Abstract
Evidence suggests that leptin is involved in relevant processes in the cardiovascular system. Low serum leptin levels have been associated with increased cardiovascular events and mortality in patients with coronary artery, diabetes, or chronic kidney disease. We hypothesized that leptin is increased in pulmonary arterial hypertension (PAH) and provides prognostic information. We correlated leptin levels with clinical data and assessed its association with survival. Sixty-seven patients with PAH and 29 healthy controls were studied. Plasma leptin levels were nonlinearly associated with BMI. Leptin level <15 μg/l was associated with higher mortality in PAH patients, with an adjusted (age, gender, BMI, and smoking status) hazard ratio of 3.8 (95% CI: 1.3-11.2), P=0.016. Similarly, PAH patients with leptin/BMI ratio <0.5 μg * m2/kg * l had worse survival than those with a level >0.5 μg * m2/ kg * l (P=0.046 by log-rank test). Two-year mortality in PAH patients was 24%. A receiver operating characteristic curve using leptin/BMI ratio as the test variable and 2-year mortality as the state variable showed an area under the curve of 0.74 (95% CI: 0.62–0.86). A leptin/BMI ratio cut-off of 0.6 had a high sensitivity (94%) and negative predictive value (96%) for predicting death of any cause at 2 years. In PAH, plasma leptin levels are directly associated with BMI. Lower leptin levels, when adjusted by BMI, are associated with an increased overall mortality and leptin/BMI ratio has high negative predictive value for mortality at 2 years.
Keywords: leptin, mortality, obesity, pulmonary hypertension
Leptin is a neuroendocrine peptide released by adipose tissue which enhances metabolism and acts on the hypothalamus suppressing appetite.[1–2] Mounting evidence suggests that, besides its central role in energy homeostasis regulation, leptin is involved in important processes in the cardiovascular system, including sympathetic activation,[3–4] angiogenesis,[5–7] and endothelial nitric oxide (NO) production.[6,8,9]
Low serum leptin levels have been associated with increased cardiovascular events and mortality in patients with coronary artery disease,[10] diabetes,[11] or chronic kidney disease,[12] independent of obesity.[10] However, no prior investigations have studied the relationship between plasma leptin levels and disease severity and outcomes in pulmonary arterial hypertension (PAH).
On the basis of these observations, we hypothesized that leptin is decreased in PAH and provides prognostic information. To test our hypothesis, we prospectively determined plasma leptin levels in patients with PAH and healthy controls. We correlated leptin levels with clinical, echocardiographic, and hemodynamic data and assessed its association with survival.
MATERIALS AND METHODS
Study population
Patients were recruited from the Pulmonary Vascular Program at Cleveland Clinic. Consecutive patients were asked at the time of right heart catheterization to donate blood for biobank. For the present study, we included all adult (≥ 18 years) PAH patients (n=67) and healthy controls (n=29) who donated blood from January of 2003 to August of 2008. PAH was confirmed by right heart catheterization. All participants signed a consent form that was approved by the Cleveland Clinic Institutional Review Board (IRB) prior to participation in the study.
Clinical evaluation
As part of their clinical evaluation, all patients underwent a comprehensive testing to exclude other etiologies of pulmonary hypertension. Clinical and outcome information were prospectively collected.
Height and weight were obtained at the time of the plasma leptin determination. We defined “current smoker” as an individual who was smoking at the time of the inclusion in the study, "ex-smoker" as a person who had not smoked for at least three months prior to leptin determination, and “nonsmoker” as a subject who smoked less than 20 packs in his or her lifetime.
