Abstract
Objectives:
This study sought to determine the high risk factors for severe hand, foot, and mouth disease (HFMD).
Materials and Methods:
Retrospective 229 severe HFMD cases from four hospitals in FuYang, HeFei, and BoZhou (Anhui Provincial Hospital, Fuyang City People's Hospital, No. 2 People's Hospital of Fuyang and Bozhou city People's Hospital) in 2008-2009 were studied, with 140 mild HFMD cases in the same area. Using univariate and multivariate logistic regression analyses, the high risk factors of HFMD were identified by comparing clinical and laboratory findings between severe cases and mild cases.
Results:
There was a significant difference in age, total duration of fever, rate of respiratory and heart, shake of limbs, white blood cell count, blood sugar, and CK-MB between the two groups. Univariate logistic regression analysis showed that severe cases were associated with age (<3 years), withdrawnness and lethargy, shake of limbs, tachycardia, total leukocyte count (≥17×109/l), blood sugar (≥7 mmol/l), and CK-MB (≥16 mmol/l). Furthermore, age (<3 years), withdrawnness, and lethargy, shake of limbs, WBC (≥17×109/l), and CK-MB (≥16 mmol/l) were found to be high risk factors for severe cases after multivariate logistic regression analysis.
Conclusions:
Clinicians should give importance to these risk factors. Early recognition of children at risk and timely intervention is the key to reduce acute mortality and morbidity.
Keywords: Complication, hand foot and mouth disease, risk factor
Introduction

Hand, foot, and mouth disease (HFMD) was a common acute infectious disease in children[1] caused by a group of enteroviruses, including coxsackievirus (Cox) A16, A5, A7, A9, A10, B2, B5, and enterovirus71 (EV71)[2–5] with CoxA16 being the most common etiologic agent. Its clinical manifestation was cutaneous lesions over hands, feet, and buttocks along with oral. In most instances, it was mild and self-limited over 3-5 days with no complications. However, severe complicated forms involving encephalitis, meningitis, encephalomyelitis, pulmonary edema, or circulatory failure occurred in the Fuyang HFMD outbreak in 2008.[6] In the same year, the epidemic spread to other areas of Anhui province; fatal cases were the most in Anhui province so far. Of 229 severe and fatal HFMD cases, the 19 EV71-positive children died of pulmonary edema, 10 fatal HFMD cases were selected for a closer investigation. The chief causes of death were found: severe cases being escaped from early recognition; lack of detection for important indicator; fulminant pulmonary edema being not correctly assessed and severe cases being not treated timely. This was consistent with the other.[7–9] In order to detect severe cases earlier, prevent deterioration, increase the ratio of successful rescue, and reduce acute mortality and morbidity, high risk factors for severe HFMD disease were determined in this article.
Materials and Methods
Diagnostic criteria
HFMD cases in 2008-2009 were divided into two groups according to Guidelines on the Diagnosis and Treatment of HFMD.[10]
Mild cases
The clinical features of the patients included acute onset, lesions over buccal, and labial mucosae, and erythematous vesicles with a red areola over palms, soles, and buttocks. They may develop cough, nasal discharge, anorexia, etc. Part of them only showed cutaneous lesions or herpangina. Most subjects recovered within a week. Some of them had atypically manifestations of cutaneous lesions.
Severe cases
The subjects had the same clinical manifestation with the mild cases, but progressed rapidly. On 1-5 days of illness, neurologic complications including lethargy, drowsiness, irritability, delirium; headache, vomiting; shake of limbs, myoclonus, ocular flutter, ataxia; asthenia, seizures. Some patients presented with meningeal irritation, diminished, or disappeared tendon reflexes. Fatal cases: Children with HFMD were considered to have more serious illness if they had at least one of the following features: (1) seizures, coma or cerebral hernia; (2) respiratory distress, cyanosis, bloody frothy sputum, lung rales, etc; (3) poor peripheral perfusion such as shock.
