Abstract
Partial anomalous pulmonary venous return (PAPVR) is a rare cause of adult onset pulmonary arterial hypertension (PAH) that can present with a wide spectrum of severity from early childhood throughout adult life. We present two patients with PAH secondary to PAPVR who reflect this range of disease. The diagnosis and treatment of PAPVR and its role in pulmonary vascular disease is discussed. Cardiac and pulmonary physicians should be aware of this entity and its diagnosis and management options.
Keywords: pulmonary arterial hypertension, congenital disease, vascular abnormalities
Partial anomalous pulmonary venous return is an uncommon congenital abnormality in which some, but not all, of the pulmonary veins connect to the right atrium or one of its venous tributaries. We discuss two adult patients who presented with pulmonary hypertension, and evidence of right ventricular hypertrophy and dysfunction.
CASE REPORTS
Case 1
A 55-year-old man with no significant past medical history presented to our institution with several months of episodic exertional lightheadedness associated with neck pain and diaphoresis. His outpatient workup included a normal EKG, conventional and stress echocardiograms, and a cardiac event monitor which revealed no arrhythmias. He was admitted to the hospital as his symptoms were becoming more frequent and was found to be in atrial fibrillation. Laboratory studies, including cardiac enzymes, thyroid function tests, liver function tests, electrolytes, and complete blood count, were all within normal limits.
Echocardiography revealed right ventricular hypokinesis and dilation, pulmonary arterial hypertension with an estimated pulmonary artery systolic pressure of 45–55 mmHg, and a normal left ventricular size and function. These findings were new compared with the echocardiogram performed 18 months prior. Pulmonary function tests, including diffusion capacity of the lung for carbon monoxide (DLco), were normal but a 6-Minute Walk test revealed a fall in oxygen saturation from 97–91% on room air. A CT pulmonary angiogram demonstrated no evidence of thromboembolic disease; however, a pulmonary vein communicating from the left upper lobe to the left brachiocephalic vein was discovered (Fig. 1).
Figure 1.
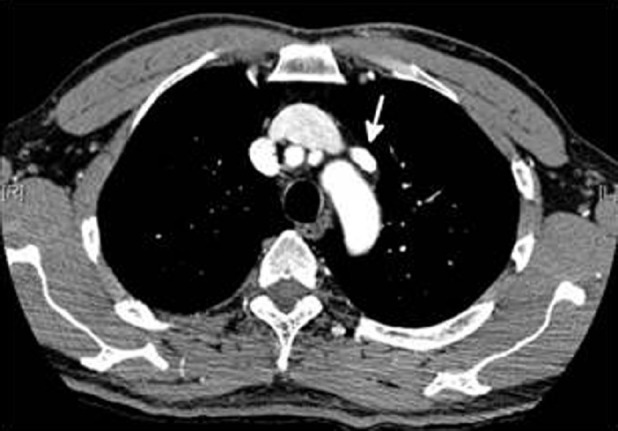
Contrast-enhanced CT scan of the chest demonstrating the presence of an anomalous pulmonary vein (white arrow) arising from the left upper lobe of the lung and connecting to the left brachiocephalic vein.
Right heart catheterization was performed revealing mean pulmonary artery pressure (PAM) of 16 mmHg; pulmonary artery systolic pressure (PAS) of 27 mmHg, pulmonary artery diastolic pressure (PAD) of 10 mmHg, and pulmonary capillary occlusion pressure (PAOP) of 12 mmHg. Cardiac output was 6.64 l/minute when measured by thermodilution and 5.46 l/minute when measured using the Fick equation. With exercise, mean pulmonary artery pressure increased to 39 mmHg, with wedge remaining at 12 mmHg, indicating the presence of exercise-induced pulmonary hypertension.
A cardiac MRI with gadolinium enhancement revealed the presence of the anomalous pulmonary vein arising from the left apical posterior and anterior segments of the left upper lobe and draining into the left brachiocephalic vein (Fig. 2), as well as right ventricular hypertrophy and dilation. The estimated shunt fraction (Qp:Qs), by using volumetric measurements and velocity-encoded imaging, was 1.28:1.