Clinical transthoracic Doppler echocardiography was performed with commercially available equipment in the left lateral recumbent position. Experienced operators, blinded to the results of leptin levels, reviewed the echocardiograms and assessed right ventricular size and function subjectively. Right ventricular systolic pressure was estimated by the Bernoulli equation with addition of right atrial pressure estimated by assessing inferior vena cava size and inspiratory collapse.[13–14] Right heart catheterization was performed in the standard manner using a 7F pulmonary artery catheter. Pressure measurements were obtained at end-expiration. Cardiac output (CO) was obtained by the thermodilution method; additionally, transpulmonary gradient (mean PAP – pulmonary artery occlusion pressure) and pulmonary vascular resistance (transpulmonary gradient/CO) were calculated.
Plasma leptin levels
Fasting venous blood was obtained in the morning of the right heart catheterization. Plasma leptin levels were determined in duplicate by enzyme-linked immunosorbent assay (ELISA; R&D Systems catalog # DY398, Minneapolis, MN, USA). The mean intra-assay variation (standard deviation [SD]) was 3% (4%). Laboratory personnel were blinded to patient characteristics and outcomes. In 53 patients, brain natriuretic peptide (BNP) was also determined in the same blood sample.
Statistics
Continuous variables were summarized using mean and standard deviation. We compared two-group numerical variables by mean difference, and 95% confidence interval by bootstrapping (2,000 repetitions). We compared categorical variables with Pearson's chi-square test. We tested associations involving the leptin plasma level using linear regression, adjusted for independent variables known to be associated with this measurement (age, gender, BMI, and smoking status).[15–17] We used binary logistic regression to test whether leptin was different in PAH versus controls, adjusted by age, gender, BMI, and smoking status. We utilized the restrictive cubic spline (three knots with fixed percentiles at 10%, 50%, and 90% of the distribution) and Lowess model to test nonlinear association between leptin level and BMI (Fig. 1).
Figure 1.
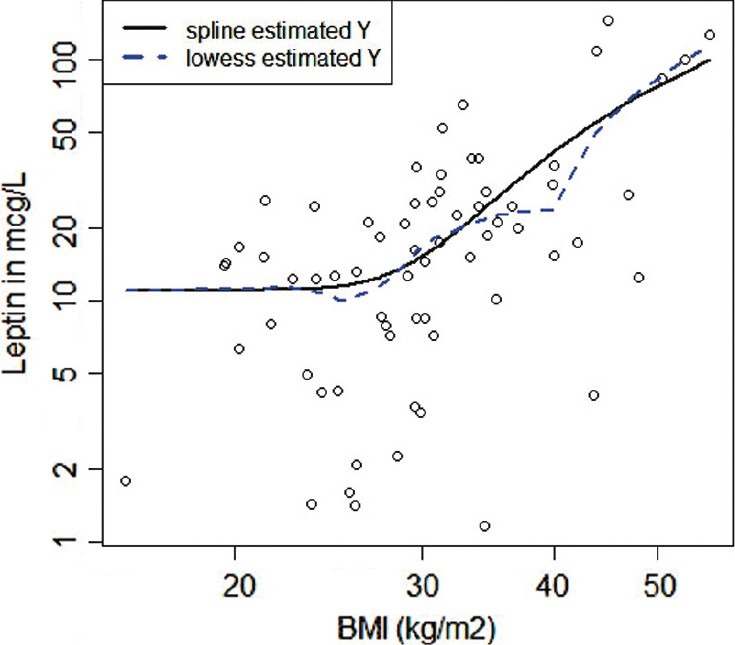
Scatterplot of leptin level and BMI. Both axes in the plot are logged. Restrictive cubic spline (black, solid) and Lowess (gray, dash) lines are displayed.
Overall survival was analyzed by the Kaplan–Meier method. Time 0 was the date of leptin determination. Censoring was performed at the time of transplantation or end of the study (20 July 2011). The date of death of the study participants was ascertained by reviewing patient records or by querying the U.S. Social Security Death Index. No patient was lost to follow-up. Survival differences between patients with and without PAH were assessed by the log-rank test and Cox regression analysis. For the log-rank test, leptin plasma levels were dichotomized using the median as cut-off. The Cox proportional hazards model was adjusted for age, gender, and smoking status; BMI was included when we used leptin level instead of leptin/BMI ratio. In addition to the aforementioned covariates, we tested Cox models that included etiology of PAH (idiopathic/heritable vs. PAH associated with other diseases), NYHA functional class, hemodynamic (right atrial pressure, cardiac output, pulmonary vascular resistance, or transpulmonary gradient), and echocardiographic (right ventricular systolic function) parameters.
