Abstract
Pulmonary hypertension (PH) is commonly seen in patients who present with left ventricular diastolic dysfunction (LVDD) and is considered a marker of poor prognosis. While PH in this setting is thought to result from pulmonary venous congestion, there is a subset of patients in which pulmonary pressures fail to improve with appropriate management of diastolic heart failure and go on to develop a clinical picture similar to that of patients with pulmonary arterial hypertension (PAH). Despite the utility of Doppler echocardiography and exercise testing in the initial evaluation of patients with suspected PH-LVDD, the diagnosis can only be confirmed using right heart catheterization. Management of PH-LVDD centers on both optimizing fluid management and afterload reduction to reducing left ventricular diastolic pressures and also increase pulmonary venous return. To date, there is no clear evidence that addition of PH-specific drugs can improve clinical outcomes, and their use should only be considered in the setting of clinical trials. In conclusion, PH-LVDD remains a challenging clinical entity that complicates the management of left ventricular dysfunction and significantly contributes to its morbidity and mortality. Determination of the optimal diagnostic and treatment strategies for this form of PH should be the goal of future studies.
Keywords: congestive heart failure, pulmonary hypertension, hemodynamics, echocardiography, therapeutics
Pulmonary hypertension (PH) is often associated with left heart failure.[1] The 2008 revised WHO classification recognizes PH associated with left heart disease as a unique disease category (WHO Class II) that is distinct from other forms of PH such as those associated with pulmonary arterial hypertension (WHO Group I), hypoxic lung (WHO Group III), and chronic thromboembolic diseases (WHO Group IV).[2,3] Awareness of this form of PH is relevant to practitioners as heart failure is the most common cause of PH in the United States. For instance, it has been estimated that over five million people in the United States suffer from heart failure and over 500,000 cases are newly diagnosed each year.[4,5] Congestive heart failure (CHF) is also the most common admission diagnosis among the elderly population and a growing source of significant morbidity and mortality for this age group[6] Given the progressive rise in cases of CHF over the last decade, it is likely that PH associated with heart failure will become the most prevalent form of PH seen in the clinical setting.
While most cases of heart failure are thought to be due to depressed systolic function, about 40–50% of symptomatic patients have preserved ejection fractions and are diagnosed with heart failure with preserved ejection fraction (HFPEF) or left ventricular diastolic dysfunction (LVDD)[7–9] In contrast to systolic heart failure, the diagnosis of LVDD is challenging as no clear diagnostic criteria or definite noninvasive tests to assess diastolic function are currently available; thus, the impact of the current management strategies on mortality is questionable. Among known risk factors, aging is strongly correlated with development of LVDD.[6,10,11] With normal aging, there is progressive development of ventricular stiffening and reduced relaxation,[12,13] which may predispose to development of LVDD in patients who also suffer from chronic conditions such as ischemic cardiomyopathy, hypertension, or diabetes. However, as the epidemic of diabetes and systemic hypertension continues to grow and the population ages, it is likely that incidence and prevalence of LVDD will also continue to increase.[6]
A recent study by Lam and colleagues has demonstrated that the prevalence of PH in the setting of LVDD is high. Using Doppler echocardiography in a group of 244 patients with LVDD, it was found that the prevalence of PH (defined as pulmonary artery systolic pressure or PASP of >35 mmHg) was estimated to be 83% with a median PASP of 48 mmHg. Moreover, just as with post myocardial infarction[14,15] and idiopathic dilated cardiomyopathy,[16] the presence of PH in this group was a shown marker for increased mortality[14] (Fig. 1). In addition, it has been proposed that there is a subset of these patients who exhibit PH “out of proportion” to the degree of left ventricular dysfunction and suffer from a form of PH which resembles pulmonary arterial hypertension (WHO Group I); whether these patients have a worse prognosis or respond differently to heart failure therapy, however, is unknown.[15,17,18]
Figure 1.
