Abstract
Pulmonary hypertension as a result of chronic thromboembolic disease (CTEPH) is potentially curable with pulmonary endarterectomy surgery. Consequently, correctly diagnosing patients with this type of pulmonary hypertension and evaluating these patients with the goal of establishing their candidacy for surgical intervention is of utmost importance. And as advancements in surgical techniques have allowed successful resection of segmental-level chronic thromboembolic disease, the number of CTEPH patients that are deemed suitable surgical candidates has expanded, making it even more important that the evaluation be conducted with greater precision. This article will review a diagnostic approach to patients with suspected chronic thromboembolic disease with an emphasis on the criteria considered in selecting patients for pulmonary endarterectomy surgery.
Keywords: chronic thromboembolic pulmonary hypertension, pulmonary endarterectomy, chronic thromboembolic disease, pulmonary hypertension
Several prospective studies have reported that between 0.57% and 4.6% of acute pulmonary embolic survivors will develop symptomatic, chronic thromboembolic pulmonary hypertension (CTEPH).[1–3] As it is also reported that from 42% to 63% of patients with the established diagnosis of chronic thromboembolic disease have no previously documented acute venous thromboembolism,[4–6] the prevalence of CTEPH cases exceeds those estimates that have resulted from following patients with known thromboembolic events. The importance of correctly establishing the diagnosis of CTEPH is underscored by the understanding that, without appropriate therapy, patients with this disorder typically experience profound functional disability with a relatively poor long-term survivorship.[7,8] However, for selected CTEPH patients, pulmonary endarterectomy (PEA) surgery offers the potential for reversing the debilitating pulmonary hypertension and right heart failure that characterizes this disease.[9]
The evaluation of patients with suspected chronic thromboembolic disease has the principal goal of identifying those who are candidates for an endarterectomy. It is meant to (1) confirm the diagnosis of chronic thromboembolic disease and define the extent of surgically accessible chronic thromboembolic residua; (2) to establish the degree of pulmonary hypertension and cardiac compromise; (3) to delineate the comorbidities that might limit the benefits expected with an endarterectomy; and (4) to estimate the extent of coexisting, small-vessel pulmonary vascular disease, which might similarly impact the anticipated hemodynamic benefit with surgery.
CLINICAL PRESENTATION
Especially early in the course of the disease, the clinical presentation of CTEPH can be subtle. This subtlety contributes to the delay in diagnosis, making it necessary to maintain a high index of suspicion in those patients presenting with exertional dyspnea without apparent cause or without a prior history of venous thromboembolism. Atypical chest pain, episodic hemoptysis, a nonproductive cough, and palpitations are rarely presenting complaints. Evidence of right heart dysfunction such as peripheral edema, severe exercise limitation and associated chest discomfort, exertional dizziness, or syncopal episodes can be manifest late in the disease.
Physical examination findings can be equally deceptive early in the natural history of CTEPH. However, with advancing pulmonary hypertension, clinical presentation and examination findings are similar to that seen in other forms of pulmonary hypertension: discernible right ventricular impulse, a split second heart sound with accentuation of the pulmonic component, varying degrees of tricuspid regurgitation, and a right ventricular S4 gallop. As right ventricular failure develops, jugular venous distension, peripheral edema, hepatomegaly, ascites, and a right-sided S3 may become evident. The presence of pulmonary flow murmurs or bruits is often a physical examination finding that can be used to distinguish small vessel from large vessel variants of pulmonary hypertension.[10] An auscultatory finding in approximately 30% of patients with CTEPH, the bruit results from turbulent flow across partially obstructed, medium- to large-sized pulmonary vessels. They have not been described in pulmonary hypertensive disorders arising from the microvasculature. However, they are not unique to patients with chronic thromboembolic disease, having been described in other disease states which involve large pulmonary arteries, such as congenital branch stenosis or large vessel pulmonary arteritis. Additional examination findings in the CTEPH patient might include peripheral cyanosis, alerting the clinician to the possibility of a right-to-left shunt through a patent foramen ovale. Examination of the lower extremities may disclose superficial varicosities and venous stasis skin discoloration in those individuals who have experienced prior venous thrombosis.
DIAGNOSTIC EVALUATION
Defining a procoagulant state in patients evaluated for CTEPH has important implications as certain thrombophilias such as the presence of antiphospholipid antibodies might warrant a more intense level of chronic anticoagulation to prevent thrombosis. Moreover, these patients are at greater risk for thrombosis postendarterectomy. Antithrombin III, Protein C, and Protein S deficiencies, as well as Factor II (prothrombin) and Factor V Leiden mutations, are among the hereditary thrombophilic states which should be pursued in the evaluation of the CTEPH patient. However, in a large study investigating this issue, Wolf and colleagues showed that hereditary thrombophilia was not more prevalent in samples analyzed in 46 CTEPH patients or 64 patients with idiopathic pulmonary hypertension compared to control subjects (N=100). The same study, however, demonstrated that 20% of patients diagnosed with CTEPH exhibited antiphospholipid antibodies.[11] Subsequent studies have revealed similar results, with the presence of antiphospholipid antibodies frequently associated with chronic thromboembolic disease.[12,13] Bonderman and colleagues also showed increased levels of Factor VIII in 41% of 122 patients with CTEPH, levels that were substantially higher than those in control subjects and patients with nonthrombotic pulmonary hypertension.[14]
In the evaluation of the patient suspected of having CTEPH, “routine” laboratory testing may be helpful in defining the severity of disease. For those with severe right ventricular dysfunction and coexisting liver congestion, elevation of transaminase and bilirubin levels can be expected. In the same subgroup of patients where renal blood flow and glomerular perfusion may be compromised, either from a low cardiac output or the use of diuretics (or both), elevation of serum creatinine and blood urea nitrogen may result.