Receiver operating characteristic curves (ROC) were used to determine sensitivity, specificity, and positive and negative predictive values for leptin plasma level at different cut-offs (test variable), with 2-year mortality as the state variable. All patients were followed for at least 2 years. All the P values were reported as two tailed. A P value of <0.05 was prespecified as indicative of statistical significance. The statistical analyses were performed using the statistical package SPSS, Version 20 (IBM; Armonk, N.Y., USA), and R version 2.13.0 (The R Foundation for Statistical Computing).[18]
RESULTS
Baseline data
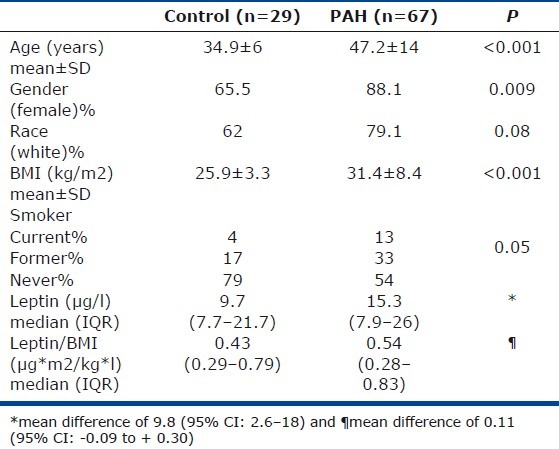
A total of 67 patients with pulmonary arterial hypertension and 29 healthy controls were studied. All patients had a diagnosis of PAH by right heart catheterization.[19] Of patients with PAH, 35 (52%), 6 (9%), and 26 (39%) had idiopathic, heritable, or PAH associated with a variety of conditions, respectively. Conditions associated with PAH were connective tissue disease (n=19; scleroderma: 10; systemic lupus erythematosus: 5; mixed tissue connective disease: 3; dermatomyositis: 1), congenital heart disease (n=4), drug-induced (n=1), hereditary hemorrhagic telangiectasia (n=1), and portal hypertension (n=1). Seventy-six percent of the patients (n=51) were receiving PAH-targeted treatment at the time of the blood sample (monotherapy in 39%, dual therapy in 41%, and triple therapy in 20%). New York Heart Association (NYHA) functional class was I, II, III, and IV in 3%, 33%, 52%, and 12% of the patients, respectively. Characteristics of the patients and control groups are shown in Table 1.
Table 1.
Baseline characteristics of PAH patients and control groups
Plasma leptin determination
Plasma leptin concentration was higher in patients with PAH (median of 15.3 with an interquartile range [IQR] of 7.9–26 μg/l) than in controls (median 9.7 [IQR: 7.7–25.7 μg/l]), with a mean difference of 9.8 (95% CI: 2.6–18) μ/l (Table 1). Leptin was directly associated with BMI with an R of 0.65 (P<0.001), an association that was seen in controls (R=0.41, P=0.03) as well as patients with PAH (R=0.65, P<0.001). The association between leptin and BMI in PAH patients followed a nonlinear relationship best explained by the restrictive cubic spline model (P for nonlinearity=0.005).
No difference was observed in plasma leptin concentration between PAH patients and controls when adjusted by age, gender, BMI, and smoking status (OR: 0.97; 95% CI: 0.93–1.01). Similarly, a nonsignificant difference was observed between the leptin/BMI ratio in PAH (0.54 [IQR: 0.28–0.83]) and controls (0.43 [IQR: 0.29–0.79]) with a mean difference of 0.11 (95% CI: -0.09 to +0.30).