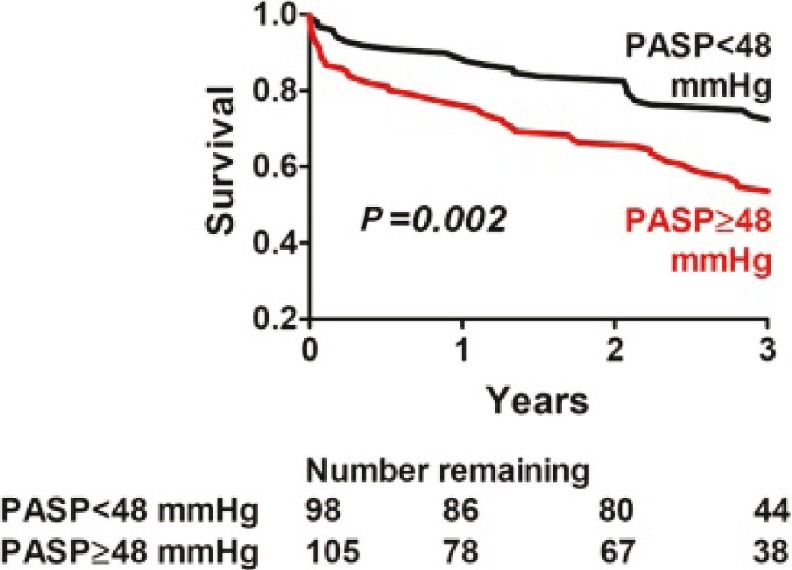
Survival of patients with PH-LVDD is inversely correlated to degree of pulmonary artery systolic pressure (PASP) elevation (Adapted from Lam et al[14]).
An understanding of the pathophysiology of PH in the setting of LVDD is mandatory prior to deciding on the optimal diagnosis and treatment strategies. In this review, we will summarize the current evidence for the various diagnostic studies and therapies available for PH and LVDD and will present an approach that will hopefully facilitate the proper management of patients with these conditions.
PATHOPHYSIOLOGY OF PH-LVDD
PH-LVDD is a consequence of abnormally elevated left ventricular diastolic pressures (LVDP) and pulmonary venous congestion.[19] In LVDD, the curve for LV diastolic pressure in relation to volume is shifted upward and to the left with a resultant increase in diastolic LV filling. Elevation of LVDP is the result of both abnormal active relaxation and increased passive stiffness of the left ventricle.[6,8,11,15,18,20] The face that both mechanisms play an active role in the pathophysiology of elevated LVDP was demonstrated in a study in which, despite correction of slow relaxation, LV pressures failed to normalize as a result of increased chamber stiffness.[21] Given the decreased pressure gradient between the left ventricle and the pulmonary venous circulation, venous return is reduced and pulmonary venous pressures increase resulting in progressive distension and damage to the pulmonary veins.[22,23] In this setting, patients can progress to pulmonary edema and alveolar hemorrhage unless the LVDP is reduced with targeted therapy. Moreover, just as with idiopathic dilated cardiomyopathy[16] and post myocardial infarction[14,15], the presence of PH in this group was a shown marker of increased mortality (Fig. 1).[14]
While most cases of PH-LVDD result from passive venous congestion, a subset of patients suffer from a more severe form of PH-LVDD characterized by findings of increased vascular tone and abnormal pulmonary artery remodeling. Vasoconstriction is thought to be secondary to endothelin release from the pulmonary endothelium in response to venous congestion and subsequent smooth muscle contraction in the neighboring medial layer. While potentially reversible if treated early, vasospasm may progress to a “remodeling” of the pulmonary arteries possibly leading to irreversible vascular disease. The vascular pathology in these cases can be indistinguishable from WHO Class I PAH and is postulated to occur from changes in the elastic fibers of the pulmonary arterial wall, intimal fibrosis, and medial hypertrophy. In addition, abnormal thickening of the veins and neointima formation can also be seen in these cases. In contrast to WHO Class I patients, there is great potential for reversibility of these changes in PH-LVDD with specific treatment of LVDD and improvement of pulmonary venous hypertension.[15,18,24]
DIAGNOSIS OF PH-LVDD
Clinically, patients with PH-LVDD usually present with worsening dyspnea, weight gain, and progressive limitation in exercise capacity. In contrast to patients with PAH, patients with PH-LVDD may also complain of orthopnea and paroxysmal nocturnal dyspnea. The physical examination may reveal signs of fluid retention such as peripheral edema, ascites, and inspiratory crackles; additionally, the cardiac examination typically will reveal presence of an S3, distented jugular veins, and hepatojugular reflux. Further clinical clues that can help differentiate PAH from PH-LVDD include the presence of left atrial enlargement, left rather than right ventricular hypertrophy on EKG, and the finding of Kerley B lines, pleural effusion, and pulmonary edema on a chest X-ray.[22]
Despite the wealth of information that can be obtained through the use of history, physical examination, and CXR, it can still be difficult for the clinician to differentiate between systolic and diastolic left ventricular impairment without further investigation. Among the imaging studies available for the evaluation of patients with possible PH-LVDD, transthoracic echocardiography (TTE) provides invaluable insight on the anatomy and function of the left ventricle and can help differentiate between systolic and diastolic failure (Fig. 2). Current diagnostic criteria for diastolic dysfunction center on estimating left ventricular filling pressures or abnormal diastolic filling profile. The most useful criteria include left atrial volume, mitral inflow filling profile and tissue Doppler annular velocities profiles. In addition, TTE can provide an estimate of the pulmonary pressures via measurement of right ventricular systolic pressure (RVSP), and can also help identify cardiac valve problems that can also impact left ventricular function.