Case definitions
The study protocol has been approved by the Ethics Committee, Anhui Medical University, Hefei, China. Written consent was obtained from each child's accompanying parent. A total of 369 HFMD cases from four hospitals in FuYang and HeFei (Anhui Provincial Hospital, Fuyang City People's Hospital, No. 2 People's Hospital of Fuyang and Bozhou city People's Hospital) in 2008-2009 were divided into two groups according to Guidelines on the Diagnosis and Treatment of HFMD. A total of 229 children were labeled as severe HFMD cases because their clinical findings were in accordance with diagnostic criteria of severe and fatal HFMD cases. A total of 140 children were classified as mild HFMD cases because their clinical findings were in accordance with diagnostic criteria of mild HFMD cases.
Risk factor analysis
Many factors such as gender, age, clinical manifestation, laboratory findings, electroencephalogram, and imaging finding were analyzed by univariate logistic regression analyses. The high risk factors of HFMD were identified by comparing clinical and laboratory findings between severe cases and mild cases using multivariate logistic regression analyses.
Statistical analysis
All analyses were conducted by SPSS for Windows 11.5 software package. Measurement data were presented in mean values with standard deviations (x ± s) and compared by a chi-square test. In case, count data were presented, they were compared by Student's t-test. Factors were analyzed by univariate logistic regression analyses. Statistically significant variables were then analyzed by multivariate logistic regression analyses with a significant (P<0.05).
Results
General data
A total of 369 HFMD cases were recruited according to guidelines on the diagnosis and treatment of HFMD (published by Chinese Ministry of Health in 2010). Of 229 severe children, 147(64.19%) children were male, 82(35.81%) children were female; their mean age was (1.94±1.31) years, and 193(84.28%) cases were at age of 0-3 years old. Of 140 mild cases, 94(67.14%) cases were male, 46(32.86%) cases were female; the mean age was (4.17±2.50) years, and 60(42.8%) cases were at age of 0-3 years old. There was no significant difference in the male:female ratio between the two groups (P>0.05). There was a significant difference in the age between the two groups, especially at age of 0-3 years (P<0.01).
Clinical features
Fever
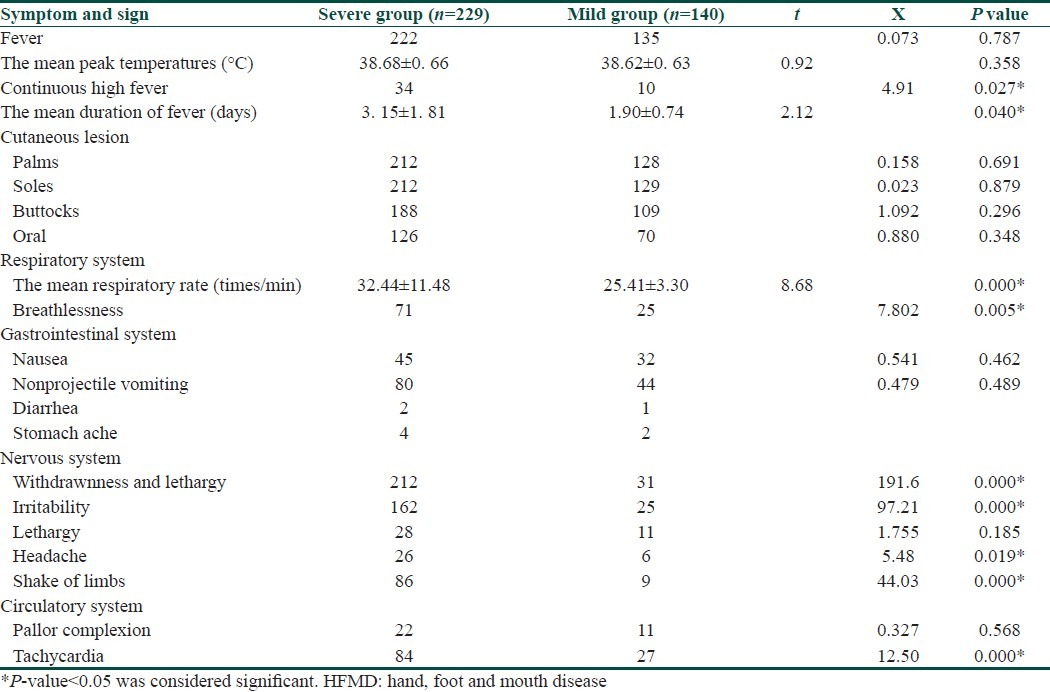
Of 229 severe cases, 222(96.9%) had fever, and 7(3.1%) had no fever. The peak range of fever was 36.7-41.0°C (mean, 38.68±0.66). A total of 34(14.8%) children had continuous high fever, and the mean duration of fever was (3.15±1.81) days. Of 140 mild cases in the control group, 135(96.4%) had fever, and 5(3.6%) had no fever. The mean peak temperature was 38.62±0.63°C. A total of 10(7.1%) children had continuous high fever, and the mean duration of fever was 1.90±0.74 days. There was no significant difference in the mean peak temperature and fever between the two groups (P>0.05). However, the mean duration of fever and continuous high fever between the two groups was significant (P<0.05) [Table 1].
