our Cochrane systematic review are to a large extent in agreement with those reported in DeCamp et al.24 This review adds to the evidence by considering two clinically important time points: outcomes occurring during the ED stay and those up to 72 h following discharge from the ED stay. While these would appear to strengthen the conclusions relevant to the ED stay, questions still remain if oral ondansetron does reduce the hospital admission rate in the period up to 72 h following discharge from the ED stay. This will change the calculation of the cost-effectiveness of ondansetron and will influence the debate on its use.24 26
Clinical practice guidelines for the treatment of children with gastroenteritis recommend supportive care using ORT for mild-to-moderate dehydration but provide no recommendations on the additional use of antiemetic medication for vomiting.5 However, in USA and Canadian practice, it would appear that there is now an increased tendency towards the prescribing of antiemetic medication by clinicians.27 28 A recent Canadian cohort study demonstrated the potential benefits of increasing the use of ondansetron which was linked to a decrease in intravenous administration by 50%.29 Clinical practice in the UK is more conservative with very few physicians happy to prescribe in either a primary or secondary care setting.
This systematic review provides evidence that supports the use of ondansetron as an adjunct to standard ORT in the treatment of children with AGE exhibiting mild-to-moderate dehydration. Ondansetron given to children with mild-to-moderate dehydration appears to decrease the number of children who have persistent vomiting as a barrier to ORT. In addition, it decreases the number of children requiring intravenous rehydration and immediate hospitalisation. Oral ondansetron may also prove to be useful as an adjunctive measure to ORT in the outpatient or home care setting.
This review shows that there is an increased incidence of diarrhoea when using ondansetron, but this is likely to vary according to the dosage. It has also been postulated that the increase in diarrhoea in the ondansetron arm is the result of a retention of fluids related to the suppression of vomiting, which would otherwise been removed from the body through vomiting.12 The implications for future research are that there is justification for more evidence to investigate the effects of ondansetron in the UK to investigate the following: primary and secondary care administration, dosage regimes, hydration status and age of child.
Future research is needed to investigate the use of antiemetics for AGE in primary care. Second, the more ready availability of ondansetron during ambulatory care or prior to presentation at secondary care might be beneficial.
We recommend that future studies should consider using outcomes that are of greater relevance to patients and their carers and should include studies designed to explore the association between ondansetron and the increased incidence of diarrhoea and the possible combination of antiemetic therapy with other treatments.
Although the majority of the included studies were conducted in an ED, the scope of the single study carried out in a community setting highlights the potential therapeutic benefits of antiemetic use in the outpatient or general practice setting.13 Currently, any child presenting to primary care with a diagnosis of AGE and who are at least moderately dehydrated would be sent to secondary care for IVT; however, the delay between primary and secondary care might provide enough time for oral ondansetron to stop the child from vomiting and challenge by ORT on presentation to secondary care could be satisfactory.8
Any future research should include a formal cost-effectiveness analysis across treatments, differentiating between routes of administration. Analysis should be evaluated separately for developed and developing countries because clinical decision making, patients' preferences and carer expectations of outcomes do differ across these variables. Future RCT must be well designed, well conducted and adequately powered to account for the challenges faced in these research areas.
Conclusions
When antiemetics are used for treatment of vomiting in children with AGE and mild-to-moderate dehydration presenting to the ED, the following was found:
There was clear evidence to support the effectiveness (and likely cost-effectiveness) of ondansetron to increase the cessation of vomiting, reduce the need for IVT and reduce the need of immediate hospitalisation.
There was evidence of an association between ondansetron and an increased incidence of diarrhoea, but further studies are needed to investigate this.
There was limited evidence for the use of dimenhydrinate or granisetron as effective antiemetic treatments.
There was no evidence to support the antiemetic effectiveness of dexamethasone or metoclopramide or even though there may be an increase in side effects with these treatments.
There was good evidence to support the use of ondansetron over dexamethasone and limited evidence to suggest that ondansetron had a greater antiemetic effect compared with metoclopramide.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the earlier contribution of Dunia Alhashimi, Hakima Alhashimi and Vanitha Jagannath to previous versions of this review. The authors would also like to thank Janet Lilleyman and Karin Dearness, Managing Editors of the Cochrane UGPD Group, and Mike Clarke (Director of the all Ireland methodology hub) for their support throughout this review. We would also like to thank Jan Schoones for conducting the most recent searches and to Ruth Lewis for her support and advice with the MTC analysis. As well as very helpful peer reviewer comments from Professor Stephen Freedman and Dr Lisa Hartling, which benefited the manuscript.
Footnotes
To cite: Carter B, Fedorowicz Z. Antiemetic treatment for acute gastroenteritis in children: an updated Cochrane systematic review with meta-analysis and mixed treatment comparison in a Bayesian framework. BMJ Open 2012;2:e000622. doi:10.1136/bmjopen-2011-000622
Contributors: BC and ZF made a substantial contribution to the conception, acquisition, data analysis, and interpretation.
Funding: This research received no specific grant from any funding agency in public, commercial or not-for-profit sectors.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data available.