Pulmonary function testing is most useful in evaluating for coexisting parenchymal lung disease or airflow obstruction. Approximately 20% of CTEPH patients with parenchymal scarring from prior lung infarction, a mild to moderate restrictive defect may be detected.[15] Similarly, a modest reduction in single breath diffusing capacity for carbon monoxide (DLco) may be present in some CTEPH patients, though a normal value does not exclude the diagnosis.[16] A severe reduction in DLco should raise concerns that the distal pulmonary vascular bed is significantly compromised, making it imperative that an alternative diagnosis other than CTEPH be considered. Furthermore, CTEPH patients will frequently exhibit some degree of hypoxemia, and if measured, elevated dead-space ventilation,[17] both worsening with exercise. These findings reflect a moderate ventilation-perfusion mismatch in CTEPH and an inadequate cardiac output response to exercise resulting in a low mixed venous oxygen saturation.[18] Marked hypoxemia at rest implies severe right heart dysfunction or the presence of a considerable right-to-left shunt, such as through a patent foramen ovale.
The chest radiograph in patients with chronic thromboembolic disease may be deceptively unremarkable early on. With disease progression and the development of pulmonary hypertension, enlargement of the proximal pulmonary vascular bed typically occurs. With chronic thromboembolic involvement of the main or lobar pulmonary arteries, this central PA enlargement can be asymmetric. This is not a radiographic finding in those patients with small-vessel disease pulmonary hypertension.[19] As the right ventricle adapts to the rise in pulmonary vascular resistance, radiographic signs of chamber enlargement such as obliteration of the retrosternal space and prominence of the right heart border, can be observed.[20] Without coexisting parenchymal lung disease, interstitial-alveolar markings within the lung fields are atypical. However, relatively avascular lung regions can be appreciated if an organized thrombus has compromised blood flow to that area. In these poorly perfused lung regions, the sequela of lung injury such as peripheral alveolar opacities, linear scar-like lesions, and pleural thickening may be found.
Following an episode of pulmonary embolism, routine cardiopulmonary screening has a low yield in the detection of CTEPH.[21] Frequently, the first objective indication as to the presence of elevated pulmonary pressures or right ventricular compromise is provided with transthoracic echocardiography. Current technology allows for estimates of pulmonary artery systolic pressure (using Doppler analysis of the degree of tricuspid regurgitation), along with cardiac output and RV performance.[22] Enlargement of the right heart chambers, tricuspid regurgitation as a result of this chamber enlargement, flattening or paradoxical motion of the interventricular septum, encroachment of an enlarged right ventricle on the left ventricular cavity, and impaired left ventricular diastolic dysfunction not the result of primary left ventricular or valvular heart disease are findings in patients with significant pulmonary hypertension.[23,24] Contrast echocardiography using intravenous agitated saline can detect the presence of an intracardiac shunt, such as a patent foramen ovale or a previously undetected septal defect. If detected preoperatively, the atrial septal defect can be surgically repaired at the time of an endarterectomy. Though not specifically studied in patients with CTEPH, should an echocardiogram obtained at rest demonstrate minimally elevated pulmonary artery pressures or only modest right ventricular compromise in a patient experiencing significant cardiopulmonary symptoms with exertion, exercise echocardiography may demonstrate a substantial rise in pulmonary artery pressures or dilatation of the right ventricle.
VENTILATION–PERFUSION SCAN
To a large extent, computed tomographic (CT) angiography of the pulmonary vessels has replaced ventilation–perfusion (V/Q) scintigraphy in the evaluation of patients with suspected acute pulmonary embolic disease. However, the V/Q scan continues to provide essential information in the evaluation of the pulmonary hypertensive patient. In these patients, the V/Q scan can often be the first indication that chronic thromboembolic disease should be considered, and serve as a valuable screening test for this disease. In a single-center, retrospective survey comparing V/Q scanning with CT angiography in 227 pulmonary hypertensive patients, there was a sensitivity of 97.4 % for V/Q scanning compared to 51% for CT angiography in the detection of chronic thromboembolic disease.[25] In a more recent study of 12 CTEPH patients, Soler and colleagues demonstrated that SPECT perfusion scintigraphy was more sensitive in detecting obstructed vascular segments when compared to CT pulmonary angiography, with a sensitivity of 62+4.1% versus 47.8+2.9%, respectively.[26] Furthermore, the interpretation of an abnormal perfusion pattern can assist in the differentiation between disorders involving the central or proximal vascular bed from those primarily affecting the peripheral pulmonary circulation. In chronic thromboembolic disease, at least one, but more commonly several, segmental or larger mismatched perfusion defects are present (Fig. 1). For those patients with small-vessel pulmonary vascular disease, perfusion scans either are normal or exhibit a “mottled” appearance characterized by nonsegmental defects.[27,28] Exceptions include cases of pulmonary veno-occlusive disease or pulmonary capillary hemangiomatosis in which multiple, larger mismatched defects have been reported.[29,30] Equally important has been the observation that a relatively normal perfusion pattern on V/Q scan excludes the diagnosis of surgically accessible chronic thromboembolic disease.
Figure 1.