[3,8,15,24] Using the information provided by TTE, several groups have attempted to reach a consensus definition for LVDD. In 1998, for example, the European Society of Cardiology proposed the following criteria for the diagnosis of LVDD: (1) presence of signs and symptoms of CHF; (2) normal or mildly abnormal left ventricular systolic dysfunction defined as EF>45%; and (3) evidence of abnormal left ventricular relaxation, filling, distensibility, or diastolic stiffness.[25] While these criteria attempt to resolve the confusion surrounding the diagnosis of LVDD, there is still an ongoing debate on whether cases of combined systolic and diastolic dysfunction can be reliably differentiated and whether TTE alone is enough to predict left ventricular dysfunction. For this reason, some experts have advocated for routine measurements of left ventricular diastolic filling to determine the presence of diastolic dysfunction. This can be done using the E/A ratio, a measure that incorporates passive left ventricular filling during diastole (E) and active filling following left atrial contraction (A). In normal hearts, the E/A ratio can be between 0.7 and 1.4; however, in the setting of diastolic dysfunction, this ratio reverses and values become less than 0.7.[4] Finally, there are studies showing that even the single presence of an abnormally elevated left atrial pressure[26] can serve as a reliable indicator for LVDD.
Figure 2.
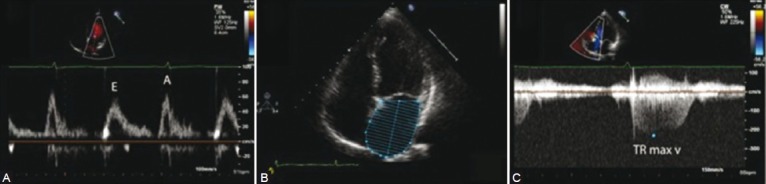
Echocardiographic findings in PH-LVDD. (A) Measurement of passive left ventricular filling during diastole (E) and active filling following left atrial contraction (A). In the setting of LVDD, ventricular filling by left atrial contraction becomes prominent and the E/A ratio is reversed (B) Distended left atrium and (C) increased tricuspid regurgitation jet in a patient with PH-LVDD.
Despite both its ability to visualize cardiac function noninvasively in real time and also its availability, there are significant limitations to the diagnostic potential of TTE in PH-LVDD. These limitations are due to the fact that TTE is both operator dependent and its reproducibility may vary from center to center. In addition, because pulmonary hypertension itself produces diastolic filling abnormalities in the LV, Doppler echocardiography cannot be relied on to distinguish between Class 1 and 2 PH. Furthermore, proper visualization of the left ventricle in some patients may be technically difficult and severely limit measurement of functional parameters.[27] Thus, given the technical limitations of TTE, confirmation of the diagnosis of PH-LVDD will require further testing.
Since its discovery in 1988, brain natriuretic peptide (BNP) has been shown to be both a sensitive and specific biomarker of altered left ventricular structure and function that increases under of atrial and/or ventricular pressure overload and with cardiomyocyte hypertrophy.[28–32] While its role of patients with systolic CHF has been demonstrated in multiple studies, the use of plasma BNP in the evaluation and management of LVDD is less clear. A major barrier to the routine use of BNP in this setting is the lack of an appropriate threshold value that can help distinguish between left ventricular hypertrophy and diastolic dysfunction or failure. This was shown in a European study where 1,678 patients were screened for diastolic dysfunction using measurements of serum BNP. In this study, despite its high negative predictive value (99.9%), plasma BNP in the detection of diastolic dysfunction had an estimated sensitivity and specificity of 61 and 55%, respectively.[33] The poor performance of the BNP test is likely related to a number of confounding factors within the target population such as presence of LVH and age. However, while age alone can increase plasma BNP, elderly patients with isolated diastolic dysfunction are likely to have higher BNP levels with advanced filling abnormalities on TTE than those with milder forms of diastolic dysfunction.[34] While use of plasma BNP measurements alone may be of limited diagnostic utility, its combination with other modalities can result in an increased diagnostic yield. Studies have shown positive correlation between BNP and echocardiographic measures of diastolic filling (i.e., E/A ratio, see above) at rest and at peak exercise.[35,36] Thus, while alterations in BNP seem to correlate with LVDD, the role of plasma BNP measurements in diagnosis need to be established further.