References
- 1.Herikstad H, Yang S, Van Gilder TJ, et al. A population - based estimate of the burden of diarrhoeal illness in the United States: FoodNet. Epidemiol Infect 2002;129:9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.OPCS Morbidity Statistics from General Practice. Forth National Study 1991–1992. London UK: HMSO, 1993 [Google Scholar]
- 3.American Academy of Pediatrics (AAP) Practice parameter: the management of acute gastroenteritis in young children. American academy of Pediatics, Provisional committee on Quality Improvement, Subcommittee on Acute Gastroenteritis. Pediatrics 1996;97:424–35 [PubMed] [Google Scholar]
- 4.National Collaborating Centre for Women's and Children's Health Diarrhoea and Vomiting Diagnosis, Assessment and Management in Children Younger than 5 years. London: RCOG Press, 2009 [PubMed] [Google Scholar]
- 5.Khanna R, Lakhanpaul M, Burman-Roy S, et al. Diarrhoea and vomiting caused by gastroenteritis in children under 5 years: summary of NICE guidance. BMJ 2009;25:1009–12 [DOI] [PubMed] [Google Scholar]
- 6.Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev 2011;(9):CD005506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration, 2011. http://www.cochrane-handbook.org [Google Scholar]
- 8.Treadwell JR, Tregear SJ, Reston JT, et al. A system for rating the stability and strength of medical evidence. BMC Med Res Methodol 2006;6:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gamble C, Hollis S. Uncertainty method improved on best-worst case analysis in a binary meta-analysis. J Clin Epidemiol 2005;58:579–88 [DOI] [PubMed] [Google Scholar]
- 10.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105–24 [DOI] [PubMed] [Google Scholar]
- 11.Al-Ansari K, Alomary S, Abdulateef H, et al. Metoclopramide versus ondansetron for the treatment of vomiting in children with acute gastroenteritis. J Pediatr Gastroenterol Nutr 2011;53:156–60 [DOI] [PubMed] [Google Scholar]
- 12.Cubeddu LX, Trujillo LM, Talmaciu I, et al. Antiemetic activity of ondansetron in acute gastroenteritis. Aliment Pharmacol Ther 1997;11:185–91 [DOI] [PubMed] [Google Scholar]
- 13.Freedman SB, Adler M, Seshadri R, et al. Oral ondansetron for gastroenteritis in a pediatric emergency department. N Engl J Med 2006;354:1698–705 [DOI] [PubMed] [Google Scholar]
- 14.Qazi K, Bin salleh HM, Shah UH, et al. Granisetron in the management of gastroenteritis related vomiting in a pediatric emergency department: a randomised placebo-controlled clinical trial. Acad Emerg Med 2011;58(4 Suppl):S323 [Google Scholar]
- 15.Ramsook C, Sahagun-Carreon I, Kozinetz CA, et al. A randomized clinical trial comparing oral ondansetron with placebo in children with vomiting from acute gastroenteritis. Ann Emerg Med 2002;39:397–403 [DOI] [PubMed] [Google Scholar]
- 16.Rerksuppaphol R, Rerksuppaphol L. Efficacy of intravenous ondansetron to prevent vomiting episodes in acute gastroenteritis: a randomised controlled trial. Pediatr Rep 2010;2:55–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roslund G, Hepps TS, McQuillen KK. The role of oral ondansetron in children with vomiting as result of acute gastritis/gastroenteritis who have failed oral rehydration therapy: a randomized controlled trial. Ann Emerg Med 2008;52:22–9 [DOI] [PubMed] [Google Scholar]
- 18.Stork CM, Brown KM, Reilly TH, et al. Emergency department treatment of viral gastritis using intravenous ondansetron or dexamethasone in children. Acad Emerg Med 2006;13:1027–33 [DOI] [PubMed] [Google Scholar]
- 19.Uhlig U, Pfeil N, Gelbrich G, et al. Dimenhydrinate in children with infectious gastroenteritis: a prospective, RCT. Pediatrics 2009;124:e622–32 [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz HL, Yildizdas RD, Sertdemir Y. A randomized clinical trial:oral ondansetron for reducing vomiting secondary to acute gastroenteritis in children. Ann Emerg Med 2010;51:482–3 [DOI] [PubMed] [Google Scholar]
- 21.Freedman SB, Sivabalasundaram V, Bohn V, et al. The treatment of pediatric gastroenteritis: a comparative analysis of pediatric emergency physicians' practice patterns. Acad Emerg Med 2010;18:38–45 [DOI] [PubMed] [Google Scholar]
- 22.Howard S. Question 1 Does oral ondansetron reduce vomiting and the need for intravenous fluids and hospital admission in children presenting with vomiting secondary to gastroenteritis? Arch Dis Child 2010;95:945–7 [DOI] [PubMed] [Google Scholar]
- 23.Colletti JE, Brown KM, Sharieff GQ, et al. The management of children with gastroenteritis and dehydration in the emergency department. J Emerg Med 2010;38:686–98 [DOI] [PubMed] [Google Scholar]
- 24.DeCamp LR, Byerley JS, Doshi N, et al. Use of antiemetic agents in acute gastroenteritis: a systematic review and meta-analysis. Arch Pediatr Adolesc Med 2008;162:858–65 [DOI] [PubMed] [Google Scholar]
- 25.Vreeman RC, Finnell SM, Cernkovich ER, et al. The effects of antiemetics for children with vomiting due to acute, moderate gastroenteritis. Arch Pediatr Adolesc Med 2008;162:866–9 [DOI] [PubMed] [Google Scholar]
- 26.Freedman SB, Steiner MJ, Chan KJ. Oral ondansetron administration in emergency departments to children with gastroenteritis: an economic analysis. PLoS Med 2010;7:pii: e1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li ST, DiGiuseppe DL, Christakis DA. Antiemetic use for acute gastroenteritis in children. Arch Pediatr Adolesc Med 2003;157:475–9 [DOI] [PubMed] [Google Scholar]
- 28.Kwon KT, Rudkin SE, Langdorf MI. Antiemetic use in paediatric gastroenteritis: a national survey of emergency physicians, paediatricians, and paediatric emergency physicians. Clin Pediatr (Phila) 2002;41:641–52 [DOI] [PubMed] [Google Scholar]
- 29.Freedman SB, Tung C, Rumantir M, et al. Time series analysis of ondansetron use in pediatric gastroenteritis. J Pediatr Gastroenterol 2012;54:381–6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.