The leptin/BMI ratio was not associated with gender, smoking status, NYHA functional class, cause of PAH (idiopathic/heritable vs. associated with other diseases), heart rate, or systemic blood pressure (data not shown). BNP measurements did not show a significant association with leptin levels or leptin/BMI ratio (P=0.63 and 0.29, respectively). However, the leptin/BMI ratio was lower in patients receiving IV prostanoids versus PH-targeted oral therapies when adjusted by gender, age, and smoking status (adjusted mean difference: -0.37 [95% CI: -0.66 to -0.08]).
Comparison of leptin with 6-minute walk distance
Fifty-one patients completed a 6-Minute Walk Distance within a month of the plasma leptin measurement. The Mean±SD distance walk was 376±129 m or 64.8±19% of predicted.[20] The distance walked in meters or percentage of predicted was not associated with leptin/BMI when adjusted by age, gender, and smoking status (P=0.8 and 0.33, respectively).
Comparison of leptin with echocardiographic parameters
All but six patients (n=61) had an echocardiogram performed within two months of the plasma leptin measurement. The Mean±SD left ventricular ejection fraction was 55±6% with right atrial area of 24.5±8 cm2. The right ventricle (RV) was dilated and dysfunctional in 56 patients (91.8%). Right ventricular dysfunction was normal, mild, moderate, moderate to severe, and severe in 5 (8%), 9 (15%), 17 (28%), 18 (29%), and 12 (20%) of the patients, respectively. The tricuspid jet velocity was 4±0.6 m/s with an estimated right ventricular systolic pressure (RVSP) of 73±20 mmHg. Leptin was not associated with tricuspid jet velocity or estimated RVSP when adjusted by age, gender, BMI, and smoking status (P=0.11 and 0.16, respectively). The degree of RV dysfunction was not associated with leptin level when adjusted by the same covariates (P=0.78).
Comparison of leptin with right heart catheterization parameters
In PAH patients, means±SD of measured parameters were as follows: right atrial pressure 11±7 mmHg; mean pulmonary artery pressure 52±13 mmHg; pulmonary artery occlusion pressure (PAOP) 11±5 mmHg; CO 4.3±1.6 l/m; and pulmonary vascular resistance (PVR) 11.4±8 Wood Units. Mixed venous oxygen saturation was 63±9%. Right atrial pressure, mean pulmonary artery pressure, PAOP, CO, PVR, and mixed venous oxygen saturation did not correlate with plasma leptin levels when adjusted by age, gender, BMI, and smoking history (P=0.3, 0.3, 0.09, 0.8, 0.9, and 0.4, respectively).
Measures of diagnostic accuracy
At 2 years, the mortality in PAH patients was 24%. The leptin/BMI ratio was higher in alive (0.69 [IQR: 0.07–2.1]) versus deceased (0.28 [IQR: 0.06–0.67]) patients with a mean (95% CI) difference of 0.4 (0.21–0.58). The receiver operating characteristic curve using the leptin/BMI ratio as the test variable and 2-year mortality as the state variable showed an area under the curve of 0.74 (95% CI: 0.62–0.86; Fig. 2 and Table 2).
Figure 2.
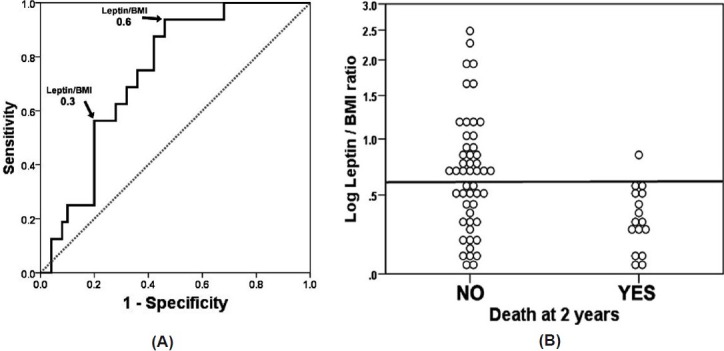
ROC curve of leptin/BMI and dot chart of log leptin/BMI with mortality at 2 years as the classification variable. (A) leptin/BMI measurements are in μg * m2/ kg * l. Area under the curve: 0.74 (95% CI: 0.62-0.86). (B) Dot chart of log leptin/BMI with line placed at a leptin/BMI ratio of 0.6. All but one of PAH patients who died at 2 years had a leptin/BMI ratio <0.6.