Figure 2.
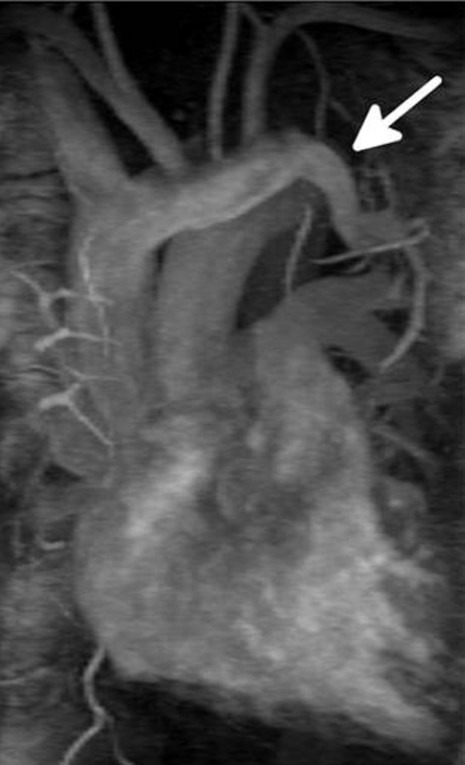
Cardiac MRI with gadolinium enhancement demonstrating the presence of the anomalous pulmonary vein (white arrow) arising from the left apical posterior and anterior segments of the left upper lobe and connecting to the left brachiocephalic vein.
His right ventricular dysfunction and exercise-induced pulmonary hypertension was felt to be the result of the additional blood volume being shunted through this anomalous circuit; however, given his stable symptoms, the patient elected to defer surgery. The patient began sildenafil treatment with good symptomatic response and continues close medical observation.
Case 2
A 33-year-old female with a history of well-controlled seizure disorder presented to a walk-in clinic with a year of increasing exertional dyspnea and was found to have cardiomegaly by chest radiography. She was sent to a local community hospital for further workup where an EKG showed right axis deviation, bundle branch block, and evidence of right ventricular hypertrophy. An echocardiogram confirmed right ventricular dilation, hypertrophy, and reduced function with an estimated peak PA pressure of 80 mmHg increasing to 90–100 mmHg with exercise. PFTs revealed moderate obstruction, but normal volumes and gas exchange. She walked 590 feet in six minutes with a slight decrease in pulse oximetry from 96% at rest to 94% on room air.
Ventilation/perfusion lung scan showed matched perfusion and ventilation without defects. A CT pulmonary angiogram revealed no evidence of pulmonary embolism, although did note an enlarged azygous and hemiazygous vein with evidence of azygous continuation of the IVC. Right heart catheterization revealed a PAM of 60, PAS of 90, PAD of 45, and PCWP of 10, with a cardiac output by thermodilution of 5.0 l/minute (CI 2.7 l/m2). A diagnosis of idiopathic pulmonary arterial hypertension was made and the patient was referred to our institution for further care.
Continuous intravenous infusion of epoprostenol was suggested, but the patient wished to try oral therapy first and was instead started on bosentan and anticoagulation with good clinical improvement, and her 6-Minute Walk Distance improved to 1,180 feet. However, repeat echocardiography continued to show elevated PAP and RV dilatation. Inhaled iloprost, 5 μg 6 times daily, was added to her bosentan therapy, but she had difficulty completing more than four treatments a day. Following addition of inhaled iloprost, her 6-Minute Walk Distance improved to 1,460 meters and peak PAP measured by echocardiogram decreased to 43 mmHg. However, her RV pressures remained grossly elevated and she began to develop signs of right heart failure and marked elevation of BNP.
She was referred for lung transplant evaluation, but was declined because of occasional tobacco use. Sildenafil was added to her treatment regiment, but her right heart failure did not improve. Repeat right heart catheterization done at our institution revealed PAM of 62 mmHg, PAS of 93 mmHg, PAD of 44 mmHg, and PAOP of 14 mmHg.