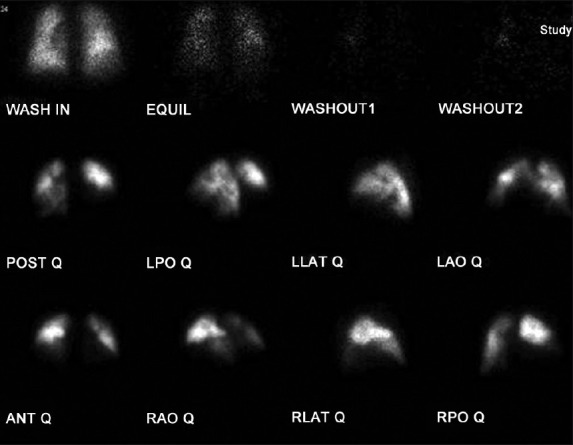
Lung ventilation-perfusion scan showing large, bilateral unmatched perfusion defects; no perfusion to right middle and lower lobes.
It has also been established that the magnitude of perfusion defects exhibited by CTEPH patients with operable disease may understate the degree of pulmonary vascular obstruction determined by angiography.[31] The plausible explanation for this finding is that during the process of thrombus organization, proximal vessel thromboemboli may recannalize, or narrow the vessel in such a manner that radiolabeled macroaggregated albumin may traverse the area of partial obstruction, creating gray zones or regions of relative hypoperfusion. Therefore, chronic thromboembolic disease should be considered and further evaluation for operable disease should proceed even if the V/Q scan demonstrates a limited number of mismatched perfusion defects, especially when accompanied by hypoperfused lung regions in a patient with pulmonary hypertension.
ASSESSMENT OF PULMONARY HEMODYNAMICS
Right heart catheterization in the evaluation of patients with suspected CTEPH objectively defines the severity of pulmonary hypertension and the degree of cardiac dysfunction at rest. This hemodynamic assessment is important in discussions with patients regarding perioperative risks should they prove to be surgical candidates. Available data would suggest that patients with severe pulmonary hypertension (pulmonary vascular resistance >1,000 dyn/s/cm-5) bear a greater perioperative mortality risk.
Hartz et al. reported that a preoperative PVR over 1,100 dyn/s/cm-5 was associated with 41% mortality, compared to less than 6% if PVR was less than 1,100 dyn/s/cm-5.[32] Dartevelle et al. reported an increased postoperative mortality of 20% for patients with preoperative PVR over 1,200 dyn/s/cm-5 compared to 4% mortality if the preoperative PVR was less than 900 dyn/s/cm-5.[33] Similarly, examining outcomes in 743 patients between 1999 and 2004, Thistlethwaite and colleagues reported a perioperative mortality rate of 10.8% in those patients with a preoperative PA systolic pressure of 100 mmHg (PVR 1299.0 + 532.6 dyn/s/cm-5), compared to 4.2% if the preoperative PA systolic pressure was less than 100 mmHg (PVR 546.4 + 365.1 dyn/s/cm-5).[34]
Additionally, for symptomatic CTEPH patients with modest pulmonary hypertension at rest, exercise hemodynamic measurements may be obtained. In these cases, it is likely that the normal compensatory mechanisms of recruitment and dilation of the pulmonary vasculature have been overcome, and with exercise, a linear elevation in pulmonary artery pressure as cardiac output increases can be observed. This hemodynamic information provides objective evidence to explain an individual's symptoms, and likely reflects a clinically relevant stage in the development of severe CTEPH in which there is coexisting, small-vessel hypertensive changes.
Furthermore, right heart catheterization has the potential to provide objective data in analyzing the degree of this small-vessel disease, information which may help predict outcomes following endarterectomy. “Partitioning” the different elements (proximal vs. distal) of pulmonary vascular resistance in CTEPH has been investigated. In a small series of 26 CTEPH patients, Kim and colleagues, utilizing pulmonary artery occlusion waveform analysis, demonstrated excellent inverse correlation between the percent upstream resistance and postoperative mean pulmonary artery pressure and pulmonary vascular resistance. In addition, all four deaths in this series occurred in patients in whom the upstream resistance was less than 60%.[35] If future investigations validate these preliminary observations, this information may identify a subgroup of CTEPH patients who might be excluded from surgical consideration.
CONVENTIONAL PULMONARY ANGIOGRAPHY
Prior to the availability of CT angiography and magnetic resonance imaging of the chest, conventional pulmonary angiography was the principal means of confirming the diagnosis of chronic thromboembolic disease and assessing the proximal extent of disease in evaluating patients for pulmonary endarterectomy surgery. In most respects, it remains the “gold standard” for achieving these diagnostic goals and in providing a “map” for surgery against which other modalities are to be measured. Conventional pulmonary angiography can be safely performed, when taking proper precautions, even in severe pulmonary hypertensive patients.[36] In terms of technique, multiple, selective injections are not required. A single injection of nonionic contrast into both proximal pulmonary arteries, the volume and injection rate adjusted based on cardiac output, appears to be sufficient. As little as 15–20 ml of contrast may be required for each pulmonary artery injection. Ideally, biplane acquisition provides optimal anatomic detail, the lateral projection providing more definition of lobar and segmental anatomy than can be achieved with an anterior–posterior view alone. Under essentially all circumstances, a properly performed biplane angiogram will provide sufficient information on which to base a decision regarding chronic thrombus location, and as a result, surgical accessibility.
The angiographic appearance of chronic thromboembolic disease bears little resemblance to that of the well-defined, intraluminal filling defects of acute pulmonary embolism. Instead, the angiographic patterns encountered in chronic thromboembolic disease reflect the complex patterns of organization and recanalization that occur following an acute thromboembolic event. Several angiographic patterns have been described in chronic thromboembolic disease which correlate with the material removed at the time of surgery.[37] These include “pouch defects,” pulmonary artery webs or bands, intimal irregularities, abrupt, frequently angular narrowing of the major pulmonary arteries, and complete obstruction of main, lobar, or segmental vessels at their point of origin (Fig. 2). In the majority of CTEPH patients, two or more of these angiographic findings are present, typically involving both lungs.