In recent years, more centers have started to use cardiopulmonary exercise testing (CPET) in the diagnosis of both systolic and diastolic LV failure. Since most patients with PH-LVDD will experience worsening symptoms with exercise, the CPET provides a unique opportunity to study cardiopulmonary interactions, measure left ventricular reserve, and determine whether a pulmonary vascular limit to exercise is present. Furthermore, in patients with no obvious diastolic filling abnormalities at rest, the CPET may help confirm the diagnosis and reveal the contribution of pulmonary, hematologic, musculoskeletal, and neurologic components to the patient's clinical picture.[18,37–39] Despite the advantages offered by the CPET, it is a labor-intensive study that few centers can perform routinely and most patients will need referral to more specialized clinics, an event that can delay the diagnosis of this condition.
Currently, the gold standard for diagnosis of PH and LVDD is right heart catheterization. In the presence of an elevated pulmonary capillary wedge pressure (PCWP) and a normal ejection fraction, a diagnosis of pulmonary venous hypertension secondary to LVDD should be strongly considered (Fig. 3). In patients in which determination of a PCWP is either technically difficult or when the presence of other conditions such as atrial myxoma, mediastinal fibrosis, or pulmonary vein stenosis may be creating false positive results, direct measurement of left ventricular end diastolic pressure (LVEDP) should be performed. Finally, it should be pointed out that changes in intrathoracic pressures affect PCWP and LVEDP measurements and reliable values can only be obtained during end expiration.[2,3,15,24]
Figure 3.
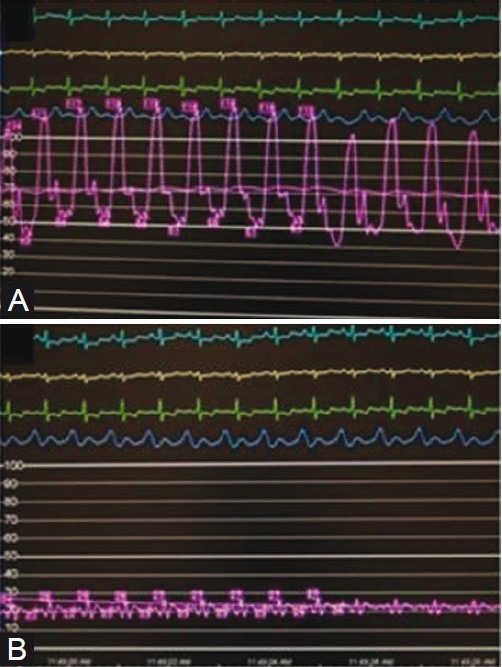
Hemodynamic profile of a patient with PH-LVDD. (A) Patient demonstrates a mean pulmonary artery pressure (in magenta) of approximately 70 mmHg in the setting of a pulmonary capillary wedge pressure (in magenta) of approximately 20 mmHg (B).
While an elevated PCWP and LVEDP in the setting of a preserved ejection fraction argues strongly in favor of LVDD, some patients can present with symptoms of LVDD without evidence of elevated PCWP and LVEDP. In these patients, differentiation of pulmonary venous from arterial hypertension is very difficult and will require the use of additional diagnostic studies to elicit evidence of diastolic dysfunction such as an exercise, fluid, or inotropic challenge.[15,40,41] Under these conditions, patients with PAH may increase their cardiac output without a significant change in the PCWP while those with PH-LVDD will show both an increase in cardiac output and PCWP supporting the presence of pulmonary venous hypertension.
As alluded to previously, there appears to be two types of patients with PH-LVDD: those whose PH is explained solely by the increase in pulmonary venous pressures; and those who, despite improvement of LV function and fluid status, will demonstrate evidence of persistent PH. In the first group, PH is a compensatory response to increase the pressure gradient between the pulmonary veins and the left ventricle, while in the second group there appears to be a combination of pulmonary arterial and venous hypertension.[14] Despite the absence of firm diagnostic criteria, it may be possible to distinguish these two disease phenotypes by using the transpulmonary gradient (TPG), defined as the difference between the mean pulmonary artery pressure (mPAP) and the PCWP. In patients with PH-LVDD secondary to passive venous congestion, the TPG will be <10 given that both mPAP and PCWP increase to a similar degree. In contrast, patients with PH-LVDD with a superimposed pulmonary arterial component will display TPG that can range between 25 and 35 mmHg, an increase that is considered to be “out of proportion” to what is expected from pulmonary venous hypertension alone (Fig. 3).[15,18] Given that a proposed component in the pathology of elevated TPG is pulmonary vasoconstriction, some experts have recommended the routine performance of vasodilator testing in these patients; however, whether the presence of a vasodilator response in this group of patients would carry the same prognostic and therapeutic implications as in cases of PAH is unclear at this time.