Table 1.
Compared symptom and sign of HFMD between the severe and mild groups
Cutaneous lesions
A total of 212(92.6%) severe cases had cutaneous lesions on palms, 212(92.6%) on soles, 188(82.8%) on buttocks, and 126(55%) on oral. A total of 128(91.4%) children in the control group had cutaneous lesions on palms, 129(92.1%) on soles, 109(78.4%) on buttocks, and 70(50%) on oral. There was no significant difference in the distribution of cutaneous lesions between the two groups (P>0.05) [Table 1].
Symptom of the respiratory system
The mean respiratory rates in the severe group were 32.44±11.48 times per minute. A total of 71(31.0%) had breathlessness, 32(14.0%) respiratory distress, 19(8.3%) changes of respiratory rhythm, 40(17.5%) cyanosis, 40(17.5%) spit white, pink or bloody frothy sputum, and 54(23.6%) crackles in the lungs. The mean respiratory rates in the control group were 25.41±3.30 times per minute. A total of 25(17.9%) had breathlessness. None of mild cases had respiratory distress, respiratory rhythm change, cyanosis, bloody frothy sputum, or crackles in the lung. The mean respiratory rates and breathlessness were significantly different between the two groups (P<0.01) [Table 1].
Symptom of the gastrointestinal system
In the severe group, 45(19.7%) children felt nausea, 80(34.9%) had nonprojectile vomiting, 2(0.9%) stomach ache, 4(1.7%) diarrhea. In the control group, 32(22.9%)children felt nausea, 44(31.4%) had nonprojectile vomiting, 1(0.7%) stomachache, 2(1.4%) diarrhea. There was no significant difference in the symptom of the gastrointestinal system between the two groups (P>0.05) [Table 1].
Symptom of the nervous system
In the severe group, 212(92.6%) withdrawnness and lethargy, 162(70.7%) irritability, 28(12.2%) lethargy, 24(10.5%) coma, 26(11.4%) headaches, 39(17.0%) seizures, 10(4.4%) myoclonus, 40(17.5%) paralysis, 86(37.6%) shake of limbs, 3(1.3%) encephaledema, 60(26.2%) stiff neck, 38(16.6%) positive Babinski sign, 11(4.8%) positive Kernig sign, 15(6.6%) positive Brudzinski sign. A total of 222(96.9%) normal tendon reflexes, 6(2.6%) tendon reflexes hyperfunction, 1(0.4%) diminished tendon reflexes. A total of 213(93.0%) normal muscular tone, 4(1.7%) hypermyotonia, 12(5.2%) hypomyotonia. In the control group, 31(22.1%) withdrawnness and lethargy, 25(17.9%) irritability, 11(7.9%) lethargy, 6(4.3%) headaches, 9(6.4%) shake of limbs. None of mild cases had the symptom of coma, seizures, myoclonus, paralysis, and encephaledema, meningeal irritation, positive Babinski sign, tendon reflexes hyperfunction, or hypofunction, hypermyotonia or hypomyotonia. There was a significant difference in withdrawnness and lethargy, irritability, headache, shake of limbs between the two groups (P<0.01) [Table 1].
Symptom of the circulatory system
In the severe group, 22(9.6%) had pallor complexion, 84(36.7%) tachycardia, 12(5.2%) bradycardia, 71(31.0%) poor peripheral perfusion, 27(11.8%) cyanosis, 37.1% hypertention, and 1.3% hypotention. None of severe cases had myocarditis or arrhythmia. In the control group, 11(7.9%) had pallor complexion, 27(19.3%) tachycardia. None of mild cases had bradycardia, poor peripheral perfusion, cyanosis, myocarditis, or arrhythmia. There was a significant difference in tachycardia between the two groups (P<0.01) [Table 1].