Figure 2.
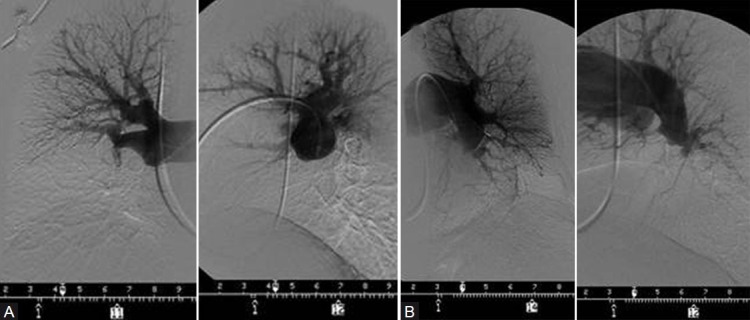
(A) PA and lateral right pulmonary angiogram of the patient whose V/Q scan is shown in Figure 1; complete obstruction of the right interlobar vessel. (B) PA and lateral left pulmonary angiogram, showing a “pouch” occlusion of the descending pulmonary vessel beyond the superior segment; appreciated on the lateral view is a small lingular artery which is difficult to discern A B on AP view.
CT ANGIOGRAPHY OF THE CHEST
With the advances in CT angiography of the chest, and greater availability and use of this technology in assessing the pulmonary vascular bed, CT is playing an increased role in the evaluation of the pulmonary hypertensive patient for chronic thromboembolic disease. There are a number of CT findings which have been described in patients with chronic thromboembolic disease. These include (1) mosaic perfusion of the lung parenchyma; (2) enlargement of the central pulmonary arteries and right heart chambers; (3) variability in the size of lobar and segmental-level vessels with a reduction in vessel caliber of those involved with chronic thrombi; and (4) peripheral, scar-like lesions in poorly perfused lung regions. With contrast enhancement of the pulmonary vasculature during CT imaging, organized thrombus can be seen to line the pulmonary vessels, often in an eccentric manner (Fig. 3). Associated narrowing of pulmonary arteries, web strictures, “pouch defects,” and other irregularities of the intima may also be appreciated[38,39] (Fig. 4); these CT findings are distinct from the intraluminal filling defects of acute thromboemboli and primary pulmonary vascular tumors.[40] And with appropriate timing of the intravenous contrast bolus for CTA, opacification of the pulmonary and systemic circulations is possible. In addition to the pulmonary vascular bed, this allows examination of a number of cardiac features including cardiac chamber size, position, and shape of the interventricular septum, the presence of congenital cardiac abnormalities, anomalous pulmonary venous drainage, and the size and distribution of collateral vessels arising from the systemic arterial circulation (bronchial arteries off the aorta, coronary vessels).[41]
Figure 3.
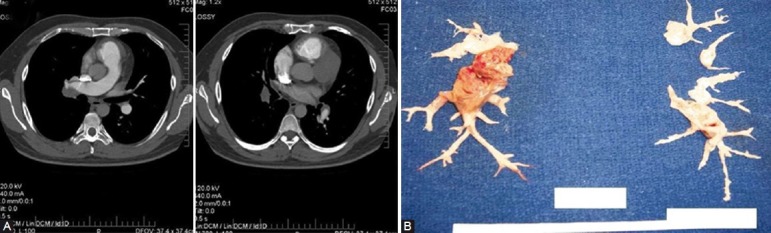
(A) Accompanying CT angiogram to the studies in Figures 1 and 2. Lining and occlusive chronic thromboembolic material observed in the right interlobar and descending PA; lining thrombus involving the left descending PA. (B) Semiorganized and chronic thromboembolic material endarterectomized from the patient.
Figure 4.
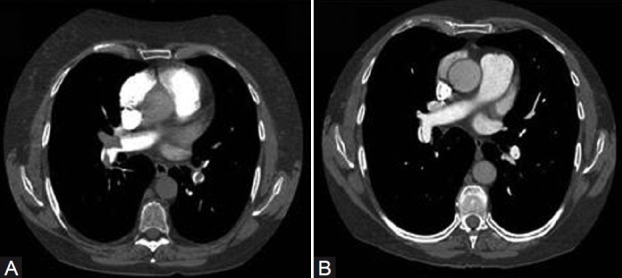
CT angiogram demonstrating the evolution of an acute thrombus to an intravascular chronic “web” in the proximal right descending PA at the level of the right middle lobe take-off. The time interval between the left (A) and right (B) images is 1 year.
What remains unvalidated is the utility of CT angiography in determining operability in certain subgroups of CTEPH patients. This is particularly important as operative techniques allow for resection of chronic thromboembolic material at the segmental vessel level (Fig. 5). Additionally, clinical experience has demonstrated that the absence of lining thrombus or thickened intima of the central vessels on CT does not exclude the diagnosis of chronic thromboembolic disease or the possibility of surgical intervention. Studies directly comparing CT with pulmonary angiography are limited. In one such study, CT and digital angiography were nearly equivalent in terms of identifying complete vessel occlusion at the segmental level. However, for nonocclusive changes, CT was significantly inferior to angiography.[42] Accuracy of CT scanning has improved with technological advances and the introduction of 64-detector row scanners. In a preliminary study involving 27 patients with suspected CTEPH, sensitivity of 64-dectector row CT was 98.3% at the main and lobar level and 94% at the segmental level when compared to digital subtraction angiography.[43]
Figure 5.