TREATMENT OF PH-LVDD
Despite the high prevalence of LVDD in the general population, few therapies have been systematically studied and their impact survival remains unproven. Initial management is centered on improving symptoms related to volume overload with the use of oral and/or intravenous diuretics. Given that most patients with LVDD suffer from systemic hypertension, aggressive control of blood pressure should be attempted with a goal of achieving less than 130/85 mmHg. Medications such as the ACE inhibitors, angiotensin receptor blockers (ARBS), beta blockers and calcium channel blockers can induce regression of left ventricular hypertrophy and improve diastolic relaxation and ventricular filling.[4,6,23]
The presence of PH has been shown to be a marker of poor prognosis and increased mortality in patients with heart failure.[18,42,43] For those patients in whom the PH fails to improve despite aggressive management of LVDD as well as for those who suffer from the “out of proportion” form of PH-LVDD, the optimal management remains unclear. Currently, there are no FDA-approved medications for the management of PH-LVDD, and the efficacy and safety of PAH specific therapies for this indication has not been critically evaluated. Available studies using prostanoid agents to treat patients with CHF have revealed conflicting data that limit enthusiasm for their routine use in this patient population. For example, one study found that, in patients with PH and end-stage CHF preparing for cardiac transplantation, inhaled iloprost was as safe and effective as nitric oxide in reducing pulmonary pressures preoperatively.[44] Several studies using IV epoprostenol have shown acute improvement in left ventricular ejection fraction,[45,46] but the duration of this effect in the long-term has not been evaluated. While these studies suggest that prostanoids can improve cardiac function in CHF patients, there is also evidence for significant adverse events in the wake of their use. The Flolan International Randomized Survival Trial (FIRST) was designed to study the long-term impact of epoprostenol (i.e., flolan) therapy on patients with refractory CHF. This trial was stopped prematurely as a result of an increased mortality in the treatment arm which was thought to be a consequence of epoprostenol-induced increased venous hypertension and worsening PCWP.[47] While most patients in the previously mentioned studies were diagnosed with systolic CHF, it is possible that these adverse effects could also occur in PH-LVDD patients in which prostanoid therapies are introduced. Therefore, at this time, we advise against using prostanoids to treat PH in the setting of systolic and/or diastolic heart failure until further characterization is undertaken.
Endothelin receptor antagonists (ERAs) and phosphodiesterase 5 (PDE5) inhibitors are two classes of oral therapies used in the management of patients with WHO Class 1 PAH. As with prostanoids, there have been a few studies that have looked at their impact on left ventricular performance in patients with heart failure. The Endothelin A Receptor Antagonist Trial in Heart Failure Trial recruited 642 patients who had LVEF less than 35% and persistent symptoms of CHF despite use of standard CHF therapies and randomized them to either placebo or the ERA darusentan. After 24 weeks, there was no evidence of regression in left ventricular remodeling nor improvement in left ventricular function.[48] Another study using bosentan to treat patients with severe CHF was terminated early as a result of increased liver toxicity and failure of the drug to demonstrate any clinical benefit.[48] At present, a study looking at the safety and efficacy of bosentan in patients with diastolic heart failure and secondary PH (BADDHY trial, NCT00820352) is currently enrolling participants and should hopefully help clarify the role of ERAs in PH-LVDD.
Of all the FDA-approved therapies for treatment of PAH, the PDE5 inhibitors hold the most promise for management of PH-LVDD. In a small case series, patients with PH and left heart disease treated with the PDE5 inhibitor sildenafil demonstrated a reduction in pulmonary artery pressures and an increase in exercise capacity without changing cardiac index or PCWP.[49] A more recent study by the same group showed that in a group of 45 patients with stable systolic failure treated with sildenafil for one year, there was evidence for improved diastolic filling and regression of LV hypertrophy and left atrial distension.[50,51] While these data are very encouraging, more systematic studies are required prior to recommending the routine use of PDE5 agents on PH-LVDD. Of note, a Phase II/III randomized double-blind clinical trial looking at the impact of sildenafil in pulmonary hemodynamics in patients with PH-LVDD over a 1-year period (NCT01156636) has been completed and the data are currently being analyzed.