Table 2.
Leptin/BMI diagnostic accuracy
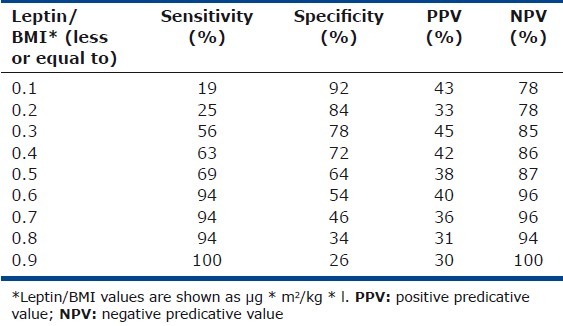
A leptin/BMI ratio of ≤ 0.6 had a high sensitivity (94%) and negative predictive value (96%) for predicting death of any cause at 2 years, with relatively low sensitivity (54%) and positive predictive ability (40%; Table 2). The positive and negative likelihood ratios of this cut-off were 2.04 and 0.12, respectively.
Survival analysis
All control patients were alive at the end of the study. A total of five patients with PAH were transplanted and censored for the analysis. Of the patients with PAH, 23.9% (n=16) died during a mean follow-up of 4.6 years. Causes of death were PAH/RV failure (n=6), respiratory failure (pneumonia [n=1], pulmonary embolism [n=1]), septic shock (n=3), and unknown (n=5). Overall, 1-, 2-, 3-, and 5-year survival was 85%, 76%, 72.9%, and 61.4%, respectively.
When survival was adjusted by age, gender, BMI, and smoking status, a plasma level leptin <15 μg/l was associated with higher mortality, with an adjusted hazard ratio (HR) of 3.8 (95% CI: 1.3–11.2), P=0.016. When leptin was added to the model as a continuous variable, the adjusted HR was 0.96 (95% CI: 0.93-0.99, P=0.024), suggesting that higher levels of leptin appear to be protective in PAH patients.
When we excluded patients that died during the first year to reduce the possibility of reverse causality (i.e., presence of comorbidities leads to low leptin levels and higher mortality[21]), our results were unchanged (HR of 3.8 [95% CI: 1–14.7], P=0.05).
Patients with a leptin/BMI ratio <0.5 μg * m2/kg * l had worse survival with log rank test (P=0.046; Fig. 3, panel A) and Cox regression analysis HR: 3.9, P=0.006 (adjusted by age, gender, and smoking status [95%CI: 1.5 – 10.2; Fig. 3, panel B). Leptin/BMI >0.5 μg * m2/ kg * l remained a significant predictor of survival even after adding to the Cox model the etiology of PAH (idiopathic/heritable vs. PAH associated with other diseases), NYHA functional class, hemodynamic (RA pressure, PVR, and CO), and echocardiographic (RV function) variables (HR of 5.1 [95% CI: 1.5–17.4], P=0.01).
Figure 3.