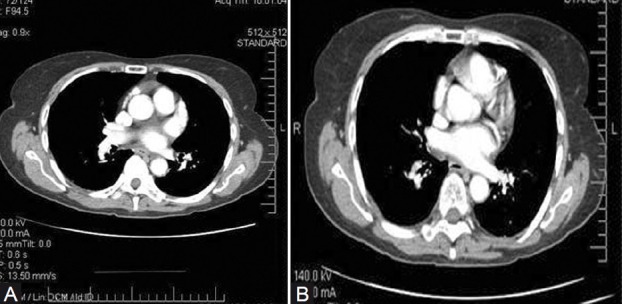
Two images from a CT angiogram demonstrating irregular intraluminal chronic thrombus and vessel narrowing of the segmental vessels. These lesions proved to be surgically resectable.
There is considerable value for CT in detecting disorders of the pulmonary parenchyma and mediastinum. For CTEPH patients with coexisting interstitial lung disease or emphysema, CT will be able to define the extent and location of the parenchymal lung process. Reperfusion of diseased lung parenchyma following an endarterectomy may result in an undesirable postoperative outcome, and thereby exclude a patient from surgical consideration. And for those patients whose V/Q scan demonstrates the absence or near complete absence of perfusion to an entire lung, CT is an essential study to rule out extrinsic pulmonary vascular compression from mediastinal adenopathy, fibrosis,[44] or neoplasm.[45]
MAGNETIC RESONANCE OF THE CHEST
Experience using magnetic resonance (MR) imaging and magnetic resonance angiography (MRA) to visualize the pulmonary vascular system in patients with chronic thromboembolic pulmonary hypertension is expanding.[46] For centers where conventional pulmonary angiography is either unavailable or felt to be too risky to perform, the evolving information on MR imaging as an alternative means to determine surgical candidacy for CTEPH patients is encouraging. At Papworth Hospital, UK, MRA has replaced conventional angiography in establishing the pulmonary vascular “map” for patients evaluated for endarterectomy surgery.[47] Kreitner and colleagues have shown that contrast-enhanced MRA is able to demonstrate the vascular changes typical for CTE disease. In a study of 34 CTEPH patients, wall-adherent thromboembolic material involving the central pulmonary arteries down to the segmental level could be demonstrated; intraluminal webs and bands, as well as abnormal vessel tapering and “cutoffs” were also detected. Furthermore, they showed that MRA was superior to digital subtraction angiography in determining the proximal location of resectable chronic thromboembolic material.[48] An additional study comparing magnetic resonance techniques with conventional contrast angiography involved 29 patients with either CTEPH or idiopathic pulmonary arterial hypertension (IPAH). Nikolaou and colleagues showed that the combined interpretation of MR perfusion imaging and MR angiography led to a correct diagnosis of IPAH or CTEPH in 26 (90%) of 29 patients when compared to the reference diagnosis based on V/Q scintigraphy, digital subtraction angiography, or CT angiography. The interpretation of MR angiography alone had a sensitivity of 71% for wall adherent thrombi, 50% for webs and bands, and between 83% and 86% for detection of complete vessel obstruction and free-floating thrombi when compared to DSA or CT angiography.[49] More recently, in a retrospective study of 53 patients with chronic thromboembolic pulmonary hypertension, the diagnostic accuracy of contrast-enhanced MR angiography (CE-MRA) and unenhanced proton MR imaging was compared to CT pulmonary angiography. The sensitivity and specificity of CE-MRA in establishing proximal and distal CTE was 98% and 94%, respectively. The sensitivity for central vessel disease rose from 50% to 88% when analysis was performed with unenhanced proton MRA. Keeping in mind that this study was comparing MR technology to CT angiography, the detection of stenotic lesions, poststenotic dilatation, and occlusive lesions was better achieved with CE-MRA.[50]
As with CT, there are other features of magnetic resonance imaging that have been demonstrated to be useful in the evaluation of CTEPH patients. Cine imaging allows an assessment of RV and LV function, providing data on end-systolic and end-diastolic volumes, ejection fraction, and muscle mass.[51,52] Furthermore, phase contrast imaging may be used to measure cardiac output, along with pulmonary and systemic arterial flow. In CTEPH patients undergoing PEA, this technique has been used to measure changes in aortic and pulmonary arterial blood flow before and after surgery.[48,53]
SUMMARY: SELECTION OF SURGICAL CANDIDATES
In the evaluation of patients with CTEPH, the goals are to establish whether or not pulmonary endarterectomy is feasible, and then to determine whether or not surgery is appropriate. And despite the advancements in imaging techniques, and the forward strides in surgical capabilities, there remains a subjective element, which to a large extent is influenced by experience, in determining surgical candidacy for any one CTEPH patient. The interpretion of conventional angiographic patterns, CT abnormalities, or MR findings that are felt to be consistent with operable CTE disease is not simply based on training and experience but needs to be viewed in the context of the capabilities of the surgical team. This is especially relevant given the greater ability and success in the resection of segmental level