Auxiliary examination
Blood tests
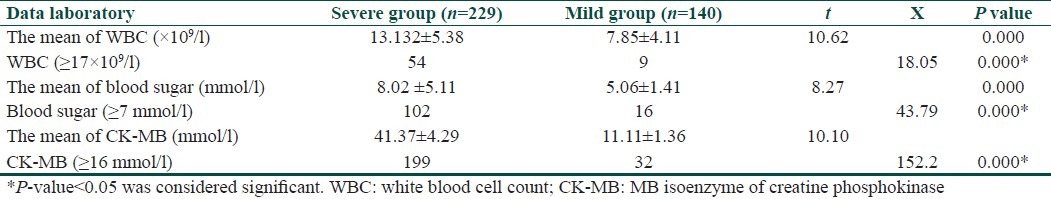
The mean white blood cell (WBC) count in the severe group and the control group was (13.132±5.38)×109/l and (7.85±4.11)×109/l, respectively. There was a significant difference in WBC count between the two groups (P<0.01). A total of 54(23.58%) severe cases and 9(6.43%) mild cases showed that the total leukocyte counts were both more than 17×109/l. The total leukocyte count (≥17×109/l) was significantly different between the two groups (P<0.01) [Table 2].
Table 2.
The data of laboratory between severe and mild cases
Biochemical examination
Blood
The mean blood sugar levels in the severe group and the control group were 8.22±5.08 mmol/l and 4.69±0.97 mmol/l, respectively. There was a significant difference in blood sugar between the two groups (P<0.01). A total of 102(44.54%) severe cases and 16(11.43%) mild cases showed that blood sugar levels were both higher than 7 mmol/l. Blood sugar (≥7 mmol/l) was significantly different between the two groups (P<0.01).
CK-MB
The mean CK-MB levels in the severe group and the control group were 41.37±4.29 mmol/l and 11.11±1.36 mmol/l, respectively. There was a significant difference in the CK-MB level between the two groups (P<0.01). A total of 199(66.56%) severe cases and 32(22.86%) mild cases showed that CK-MB levels were both higher than 16 mmol/l. CK-MB (≥16 mmol/l) was significantly different between the two groups (P<0.01) [Table 2].
In the severe group, normal findings were shown in the liver function, kidney functions, and electrolyte. There was no significant difference between the severe and control groups (P>0.05).
Cerebrospinal fluid examination
Of 138 severe cases, 114(82.6%) cases showed that cerebrospinal fluid (CSF) pleocytosis was more than 10×106/l, and in the mild HFMD patients lumbar puncture was not performed.
Physical examination in the severe group:
Chest radiograph: Of 126 severe cases, 82(65%) had increased lung markings or infectious bronchus, 34(27%) bronchopneumonia, 10(8%) exudative lesion.
ECG: Of 36 severe cases, 1(2.8%) had change of T wave, 10(27.8%) sinus tachycardia.
Brain magnetic resonance: Of 62 severe cases, 40(64.5%) showed normal findings, 22(35.5%) abnormal signal in the brainstem or medulla oblongata.
Treatment and clinical outcome
In the mild group, patients were treated with virazole under home quarantine. They would see the doctor timely whenever needed. In the severe group, patients were treated with sedative, oxygen, fluid restriction, 20% mannitol in time to keep them in mild dehydration. Large dose of r-globulin (2 g/kg) was intravenous dripped in two days. High-dose intravenous methylprednisolone (15-30 mg/kg/d) was used for 3 days. Trachea cannula and positive pressure mechanical ventilation were used in 28 fatal cases with respiratory failure. The mean hospitalization of 229 severe children was (9.0±7.3) days. A total of 199(86.9%) were cured or improved, 3(1.3%) gave up treatment and left voluntarily, 27(11.8%) died.