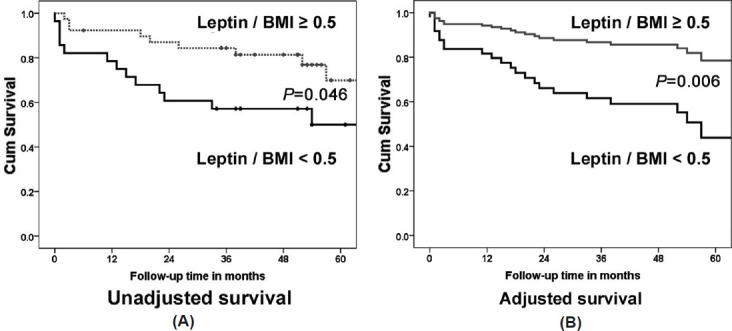
Survival analysis. Kaplan–Meier survival analysis with log-rank test comparing the ratio of leptin/BMI <0.5 and ≥ 0.5 μg * m2/ kg * l (A) and Cox regression analysis using the same cut-off of leptin/BMI adjusted for age, gender, BMI, and smoking status (B).
DISCUSSION
In the present study, we demonstrated that a low plasma leptin concentration (adjusted for BMI and smoking status) is associated with worse survival in PAH patients, independent of other clinical, echocardiographic, or hemodynamic data. In addition, patients on intravenous prostanoids had a lower leptin/BMI ratio than patients only on oral PH-specific treatment.
The plasma level of leptin is proportional to body fat mass in humans and mice,[16–17] a finding that held true in our cohort of patients with PAH. Although PAH patients had higher plasma leptin levels than healthy controls, this difference disappeared when adjusting for BMI, since in our cohort PAH patients had higher BMI than healthy controls.
Mantzoros et al.[15] found that smoking was negatively and independently associated with leptin concentration in healthy men. However, we were not able to find an association between smoking status and plasma leptin levels either in controls or PAH patients. Other factors that inhibit leptin secretion include low energy states with decreased fat stores, fasting, catecholamines, androgens, PPAR-gamma agonists, inflammatory cytokines, and exposure to cool temperature.[22] In our study, all patients were observed at room temperature at time of the blood sample and were not receiving cathecholamines, androgens, or thiazolidinediones.
While we found a lower ratio of leptin/BMI in patients on IV prostanoids versus oral PH-specific therapy as marker of PH severity, we were unable to find an association between leptin or leptin/BMI and BNP measurements, 6-Minute Walk Distance, echocardiographic, or hemodynamic parameters. Similarly, Maruna et al.[23] found no association between preoperative plasma levels of leptin and hemodynamic parameters in patients with chronic thromboembolic pulmonary hypertension.
In the present study, low plasma leptin levels were associated with higher mortality in patients with PAH when adjusted for age, gender, BMI, and smoking status. Similarly, a simple-to-use ratio of leptin/BMI was inversely associated with mortality, raising the possibility that leptin may have a protective effect in PAH in pathways that are independent of obesity.[22] Supporting our findings, low circulating leptin levels have been associated with increased cardiovascular mortality independent of obesity in patients with chronic stable coronary artery disease,[10] diabetes,[11] or chronic kidney disease.[12] However, other studies have shown a direct and independent association between leptin levels and atherosclerosis, hypertension, or thrombosis, underscoring the need to better elucidate the complex relationship between leptin and vascular disease.[24–28]
One potential explanation for the survival advantage observed in patients with higher leptin (adjusted by BMI) may be related to its known cardioprotective role. Leptin has been shown to have a cardioprotective function since it reduces cardiac apoptosis via downregulation of caspase-3 and activation of signal transducer and activator of transcription (STAT)-3 responsive antiapoptotic genes (antiapoptotic bcl-2 and surviving gene).[29] Experimentally induced myocardial infarction in ob/ob mice (leptin deficient) was associated with blunted antiapoptotic (STAT- 3) response which led to a significant increase in morbidity and mortality which was reverted by leptin treatment.[30]
We observed that a leptin/BMI ratio cut-off of 0.6 has a high negative predictive value (96%) for death of any cause at 2 years. Leptin/BMI ratio >0.6 was present in 43% of the patients and only one of them died during the 2-year follow-up period compared with 39.5% in the group with leptin/BMI index ≤ 0.6. These results support further investigation to determine whether leptin/BMI can be used to identify PAH patients who will have a more favorable prognosis.