chronic thromboembolic disease. More difficult to predict are the factors that influence perioperative mortality and postoperative outcomes. The impact of an individual's age and comorbid medical conditions on surgical risk is always difficult to assess. And for any individual patient, the level of cardiopulmonary and functional limitation experienced by them is an important consideration in the decision to proceed with surgery, which needs to be balanced against the anticipation that a hemodynamic benefit will result from an endarterectomy. If a meaningful reduction in pulmonary pressures seems unlikely, proceeding with surgery is ill-advised. This prediction of hemodynamic benefit is often based on the degree of coexisting, small-vessel disease and whether the extent of surgically accessible chronic thromboembolic disease is disproportionate to the level of pulmonary hypertension and RV dysfunction experienced by the patient. To date, this assessment is to a large extent subjective and based on the experience of the evaluation team, making it increasingly important to develop evaluative techniques to make it less so. Ongoing research and careful clinical observations are required to make this decision as precise as possible.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Klok F, van Kralingen KW, van Dijk AP, Heyning FH, Vliegen HW, Huisman MV. Prospective cardiopulmonary screening program to detect chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Haematologica. 2010;95:970–5. doi: 10.3324/haematol.2009.018960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pengo V, Lensing AW, Prins MH, Marchiori A, Davidson BL, Tiozzo F, et al. Thromboembolic pulmonary hypertension study group.Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257–64. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- 3.Korkmaz A, Ozlu T, Ozsu S, Kazaz Z, Bulbul Y. Long-term outcomes in acute pulmonary thromboembolism: The incidence of chronic thromboembolic pulmonary hypertension and associated risk factors. Clin Appl Thromb Hemost. 2012;18:281–8. doi: 10.1177/1076029611431956. [DOI] [PubMed] [Google Scholar]
- 4.Lang IM. Chronic thromboembolic pulmonary hypertension – not so rare after all. N Engl J Med. 2004;350:2236–8. doi: 10.1056/NEJMp048088. [DOI] [PubMed] [Google Scholar]
- 5.Bonderman D, Jakowitsch J, Adlbrecht C, Schemper M, Kyrle PA, Schonauer V, et al. Medical conditions increasing the risk of chronic thromboembolic pulmonary hypertension. Thromb Haemost. 2005;93:512–6. doi: 10.1160/TH04-10-0657. [DOI] [PubMed] [Google Scholar]
- 6.Condliffe R, Kiely DG, Gibbs JS, Corris PA, Peacock AJ, Jenkins DP, et al. Prognostic and aetiological factors in chronic thromboembolic pulmonary hypertension. Eur Resp J. 2009;33:332–8. doi: 10.1183/09031936.00092008. [DOI] [PubMed] [Google Scholar]
- 7.Riedel M, Stanek V, Widimsky J, Prerovsky I. Long-term follow-up of patients with pulmonary thromboembolism: late prognosis and evolution of hemodynamic and respiratory data. Chest. 1982;81:151–8. doi: 10.1378/chest.81.2.151. [DOI] [PubMed] [Google Scholar]
- 8.Lewczuk J, Piszko P, Jagas J, Porada A, Wojciak S, Sobkowicz B, et al. Prognostic factors in medically treated patients with chronic pulmonary embolism. Chest. 2001;119:818–23. doi: 10.1378/chest.119.3.818. [DOI] [PubMed] [Google Scholar]
- 9.Condliffe R, Kiely DG, Gibbs JS, Corris PA, Peacock AJ, Jenkins DP, et al. Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2008;177:1122–7. doi: 10.1164/rccm.200712-1841OC. [DOI] [PubMed] [Google Scholar]
- 10.Auger WR, Moser KM. Pulmonary flow murmurs: a distinctive physical sign found in chronic pulmonary thromboembolic disease. Clin Res. 1989;37:145A. [Google Scholar]
- 11.Wolf M, Boyer-Neumann C, Parent F, Eschwege V, Jaillet H, Meyer D, et al. Thrombotic risk factors in pulmonary hypertension. Eur Resp J. 2000;15:395–9. doi: 10.1034/j.1399-3003.2000.15b28.x. [DOI] [PubMed] [Google Scholar]
- 12.Colorio CC, Martinuzzo ME, Forastiero RR, Pombo G, Adamczuk Y, Carreras LO. Thrombophilic factors in chronic thromboembolic pulmonary hypertension. Blood Coagul Fibrinolysis. 2001;12:427–32. doi: 10.1097/00001721-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Bonderman D, Wilkens H, Wakounig S, Schafers HJ, Jansa P, Lindner J, et al. Risk factors for chronic thromboembolic pulmonary hypertension. Eur Respir J. 2009;33:325–31. doi: 10.1183/09031936.00087608. [DOI] [PubMed] [Google Scholar]
- 14.Bonderman D, Turecek PL, Jakowitsch J, Weltermann A, Adlbrecht C, Schneider B, et al. High prevalence of elevated clotting factor VIII in chronic thromboembolic pulmonary hypertension. Thromb Haemost. 2003;90:372–6. doi: 10.1160/TH03-02-0067. [DOI] [PubMed] [Google Scholar]
- 15.Morris TA, Auger WR, Ysrael MZ, Olson LK, Channick RN, Fedullo PF, et al. Parenchymal scarring is associated with restrictive spirometric defects in patients with chronic thromboembolic pulmonary hypertension. Chest. 1996;110:399–403. doi: 10.1378/chest.110.2.399. [DOI] [PubMed] [Google Scholar]