CONCLUDING REMARKS
PH-LVDD is a unique form of PH that is associated with poor prognosis and for which the optimal management strategy remains to be determined. Evaluation and approach to management of these patients are further complicated by the existence of patients whose PH is a consequence of elevated pulmonary congestion and those who may have both pulmonary arterial and venous pathology, leading to more severe PH, and possibly worse outcomes. While the early diagnostic approach can include a careful history, physical examination, performance of a TTE and/or exercise study, the only way to firmly establish the diagnosis of PH-LVDD is by right heart catheterization. Initial management of PH-LVDD should focus on optimizing blood pressure and fluid status followed by reassessment of pulmonary pressures to gauge the impact of these interventions on pulmonary hemodynamics. At this time, the use of PH-specific therapies in patients who fail to demonstrate improvement in PH with CHF management is undetermined. Use of these agents (prostanoids, ERAs, and PDE5 inhibitors) should only be done as part of clinical studies designed to evaluate their efficacy in controlling PH with LVDD.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Oudiz RJ. Secondary Pulmonary Hypertension. Curr Treat Options Cardiovasc Med. 2001;3:115–24. doi: 10.1007/s11936-001-0067-9. [DOI] [PubMed] [Google Scholar]
- 2.Hoeper MM. Definition, classification, and epidemiology of pulmonary arterial hypertension. Semin Respir Crit Care Med. 2009;30:369–75. doi: 10.1055/s-0029-1233306. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Shammas RL, Khan NU, Nekkanti R, Movahed A. Diastolic heart failure and left ventricular diastolic dysfunction: What we know, and what we don’t know! Int J Cardiol. 2007;115:284–92. doi: 10.1016/j.ijcard.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 5.Garg R, Packer M, Pitt B, Yusuf S. Heart failure in the 1990s: evolution of a major public health problem in cardiovascular medicine. J Am Coll Cardiol. 1993;22:3A–5A. doi: 10.1016/0735-1097(93)90454-9. [DOI] [PubMed] [Google Scholar]
- 6.Kitzman DW. Diastolic heart failure in the elderly. Heart Fail Rev. 2002;7:17–27. doi: 10.1023/a:1013745705318. [DOI] [PubMed] [Google Scholar]
- 7.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 8.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, et al. How to diagnose diastolic heart failure: A consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–50. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 9.Abhayaratna WP, Marwick TH, Smith WT, Becker NG. Characteristics of left ventricular diastolic dysfunction in the community: an echocardiographic survey. Heart. 2006;92:1259–64. doi: 10.1136/hrt.2005.080150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis. 2005;47:320–32. doi: 10.1016/j.pcad.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Sanders D, Dudley M, Groban L. Diastolic dysfunction, cardiovascular aging, and the anesthesiologist. Anesthesiol Clin. 2009;27:497–517. doi: 10.1016/j.anclin.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sagie A, Benjamin EJ, Galderisi M, Larson MG, Evans JC, Fuller DL, et al. Reference values for Doppler indexes of left ventricular diastolic filling in the elderly. J Am Soc Echocardiogr. 1993;6:570–6. doi: 10.1016/s0894-7317(14)80174-0. [DOI] [PubMed] [Google Scholar]
- 13.Scalia GM, Khoo SK, O’Neill S. Age-related changes in heart function by serial echocardiography in women aged 40-80 years. J Womens Health (Larchmt) 2010;19:1741–5. doi: 10.1089/jwh.2009.1752. [DOI] [PubMed] [Google Scholar]
- 14.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–26. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rich S, Rabinovitch M. Diagnosis and treatment of secondary (non- category 1) pulmonary hypertension. Circulation. 2008;118:2190–9. doi: 10.1161/CIRCULATIONAHA.107.723007. [DOI] [PubMed] [Google Scholar]
- 16.Grzybowski J, Bilinska ZT, Ruzyllo W, Kupść W, Michalak E, Szcześniewska D, et al. Determinants of prognosis in nonischemic dilated cardiomyopathy. J Card Fail. 1996;2:77–85. doi: 10.1016/s1071-9164(96)80026-1. [DOI] [PubMed] [Google Scholar]