In conclusion, in PAH, lower leptin levels, when adjusted to BMI, are associated with an increased, overall mortality. The leptin/BMI ratio cut-off of 0.6 has high negative predictive value for mortality at 2 years.
Footnotes
Source of Support: Dr. Adriano Tonelli is supported by CTSA KL2 Grant # RR024990 (A.R.T.) from the National Center for Research Resources (NCRR). Dr. Metin Aytekin is supported by 0826095H from American Heart Association (AHA). Dr. Feldstein is supported by NIH Grants (DK076852) and (DK082451). Dr. Raed Dweik is supported by the following grants: HL081064, HL107147, HL095181, and RR026231 from the National Institutes of Health (NIH), and BRCP 08-049 Third Frontier Program grant from the Ohio Department of Development (ODOD).
Conflict of Interest: None declared.
REFERENCES
- 1.Breslow MJ, Min-Lee K, Brown DR, Chacko VP, Palmer D, Berkowitz DE. Effect of leptin deficiency on metabolic rate in ob/ob mice. Am J Physiol. 1999;276:E443–9. doi: 10.1152/ajpendo.1999.276.3.E443. [DOI] [PubMed] [Google Scholar]
- 2.Campfield LA, Smith FJ, Burn P. The OB protein (leptin) pathway–a link between adipose tissue mass and central neural networks. Horm Metab Res. 1996;28:619–32. doi: 10.1055/s-2007-979867. [DOI] [PubMed] [Google Scholar]
- 3.Haynes WG, Sivitz WI, Morgan DA, Walsh SA, Mark AL. Sympathetic and cardiorenal actions of leptin. Hypertension. 1997;30:619–23. doi: 10.1161/01.hyp.30.3.619. [DOI] [PubMed] [Google Scholar]
- 4.Yang R, Sikka G, Larson J, Watts VL, Niu X, Ellis CL, et al. Restoring leptin signaling reduces hyperlipidemia and improves vascular stiffness induced by chronic intermittent hypoxia. Am J Physiol Heart Circ Physiol. 2011;300:H1467–76. doi: 10.1152/ajpheart.00604.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anagnostoulis S, Karayiannakis AJ, Lambropoulou M, Efthimiadou A, Polychronidis A, Simopoulos C. Human leptin induces angiogenesis in vivo. Cytokine. 2008;42:353–7. doi: 10.1016/j.cyto.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Bouloumie A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res. 1998;83:1059–66. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 7.Sierra-Honigmann MR, Nath AK, Murakami C, García-Cardeña G, Papapetropoulos A, Sessa WC, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–6. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 8.Winters B, Mo Z, Brooks-Asplund E, Kim S, Shoukas A, Li D, et al. Reduction of obesity, as induced by leptin, reverses endothelial dysfunction in obese (Lep[ob]) mice. J Appl Physiol. 2000;89:2382–90. doi: 10.1152/jappl.2000.89.6.2382. [DOI] [PubMed] [Google Scholar]
- 9.Lembo G, Vecchione C, Fratta L, Marino G, Trimarco V, d’Amati G, et al. Leptin induces direct vasodilation through distinct endothelial mechanisms. Diabetes. 2000;49:293–7. doi: 10.2337/diabetes.49.2.293. [DOI] [PubMed] [Google Scholar]
- 10.Ku IA, Farzaneh-Far R, Vittinghoff E, Zhang MH, Na B, Whooley MA. Association of low leptin with cardiovascular events and mortality in patients with stable coronary artery disease: The Heart and Soul Study. Atherosclerosis. 2011;217:503–8. doi: 10.1016/j.atherosclerosis.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piemonti L, Calori G, Mercalli A, Lattuada G, Monti P, Garancini MP, et al. Fasting plasma leptin, tumor necrosis factor-alpha receptor 2, and monocyte chemoattracting protein 1 concentration in a population of glucose-tolerant and glucose-intolerant women: impact on cardiovascular mortality. Diabetes Care. 2003;26:2883–9. doi: 10.2337/diacare.26.10.2883. [DOI] [PubMed] [Google Scholar]