- 16.Steenhuis LH, Groen HJ, Koeter GH, van der Mark TW. Diffusion capacity and haemodynamics in primary and chronic thromboembolic pulmonary hypertension. Eur Respir J. 2000;16:276–81. doi: 10.1034/j.1399-3003.2000.16b15.x. [DOI] [PubMed] [Google Scholar]
- 17.van der Plas MN, Reesink HJ, Roos CM, van Steenwijk RP, Kloek JJ, Bresser P. Pulmonary endarterectomy improves dyspnea by the relief of dead space ventilation. Ann Thorac Surg. 2010;89:347–52. doi: 10.1016/j.athoracsur.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Kapitan KS, Buchbinder M, Wagner PD, Moser KM. Mechanisms of hypoxemia in chronic thromboembolic pulmonary hypertension. Am Rev Respir Dis. 1989;139:1149–54. doi: 10.1164/ajrccm/139.5.1149. [DOI] [PubMed] [Google Scholar]
- 19.Woodruff III WW, Hoeck BE, Chitwood WR, Jr, Lyerly HK, Sabiston DC, Jr, Chen JT. Radiographic findings in pulmonary hypertension from unresolved embolism. Am J Roentgenol. 1985;144:681–6. doi: 10.2214/ajr.144.4.681. [DOI] [PubMed] [Google Scholar]
- 20.Satoh T, Kyotani S, Okano Y, Nakanishi N, Kunieda T. Descriptive patterns of severe chronic pulmonary hypertension by chest radiography. Respir Med. 2005;99:329–36. doi: 10.1016/j.rmed.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Surie S, Gibson NS, Gerdes VE, Bouma BJ, van Eck-Smit BL, Buller HR, et al. Active search for chronic thromboembolic pulmonary hypertension does not appear indicated after acute pulmonary embolism. Thromb Res. 2010;125:e202–5. doi: 10.1016/j.thromres.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Blanchard DG, Malouf PJ, Gurudevan SV, Auger WR, Madani MM, Thistlethwaite P, et al. Utility of right ventricular Tei index in the noninvasive evaluation of chronic thromboembolic pulmonary hypertension before and after pulmonary thromboendarterectomy. J Am Coll Cardiol Img. 2009;2:143–9. doi: 10.1016/j.jcmg.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Dittrich HC, McCann HA, Blanchard DG. Cardiac structure and function in chronic thromboembolic pulmonary hypertension. Am J Card Imaging. 1994;8:18–27. [PubMed] [Google Scholar]
- 24.Mahmud E, Sadehgi HM, Raisinghani A, Hassankhani A, Strachan GM, Auger WR, et al. Correlation of left ventricular diastolic filling characteristics with right ventricular overload and pulmonary artery pressure in chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2002;40:318–24. doi: 10.1016/s0735-1097(02)01959-9. [DOI] [PubMed] [Google Scholar]
- 25.Tunariu N, Gibbs SJ, Win Z, Gin-Sing W, Graham A, Gishen P, et al. Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic disease as a treatable cause of pulmonary hypertension. J Nucl Med. 2007;48:680–4. doi: 10.2967/jnumed.106.039438. [DOI] [PubMed] [Google Scholar]
- 26.Soler X, Kerr KM, Marsh JJ, Renner JW, Hoh CK, Test VJ, et al. Pilot study comparing SPECT perfusion scintigraphy with CT pulmonary angiography in chronic thromboembolic pulmonary hypertension. Respirology. 2012;17:180–4. doi: 10.1111/j.1440-1843.2011.02061.x. [DOI] [PubMed] [Google Scholar]
- 27.Lisbona R, Kreisman H, Novales-Diaz J, Derbekyan V. Perfusion lung scanning: differentiation of primary from thromboembolic pulmonary hypertension. Am J Roentgenol. 1985;144:27–30. doi: 10.2214/ajr.144.1.27. [DOI] [PubMed] [Google Scholar]
- 28.Powe JE, Palevsky HI, McCarthy KE, Alavi A. Pulmonary arterial hypertension: value of perfusion scintigraphy. Radiology. 1987;164:727–30. doi: 10.1148/radiology.164.3.3615869. [DOI] [PubMed] [Google Scholar]
- 29.Bailey CL, Channick RN, Auger WR, Fedullo PF, Kerr KM, Yung GL, et al. “High probability” perfusion lung scans in pulmonary venoocclusive disease. Am J Respir Crit Care Med. 2000;162:1974–8. doi: 10.1164/ajrccm.162.5.2003045. [DOI] [PubMed] [Google Scholar]
- 30.Rush C, Langleben D, Schlesinger RD, Stern J, Wang NS, Lamoureux E. Lung scintigraphy in pulmonary capillary hemangiomatosis: a rare disorder causing primary pulmonary hypertension. Clin Nucl Med. 1991;16:913–7. doi: 10.1097/00003072-199112000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Ryan KL, Fedullo PF, Davis GB, Vasquez TE, Moser KM. Perfusion scan findings understate the severity of angiographic and hemodynamic compromise in chronic thromboembolic pulmonary hypertension. Chest. 1988;93:1180–5. doi: 10.1378/chest.93.6.1180. [DOI] [PubMed] [Google Scholar]
- 32.Hartz RS, Byme JG, Levitsky S, Park J, Rich S. Predictors of mortality in pulmonary thromboendarterectomy. Ann Thorac Surg. 1996;62:1255–60. doi: 10.1016/0003-4975(96)00460-2. [DOI] [PubMed] [Google Scholar]
- 33.Darteville P, Fadel E, Mussot S, Chapelier A, Herve P, de Perrot M, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2004;23:637–48. doi: 10.1183/09031936.04.00079704. [DOI] [PubMed] [Google Scholar]