She again declined intravenous epoprostenol, but agreed to initiation of subcutaneous treprostinil infusion, and inhaled iloprost was discontinued. Despite some initial improvement on treprostinil, her condition progressed to overt right heart failure and she was hospitalized for treatment of peripheral edema and ascites. At the time of this admission, a chest radiograph showed a new right-sided aberrant pulmonary artery in a curved “scimitar” shape. Further review of her prior noncontrast chest CT obtained as part of her evaluation for lung transplantation revealed evidence of aberrant drainage of the right lung with a “scimitar vein” (Fig. 3). Cardiac MRI was obtained showing that the abnormal vein originated from aberrant right upper and middle pulmonary veins which drained into a right atrial-hepatic vein at the level of the diaphragm (Fig. 4). Moderate hypoplasia of the right lung was also noted. The Qp:Qs ratio was estimated at 1.45:1. Copies of her original contrast enhanced CT were not able to be obtained, but PAPVR was not reported.
Figure 3.
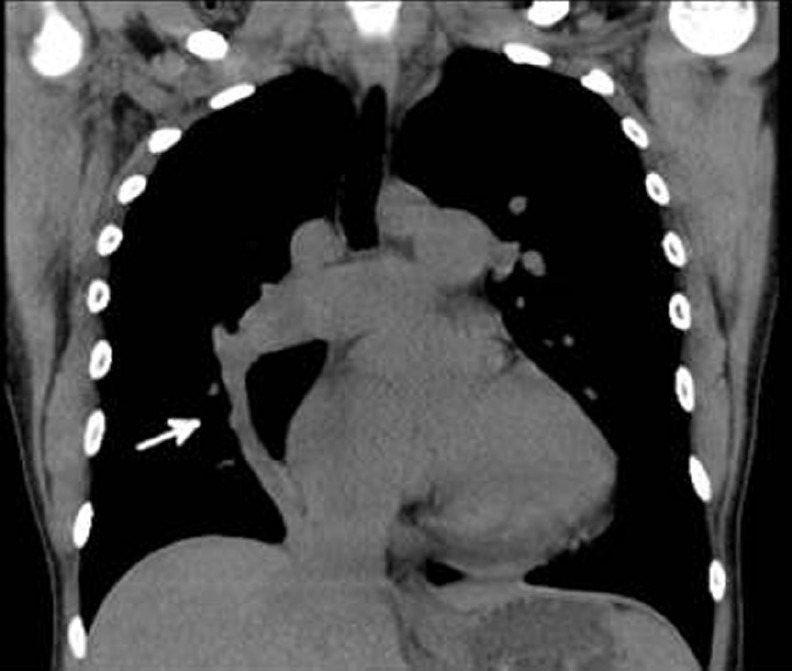
Noncontrast CT of the chest demonstrating right sided “scimitar vein” (white arrow) draining toward diaphragm.
Figure 4.
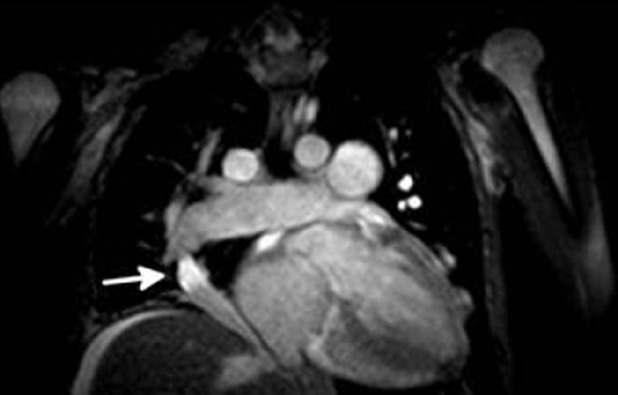
Cardiac MRI demonstrating right sided “scimitar vein” (white arrow) draining into right atrial-hepatic vein.
The patient was again referred for lung transplantation and eventually underwent heart-lung transplantation, with visualization of the scimitar vein at the time of surgery.