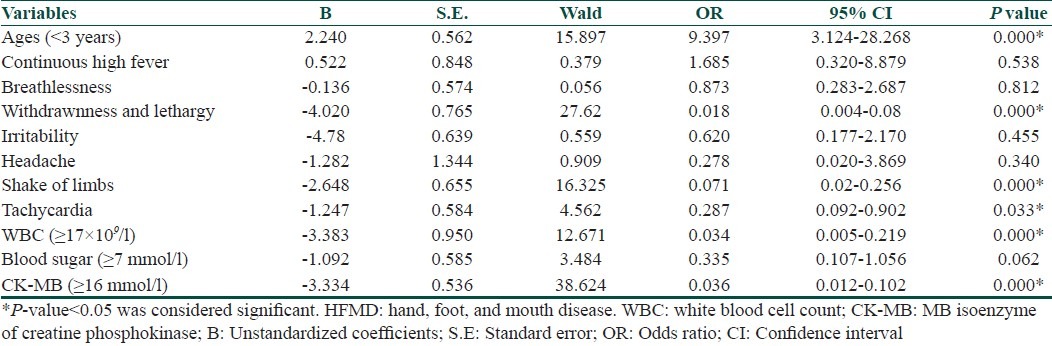
By using the one-factor logistic regression analysis method, many factors such as age, withdrawnness, and lethargy, shake of limbs, tachycardia, total leukocyte count, blood sugar, and CK-MB were significantly associated with incidence of severe HFMD. Univariate logistic regression analysis showed that severe cases were associated with age (<3 years), withdrawnness, and lethargy, shake of limbs, tachycardia, total leukocyte count (≥17×109/l), blood sugar (≥7 mmol/l), and CK-MB (≥16 mmol/l). Moreover, age (<3 years), withdrawnness, and lethargy, shake of limbs, WBC (≥17×109/l), and CK-MB (≥16 mmol/l) were found to be high risk factors for severe cases after multivariate logistic regression analysis [Table 3].
Table 3.
The result of high risk factors of HFMD under logistic regression analysis
Discussion
HFMD was a common acute infectious disease in children. EV71 was one of very few viruses that caused HFMD as well as a variety of other clinical manifestations. The most important of these was serious complications including encephalitis, parencephalitis, brainstem encephalitis, myelitis, which caused significant morbidity.[5,11–13] HFMD had outbroken in many countries and regions such as Japan, Switzerland, Australia, America, Malaysia, Singapore, Indian, China Taiwan, etc.[2,5,14–17] HFMD cases confirmed that they were mainly caused by EV71, recruited from four hospitals in FuYang, HeFei, and BoZhou during the period 2008-2009.
Almost all the severe cases had CNS involvement; some then had circulatory failure, pulmonary edema, and pneumorrhagia. The progression of the HFMD disease was very fast. The duration was about several hours, even less than 48 hours. The mortality rate was very high if it progressed to pulmonary edema. Fortunately, severe HFMD could be stopped by effective treatment without delay because the early pathological change was reversible. Early recognition of severe cases and timely intervention is key to prevent cardiorespiratory failure, increase the ratio of successful rescue, and reduce the acute mortality.
Through comparative analysis between two groups, the study showed that there were mainly patients of severe cases younger than 3 years, with continuous hyperpyrexia, withdrawnness, and lethargy, irritability, shake of limbs, breathlessness and tachycardia, total leukocyte count (≥17×109/l), and blood sugar (≥7 mmol/l). It was consistent with the other reports.[18,19]
The primary cause of aggravation was involved in the central nervous system. The study of the deaths caused by EV71 showed that the virus could injure all the central nervous system, especially cerebellum, brainstem, and spinal.[15,20] EV71 invades the central nervous system through bloodstream or cranial nerves (facial nerve or glossopharyngeal nerve) on days 2-5.[21] Rhombencephalitis was the primary fatal cause of many death. It showed that the patients would have rhombencephalitis when they occurred salivation, cough when drinking, myoclonus, nystagmus, and palpitate, etc. Rhombencephalitis was divided into three levels according to the clinical manifestation.[22] Level 1 had myoclonus, tremor, and ataxia. Level 2 had myoclonus together with vegetative nerve functional disturbance. Level 3 had vegetative nerve functional disturbance, pulmonary edema, and hemorrhage. The clinical manifestation was rapid onset of respiratory distress, cyanosis, circulatory failure, shock, coma, absence of the pupillary light reflex, apnea, etc.