- 34.Thistlethwaite P, Kemp A, Du L, Madani MM, Jamieson SW. Outcomes of pulmonary thromboendarterectomy for treatment of extreme thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg. 2006;131:307–13. doi: 10.1016/j.jtcvs.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 35.Kim HS, Fesler P, Channick RN, Knowlton KU, Ben-Yehuda O, Lee SH, et al. Preoperative partitioning of pulmonary vascular resistance correlates with early outcome after thromboendarterectomy for chronic thromboembolic pulmonary hypertension. Circulation. 2004;109:18–22. doi: 10.1161/01.CIR.0000111841.28126.D4. [DOI] [PubMed] [Google Scholar]
- 36.Pitton MB, Duber C, Mayer E, Thelen M. Hemodynamic effects of nonionic contrast bolus injection and oxygen inhalation during pulmonary angiography in patients with chronic major-vessel thromboembolic pulmonary hypertension. Circulation. 1996;94:2485–91. doi: 10.1161/01.cir.94.10.2485. [DOI] [PubMed] [Google Scholar]
- 37.Auger WR, Fedullo PF, Moser KM, Buchbinder M, Peterson KL. Chronic major-vessel chronic thromboembolic pulmonary artery obstruction: Appearance at angiography. Radiology. 1992;183:393–8. doi: 10.1148/radiology.182.2.1732955. [DOI] [PubMed] [Google Scholar]
- 38.Castaner E, Gallardo X, Ballasteros E, Andreu M, Pallardo Y, Mata JM, et al. CT diagnosis of chronic pulmonary thromboemboliam. Radiographics. 2009;29:31–50. doi: 10.1148/rg.291085061. [DOI] [PubMed] [Google Scholar]
- 39.Willemink MJ, van Es HW, Koobs L, Morshuis WJ, Snijder RJ, van Hesewijk JP. CT evaluation of chronic thromboembolic pulmonary hypertension. Clin Radiol. 2012;67:277–85. doi: 10.1016/j.crad.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Kerr KM. Pulmonary artery sarcoma masquerading as chronic thromboembolic pulmonary hypertension. Nat Clin Pract Cardiovasc Med. 2005;2:108–12. doi: 10.1038/ncpcardio0118. [DOI] [PubMed] [Google Scholar]
- 41.McKie SJ, Hardwick DJ, Reid JH, Murchison JT. Features of cardiac disease demonstrated on CT angiography. Clin Radiol. 2005;60:31–8. doi: 10.1016/j.crad.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Pitton MB, Kemmerich G, Herber S, Schweden F, Mayer E, Thelen M. Chronic thromboembolic pulmonary hypertension: Diagnostic impact of multislice CT and selective pulmonary DSA. Roto. 2002;174:474–9. doi: 10.1055/s-2002-25117. [DOI] [PubMed] [Google Scholar]
- 43.Reichelt A, Hoeper MM, Galanski M, Keberle M. Chronic thromboembolic pulmonary hypertension: Evaluation with 64-detector row CT versus digital subtraction angiography. Eur J Radiol. 2009;71:49–54. doi: 10.1016/j.ejrad.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Rossi SE, McAdams HP, Rosado-de-Christenson ML, Franks TJ, Galvin JR. Fibrosing mediastinitis. Radiographics. 2001;21:737–57. doi: 10.1148/radiographics.21.3.g01ma17737. [DOI] [PubMed] [Google Scholar]
- 45.Shields JJ, Cho KJ, Geisinger KR. Pulmonary artery constriction by mediastinal lymphoma simulating pulmonary embolus. AJR Am J Roentgenol. 1980;135:147–50. doi: 10.2214/ajr.135.1.147. [DOI] [PubMed] [Google Scholar]
- 46.Kreitner KF, Kunz RP, Ley S, Oberholzer K, Neeb D, Gast KK, et al. Chronic thromboembolic pulmonary hypertension assessment by magnetic resonance imaging. Eur Radiol. 2007;17:11–21. doi: 10.1007/s00330-006-0327-x. [DOI] [PubMed] [Google Scholar]
- 47.Coulden R. State-of-the-art imaging techniques in chronic thromboembolic pulmonary hypertension. Proc Am Thorac Soc. 2006;3:577–83. doi: 10.1513/pats.200605-119LR. [DOI] [PubMed] [Google Scholar]
- 48.Krietner KF, Ley S, Kauczor HU, Mayer E, Kramm T, Pitton MB, et al. Chronic thromboembolic pulmonary hypertension: pre- and post-operative assessment with breath-hold magnetic resonance imaging techniques. Radiology. 2004;232:535–43. doi: 10.1148/radiol.2322030945. [DOI] [PubMed] [Google Scholar]
- 49.Nikolaou K, Schoenberg SO, Attenberger U, Scheidler J, Dietrich O, Kuehn B, et al. Pulmonary arterial hypertension: Diagnosis with fast perfusion imaging and high-spatial-resolution MR angiography - Preliminary experience. Radiology. 2005;236:694–703. doi: 10.1148/radiol.2361040502. [DOI] [PubMed] [Google Scholar]
- 50.Rajaram S, Swift AJ, Capener D, Telfer A, Davies C, Hill C, et al. Diagnostic accuracy of contrats-enhanced MR angiography and unenhanced proton MR imaging compared with CT pulmonary angiography in chronic thromboembolic pulmonary hypertension. Eur Radiol. 2012;22:310–7. doi: 10.1007/s00330-011-2252-x. [DOI] [PubMed] [Google Scholar]
- 51.Alfakih K, Reid S, Jones T, Sivananthan M. Assessment of ventricular function and mass by cardiac magnetic resonance imaging. Eur Radiol. 2004;14:1813–22. doi: 10.1007/s00330-004-2387-0. [DOI] [PubMed] [Google Scholar]
- 52.Beygui F, Furber A, Delepine S, Helft G, Metzger JP, Geslin P, et al. Routine breath-hold gradient echo MRI-derived right ventricular mass, volumes and function: accuracy, reproducibility and coherence study. Int J Cardiovasc Imaging. 2004;20:509–16. doi: 10.1007/s10554-004-1097-7. [DOI] [PubMed] [Google Scholar]
- 53.Miller FN, Coulden RA, Sonnex E, Pepke-Zaba J, Dunning J. The use of MR flow mapping in the assessment of pulmonary artery blood flow following pulmonary thrombo-endarterectomy. Radiology, RSNA Proceedings. 2003:462P. [Google Scholar]