- 17.Hoeper MM, Oudiz RJ, Peacock A, Tapson VF, Haworth SG, Frost AE, et al. End points and clinical trial designs in pulmonary arterial hypertension: clinical and regulatory perspectives. J Am Coll Cardiol. 2004;43:48S–55S. doi: 10.1016/j.jacc.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Oudiz RJ. Pulmonary hypertension associated with left-sided heart disease. Clin Chest Med. 2007;28:233–41. doi: 10.1016/j.ccm.2006.12.001. x. [DOI] [PubMed] [Google Scholar]
- 19.Rogers WB, Prisant LM, Houghton JL, Frank MJ. Congestive heart failure with normal ejection fraction.A different mechanism requiring different therapy. Postgrad Med. 1992;91:207–14. doi: 10.1080/00325481.1992.11701374. [DOI] [PubMed] [Google Scholar]
- 20.Moller JE, Hillis GS, Oh JK, Pellikka PA. Prognostic importance of secondary pulmonary hypertension after acute myocardial infarction. Am J Cardiol. 2005;96:199–203. doi: 10.1016/j.amjcard.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 21.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure-abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–9. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 22.Desai A. Current understanding of heart failure with preserved ejection fraction. Curr Opin Cardiol. 2007;22:578–85. doi: 10.1097/HCO.0b013e3282f02116. [DOI] [PubMed] [Google Scholar]
- 23.Haney S, Sur D, Xu Z. Diastolic heart failure: a review and primary care perspective. J Am Board Fam Pract. 2005;18:189–98. doi: 10.3122/jabfm.18.3.189. [DOI] [PubMed] [Google Scholar]
- 24.Hoeper MM, Barbera JA, Channick RN, Hassoun PM, Lang IM, Manes A, et al. Diagnosis, assessment, and treatment of non-pulmonary arterial hypertension pulmonary hypertension. J Am Coll Cardiol. 2009;54:S85–96. doi: 10.1016/j.jacc.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 25.How to diagnose diastolic heart failure. European Study Group on Diastolic Heart Failure. Eur Heart J. 1998;19:990–1003. doi: 10.1053/euhj.1998.1057. [DOI] [PubMed] [Google Scholar]
- 26.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90:1284–9. doi: 10.1016/s0002-9149(02)02864-3. [DOI] [PubMed] [Google Scholar]
- 27.Picard MH, Popp RL, Weyman AE. Assessment of left ventricular function by echocardiography: a technique in evolution. J Am Soc Echocardiogr. 2008;21:14–21. doi: 10.1016/j.echo.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Ritchie RH, Rosenkranz AC, Kaye DM. B-type natriuretic peptide: endogenous regulator of myocardial structure, biomarker and therapeutic target. Curr Mol Med. 2009;9:814–25. doi: 10.2174/156652409789105499. [DOI] [PubMed] [Google Scholar]
- 29.Mohammed AA, Januzzi JL., Jr Natriuretic peptides in the diagnosis and management of acute heart failure. Heart Fail Clin. 2009;5:489–500. doi: 10.1016/j.hfc.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Januzzi JL, Bayes-Genis A. Evolution of amino-terminal pro-B type natriuretic peptide testing in heart failure. Drug News Perspect. 2009;22:267–73. doi: 10.1358/dnp.2009.22.5.1378643. [DOI] [PubMed] [Google Scholar]
- 31.Mohammed AA, Januzzi JL., Jr Natriuretic peptide guided heart failure management. Curr Clin Pharmacol. 2009;4:87–94. doi: 10.2174/157488409788184945. [DOI] [PubMed] [Google Scholar]
- 32.van Kimmenade RR, Januzzi JL., Jr The evolution of the natriuretic peptides - Current applications in human and animal medicine. J Vet Cardiol. 2009;11(Suppl 1):S9–21. doi: 10.1016/j.jvc.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Lukowicz TV, Fischer M, Hense HW, Döring A, Stritzke J, Riegger G, et al. BNP as a marker of diastolic dysfunction in the general population: Importance of left ventricular hypertrophy. Eur J Heart Fail. 2005;7:525–31. doi: 10.1016/j.ejheart.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Saul L, Shatzer M. B-type natriuretic peptide testing for detection of heart failure. Crit Care Nurs Q. 2003;26:35–9. doi: 10.1097/00002727-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Mottram PM, Haluska BA, Marwick TH. Response of B-type natriuretic peptide to exercise in hypertensive patients with suspected diastolic heart failure: Correlation with cardiac function, hemodynamics, and workload. Am Heart J. 2004;148:365–70. doi: 10.1016/j.ahj.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 36.Mottram PM, Leano R, Marwick TH. Usefulness of B-type natriuretic peptide in hypertensive patients with exertional dyspnea and normal left ventricular ejection fraction and correlation with new echocardiographic indexes of systolic and diastolic function. Am J Cardiol. 2003;92:1434–8. doi: 10.1016/j.amjcard.2003.08.053. [DOI] [PubMed] [Google Scholar]