- 12.Scholze A, Rattensperger D, Zidek W, Tepel M. Low serum leptin predicts mortality in patients with chronic kidney disease stage 5. Obesity (Silver Spring) 2007;15:1617–22. doi: 10.1038/oby.2007.191. [DOI] [PubMed] [Google Scholar]
- 13.Currie PJ, Seward JB, Chan KL, Fyfe DA, Hagler DJ, Mair DD, et al. Continuous wave doppler determination of right ventricular pressure: A simultaneous doppler-catheterization study in 127 patients. J Am Coll Cardiol. 1985;6:750–6. doi: 10.1016/s0735-1097(85)80477-0. [DOI] [PubMed] [Google Scholar]
- 14.Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66:493–6. doi: 10.1016/0002-9149(90)90711-9. [DOI] [PubMed] [Google Scholar]
- 15.Mantzoros CS, Liolios AD, Tritos NA, Kaklamani VG, Doulgerakis DE, Griveas I, et al. Circulating insulin concentrations, smoking, and alcohol intake are important independent predictors of leptin in young healthy men. Obes Res. 1998;6:179–86. doi: 10.1002/j.1550-8528.1998.tb00335.x. [DOI] [PubMed] [Google Scholar]
- 16.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 17.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, et al. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–61. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 18.Hmisc: Harrell Miscellaneous. 2010. [Last accessed on 2011 Sept]. Available from: http://cran.r-project.org/web/packages/Hmisc/index.html .
- 19.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–7. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 21.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 22.Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. Narrative review: The role of leptin in human physiology: Emerging clinical applications. Ann Intern Med. 2010;152:93–100. doi: 10.1059/0003-4819-152-2-201001190-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maruna P, Lindner J, Kubzova KM. Leptin and soluble leptin receptor changes after pulmonary endarterectomy: Relations to cortisol and cytokine network. Physiol Res. 2009;58:569–76. doi: 10.33549/physiolres.931523. [DOI] [PubMed] [Google Scholar]
- 24.Beltowski J. Leptin and atherosclerosis. Atherosclerosis. 2006;189:47–60. doi: 10.1016/j.atherosclerosis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Koh KK, Park SM, Quon MJ. Leptin and cardiovascular disease: Response to therapeutic interventions. Circulation. 2008;117:3238–49. doi: 10.1161/CIRCULATIONAHA.107.741645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werner N, Nickenig G. From fat fighter to risk factor: The zigzag trek of leptin. Arterioscler Thromb Vasc Biol. 2004;24:7–9. doi: 10.1161/01.ATV.0000110908.43721.ad. [DOI] [PubMed] [Google Scholar]
- 27.Sweeney G. Cardiovascular effects of leptin. Nat Rev Cardiol. 2010;7:22–9. doi: 10.1038/nrcardio.2009.224. [DOI] [PubMed] [Google Scholar]
- 28.Wallace AM, McMahon AD, Packard CJ, Kelly A, Shepherd J, Gaw A, et al. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS) Circulation. 2001;104:3052–6. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- 29.McGaffin KR, Zou B, McTiernan CF, O’Donnell CP. Leptin attenuates cardiac apoptosis after chronic ischaemic injury. Cardiovasc Res. 2009;83:313–24. doi: 10.1093/cvr/cvp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGaffin KR, Sun CK, Rager JJ, Romano LC, Zou B, Mathier MA, et al. Leptin signalling reduces the severity of cardiac dysfunction and remodelling after chronic ischaemic injury. Cardiovasc Res. 2008;77:54–63. doi: 10.1093/cvr/cvm023. [DOI] [PubMed] [Google Scholar]