Neurogenic pulmonary edema (NPE) was the major and direct reason to give rise to death of HFMD patients.[23] Autopsy and histopathology showed that pulmonary edema caused by EV71 was neurogenic.[24] EV71 destroyed the very regions of the brainstem with regulatory function which stimulated the sympathetic nervous system, caused the blood vessel to contract intensely, and the bloodstream flow from systemic circulation to pulmonary circulation with little resistance. Pulmonary edema was the result of all the points mentioned together with increased pulmonary capillary.[25] Almost all the deaths showed vasoconstriction, ochrodermia, cold sweat, and weak pulse besides anoxia and bloody frothy sputum. The mortality of NPE was high. Its prognosis was closely related to early treatment, mainly supporting treatment, including mechanical ventilation, oxygen supply, antisympathetic drugs, etc. Therefore, early recognition and rigorous monitoring is critical to rescue the severe HFMD patients with rhombencephalitis successfully.
According to the analysis of these data and the experience of clinical treatment, high risk factors were significantly associated with incidence of severe HFMD, including age (<3 years), withdrawnness and lethargy, shake of limbs, WBC (≥17×109/l), and CK-MB (≥16 mmol/l).

Footnotes
Source of Support: This work was supported by a grant from the Foundation of Health Department of Anhui Province of China (No. 2010C041)
Conflict of Interest: Nil.
References
- 1.Richardson HB, Jr, Leibovitz A. Hand, foot and mouth disease in children. J Pediatr. 1965;67:6–12. doi: 10.1016/s0022-3476(65)80297-9. [DOI] [PubMed] [Google Scholar]
- 2.Wu Y, Yeo A, Phoon MC, Tan EL, Poh CL, Quak SH, et al. The largest outbreak of hand, foot and mouth disease in Singapore in 2008: The role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010;14:e1076–81. doi: 10.1016/j.ijid.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Bârlean L, Avram G, Pavlov E, Cotor F. Investigation of five cases of vesicular enteroviral stomatitis with exanthema induced by coxsackie A5 virus. Rev Roum Virol. 1994;45:3–9. [PubMed] [Google Scholar]
- 4.Itagaki A, Ishihara J, Mochida K, Ito Y, Saito K, Nishino Y, et al. A clustering outbreak of hand, foot, and mouth disease caused by Coxsackie virus A10. Microbiol Immunol. 1983;27:929–35. doi: 10.1111/j.1348-0421.1983.tb00658.x. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert GL, Dickson KE, Waters MJ, Kennett ML, Land SA, Sneddon M. Outbreak of enterovirus infection in Victoria, Australia, with a high incidence of neurologic involvement. Pediatr Infect Dis J. 1988;7:484–8. doi: 10.1097/00006454-198807000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Zhu Z, Yang W, Ren J, Tan X, Wang Y, et al. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand foot and mouth disease in Fuyang city of China. Virol J. 2010;7:94. doi: 10.1186/1743-422X-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muir P, van Loon AM. Enterovirus infections of the central nervous system. Intervirology. 1997;40:153–66. doi: 10.1159/000150542. [DOI] [PubMed] [Google Scholar]
- 8.Hall WA. Infectious lesions of the brain stem. Neurosurg Clin North Am. 1993;4:543–51. [PubMed] [Google Scholar]
- 9.Sun LM, Zheng HY, Zheng HZ, Guo X, He JF, Guan DW, et al. An Enterovirus 71 Epidemic in Guangdong Province of China, 2008: Epidemiological, Clinical, and Virogenic Manifestations. Jpn J Infect Dis. 2011;64:13–8. [PubMed] [Google Scholar]
- 10.Guidelines on the diagnosis and treatment of hand, foot, and mouth disease, published by Chinese Ministry of Health in 2010. Chinese Community Doctors. 2010:21. [Google Scholar]