- 37.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–54. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–95. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lam CS, Grewal J, Borlaug BA, Ommen SR, Kane GC, McCully RB, et al. Size, shape, and stamina: The impact of left ventricular geometry on exercise capacity. Hypertension. 2010;55:1143–9. doi: 10.1161/HYPERTENSIONAHA.109.146845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Costard-Jackle A, Fowler MB. Influence of preoperative pulmonary artery pressure on mortality after heart transplantation: Testing of potential reversibility of pulmonary hypertension with nitroprusside is useful in defining a high risk group. J Am Coll Cardiol. 1992;19:48–54. doi: 10.1016/0735-1097(92)90050-w. [DOI] [PubMed] [Google Scholar]
- 41.Nootens M, Wolfkiel CJ, Chomka EV, Rich S. Understanding right and left ventricular systolic function and interactions at rest and with exercise in primary pulmonary hypertension. Am J Cardiol. 1995;75:374–7. doi: 10.1016/s0002-9149(99)80557-8. [DOI] [PubMed] [Google Scholar]
- 42.Costanzo MR, Augustine S, Bourge R, Bristow M, O’Connell JB, Driscoll D, et al. Selection and treatment of candidates for heart transplantation.A statement for health professionals from the Committee on Heart Failure and Cardiac Transplantation of the Council on Clinical Cardiology, American Heart Association. Circulation. 1995;92:3593–612. doi: 10.1161/01.cir.92.12.3593. [DOI] [PubMed] [Google Scholar]
- 43.Grady KL, Jalowiec A, White-Williams C, Pifarre R, Kirklin JK, Bourge RC, et al. Predictors of quality of life in patients with advanced heart failure awaiting transplantation. J Heart Lung Transplant. 1995;14:2–10. [PubMed] [Google Scholar]
- 44.Braun S, Schrotter H, Schmeisser A, Strasser RH. Evaluation of pulmonary vascular response to inhaled iloprost in heart transplant candidates with pulmonary venous hypertension. Int J Cardiol. 2007;115:67–72. doi: 10.1016/j.ijcard.2006.01.067. [DOI] [PubMed] [Google Scholar]
- 45.Virgolini I, Kaliman J, Fitscha P, O’Grady J, Rogatti W, Sinzinger H. Beneficial effect of long-term PGE1-treatment in left ventricular heart failure. Prostaglandins Leukot Essent Fatty Acids. 1989;38:177–80. doi: 10.1016/0952-3278(89)90069-0. [DOI] [PubMed] [Google Scholar]
- 46.Auinger C, Virgolini I, Weissel M, Bergmann H, Sinzinger H. Prostacyclin I2 (PGI2) increases left ventricular ejection fraction (LVEF) Prostaglandins Leukot Essent Fatty Acids. 1989;36:149–54. doi: 10.1016/0952-3278(89)90054-9. [DOI] [PubMed] [Google Scholar]
- 47.Califf RM, Adams KF, McKenna WJ, Gheorghiade M, Uretsky BF, McNulty SE, et al. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: The Flolan International Randomized Survival Trial (FIRST) Am Heart J. 1997;134:44–54. doi: 10.1016/s0002-8703(97)70105-4. [DOI] [PubMed] [Google Scholar]
- 48.Anand I, McMurray J, Cohn JN, Konstam MA, Notter T, Quitzau K, et al. Long-term effects of darusentan on left-ventricular remodelling and clinical outcomes in the Endothelin – A Receptor Antagonist Trial in Heart Failure (EARTH): Randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:347–54. doi: 10.1016/S0140-6736(04)16723-8. [DOI] [PubMed] [Google Scholar]
- 49.Bocchi EA, Guimaraes G, Mocelin A, Bacal F, Bellotti G, Ramires JF. Sildenafil effects on exercise, neurohormonal activation, and erectile dysfunction in congestive heart failure: A double-blind, placebo-controlled, randomized study followed by a prospective treatment for erectile dysfunction. Circulation. 2002;106:1097–103. doi: 10.1161/01.cir.0000027149.83473.b6. [DOI] [PubMed] [Google Scholar]
- 50.Guazzi M, Tumminello G, Di Marco F, Fiorentini C, Guazzi MD. The effects of phosphodiesterase-5 inhibition with sildenafil on pulmonary hemodynamics and diffusion capacity, exercise ventilatory efficiency, and oxygen uptake kinetics in chronic heart failure. J Am Coll Cardiol. 2004;44:2339–48. doi: 10.1016/j.jacc.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 51.Guazzi M, Tumminello G, Di Marco F, Guazzi MD. Influences of sildenafil on lung function and hemodynamics in patients with chronic heart failure. Clin Pharmacol Ther. 2004;76:371–8. doi: 10.1016/j.clpt.2004.06.003. [DOI] [PubMed] [Google Scholar]