DISCUSSION
Partial anomalous pulmonary venous return (PAPVR) occurs when some of the pulmonary veins connect to the right atrium or one of its venous tributaries rather than the left atrium. PAPVR has traditionally been associated with atrial septal defects (ASD), found in 80% of patients in one pediatric series.[1] It has also been thought that most anomalous pulmonary veins arise mainly from the right lung; 80% of those studied in the above series had a right-sided anomaly, connecting primarily to the superior vena cava (SVC), less commonly to the right atrium (RA) or inferior vena cava (IVC). Only 3–8% have been reported to originate from the left lung, connecting to the left brachiocephalic in all cases.[1,2] PAPVR is often associated with other cardiac defects and is reported in certain congenital syndromes, such as Turner's syndrome (monosomy X).[3]
The scimitar syndrome, as seen in the second case, is a clinical association of a right-sided anomalous pulmonary vein connecting to the RA or IVC with other anatomic abnormalities, including hypoplasia of the right lung and right pulmonary artery, dextroposition, and/or dextrorotation of the heart.
PAPVR is often clinically silent, and an autopsy series in the 1950s found it in 0.4% of postmortem examinations.[4] More recently, retrospective reviews of computed tomography (CT) series in adults receiving imaging for other indications have identified rates of 0.1–0.2% in the adult population.[5,6] Interestingly in these studies, almost half of the identified anomalies were left sided, and only right upper lobe PAPVR was associated with ASD6. This indicates the pediatric and adult populations who present with PAPVR may be significantly different.
Embryologically, PAPVR arises from failure of primitive lung drainage to regress properly (Fig. 5). In the human embryo, the primordial lung bud has no connection to the heart, draining venous blood from the pulmonary vascular bed through systemic veins (Days 27–29 of gestation). By Days 32–33, the common pulmonary vein forms from the left atrium, establishing a connection with the pulmonary vascular bed. Once a direct connection with the heart is established, connections with systemic veins begin to disappear (Day 40), allowing venous blood from the developing lung to drain into the common pulmonary vein and left atrium via four individual pulmonary veins. By term, the common pulmonary vein incorporates into the left atrium and the pulmonary veins connect directly to the heart. Failure of one or more of the pulmonary veins to establish a connection with the common pulmonary vein, instead maintaining persistent systemic venous connection, results in PAPVR.[7]
Figure 5.
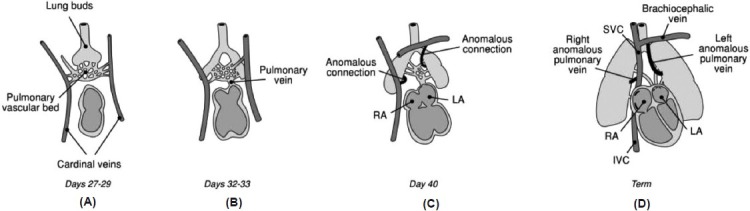
Embryology of PAPVR. (A) At post-conceptional Days 27–29, the primordial lung buds drain through a vascular bed to the cardinal veins, which will develop into systemic veins. (B) By Days 32–33, the common pulmonary vein forms from the left atrium and establishes a connection with the pulmonary venous circulation. Pulmonary venous connections to systemic veins begin to regress and pulmonary venous blood drains into the common pulmonary vein. (C) By Day 40, the primitive connections from the pulmonary vascular bed to the cardinal veins should have regressed, but in PAPVR anomalous connections persist. (D) At term, the anomalous connection will have developed into anomalous pulmonary veins draining most commonly into the SVC on the right, or the brachiocephalic vein on the left.
This persistent systemic venous connection acts similarly to a left-to-right shunt, in that a portion of right ventricular output is continuously recirculated and oxygenated blood is returned to the right heart without traveling to the systemic circulation. Over time, which may be years to decades, the increase in pulmonary blood flow can lead to progressive remodeling of the pulmonary circulation and increased pulmonary vascular resistance. If severe enough, pulmonary arterial hypertension (PAH) and right ventricular volume overload occur, leading to RV failure.[8] Patients can present anytime from infancy to the seventh decade, depending on the size of the shunt, as well as the presence of other cardiovascular anomalies and medical conditions.