- 11.Melnick JL. Enterovirus type 71 infections: A varied clinical pattern sometimes mimicking paralytic poliomyelitis. Rev Infect Dis. 1984;6:S387–90. doi: 10.1093/clinids/6.supplement_2.s387. [DOI] [PubMed] [Google Scholar]
- 12.Samuda GM, Chang WK, Yeung CY, Tang PS. Monoplegia caused by enterovirus 71: An outbreak in Hong Kong. Pediatr Infect Dis J. 1987;6:206–8. doi: 10.1097/00006454-198702000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Landry ML, Fonseca SN, Cohen S, Bogue CW. Fatal enterovirus type 71 infection: Rapid detection and diagnostic pitfalls. Pediatr Infect Dis J. 1995;14:1095–100. doi: 10.1097/00006454-199512000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Tan XJ, Wang HY, Yan DM, Zhu SL, Wang DY, et al. An outbreak of hand, foot, and mouth disease associated with subgenotype C4 of human enterovirus 71 in Shandong, China. J Clin Virol. 2009;44:262–7. doi: 10.1016/j.jcv.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Ishimaru Y, Nakano S, Yamaoka K, Takami S. Outbreaks of hand, foot and mouth disease by enterovirus 71: High incidence of complication disorders of central nervous system. Arch Dis Child. 1980;55:583–8. doi: 10.1136/adc.55.8.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chatproedprai S, Theanboonlers A, Korkong S, Thongmee C, Wananukul S, Poovorawan Y. Clinical and molecular characterization of hand-foot-and-mouth disease in Thailand, 2008-2009. Jpn J Infect Dis. 2010;63:229–33. [PubMed] [Google Scholar]
- 17.Sarma N, Sarkar A, Mukherjee A, Ghosh A, Dhar S, Malakar R. Epidemic of hand, foot and mouth disease in West Bengal, India in August, 2007: A multicentric study. Indian J Dermatol. 2009;54:26–30. doi: 10.4103/0019-5154.48982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang LY, King CC, Hsu KH, Ning HC, Tsao KC, Li CC, et al. Risk factors of enterovirus 71 infection and associated hand, foot, and mouth disease/herpangina in children during an epidemic in Taiwan. Pediatrics. 2002;109:e88. doi: 10.1542/peds.109.6.e88. [DOI] [PubMed] [Google Scholar]
- 19.Ooi MH, Wong SC, Mohan A, Podin Y, Perera D, Clear D, et al. Identification and validation of clinical predictors for the risk of neurological involvement in children with hand, foot, and mouth disease in Sarawak. BMC Infect Dis. 2009;9:3. doi: 10.1186/1471-2334-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho HK, Lee NY, Lee H, Kim HS, Seo JW, Hong YM, et al. Enterovirus 71-associated hand, foot and mouth diseases with neurologic symptoms, a university hospital experience in Korea, 2009. Korean J Pediatr. 2010;53:639–43. doi: 10.3345/kjp.2010.53.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong KT, Munisamy B, Ong KC, Kojima H, Noriyo N, Chua KB, et al. The distribution of inflammation and virus in human enterovirus 71 encephalomyelitis suggests possible viral spread by neural pathways. J Neuropathol Exp Neurol. 2008;67:162–9. doi: 10.1097/nen.0b013e318163a990. [DOI] [PubMed] [Google Scholar]
- 22.Huang CC, Liu CC, Chang YC, Chen CY, Wang ST, Yeh TF. Neurologic complications in children with enterovirus 71 infection. N Engl J Med. 1999;341:936–42. doi: 10.1056/NEJM199909233411302. [DOI] [PubMed] [Google Scholar]
- 23.Wang SM, Lei HY, Huang KJ, Wu JM, Wang JR, Yu CK, et al. Pathogenesis of enterovirus 71 brainstem encephalitis in pediatric patients: Roles of cytokines and cellular immune activation in patients with pulmonary edema. J Infect Dis. 2003;188:564–70. doi: 10.1086/376998. [DOI] [PubMed] [Google Scholar]
- 24.Lum LC. Fatal enterovirus 71 encephalomyelitis. Pediatrics. 1998;133:795–8. doi: 10.1016/s0022-3476(98)70155-6. [DOI] [PubMed] [Google Scholar]
- 25.Theodore J, Robin ED. Pathogenesis of neurogenic pulmonary oedema. Lancet. 1975;2:749–51. doi: 10.1016/s0140-6736(75)90729-1. [DOI] [PubMed] [Google Scholar]