Noninvasive imaging and diagnosis of PAPVR continues to be an evolving field. Chest radiography has limited sensitivity, but CT scanning is an extremely effective diagnostic modality, especially when iodinated contrast is used.[6] Transthoracic echocardiography (TTE), which is often obtained in patients being evaluated for cardiac symptoms, cannot reliably delineate pulmonary venous anatomy due to technical limitations. In pediatric patients, approximately one third of cases of PAPVR are missed by TTE[9] and the proportion is likely higher in adults although it has not been specifically studied. Transesophageal echocardiography (TEE), by contrast, is quite sensitive and specific in the hands of experienced operators.[10] Finally, cardiac MRI is increasingly being used as it can identify multiple defects and better define structural abnormalities without ionizing radiation. It has also proven useful in providing noninvasive, but accurate, quantification of shunt volume.[11,12]
Shunt volume is expressed as a ratio of the flow through the pulmonary arterial bed to the flow through the systemic arterial bed (Qp:Qs). The “gold standard” for quantifying Qp:Qs has been by determining blood flow using a modified Fick equation during right and left heart catheterizations. However, shunt quantification using MRI phase velocity mapping correlated very well with ventricular volumetric data obtained by MRI as well as oximetry data from cardiac catheterizations.[12] Additionally, cardiac MRI may provide better anatomic definition. A study of 13 patients with PAPVR showed that the diagnosis was made with cardiac MRI in three patients whose previous echocardiographic or catheter studies were either nondiagnostic or misinterpreted.[13]
In pediatric patients, PAPVR is usually treated with surgical correction if large enough to create a significant shunt. In general, patients that have a Qp:Qs of 1:1.5 or more are considered for surgical repair as they are more likely to develop pulmonary hypertension and right ventricular failure,[14] although this cutoff has not been subject to rigorous study. In adult patients, the criteria for surgical repair are less clear cut. Those who have already developed symptoms due to shunting, or have evidence of right-sided volume overload, regardless of the magnitude of the shunt, are also considered for surgery. However, in asymptomatic patients with a low shunt fraction and no clinical or echocardiographic evidence of right heart overload, pulmonary hypertension, or other symptoms, surgery may be unnecessary.[15]
The general principle of surgical repair is to separate the pulmonary venous system from the systemic system. This involves anastomosing the aberrant pulmonary vein or veins either to the left atrium (recreating normal anatomy) or, more commonly, to the right atrial appendage. In the latter case, a GORE-TEX™ graft or intracardiac baffle and ASD would be used to direct blood to the left atrium (Fig. 6).
Figure 6.
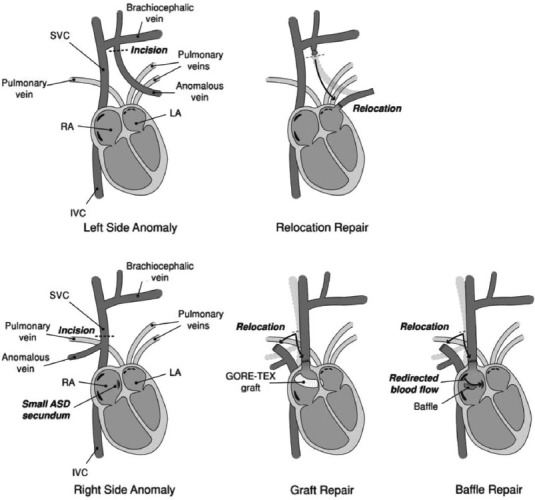
Surgical approaches for correcting PAPVR. For anomalous veins connecting portions of the left lung to the left brachiocephalic vein, the anomalous vein is reconnected directly to the LA. For anomalous veins connecting portions of the right lung to the SVC, the SVC is reconnected to the right atrial appendage and blood from the anomalous vein is shunted, with the help of a pericardial patch, though a newly-created (or enlarged) ASD into the LA. Alternatively, there have been reports of GORE-TEX™ grafts being used to create a conduit crossing the right atrium.
Surgery is generally effective at correcting the abnormal shunt and its associated symptoms; moreover, the complication rate reported in literature is low. In one of the largest series consisting of 306 patients, both adult and pediatric, who underwent surgical correction of PAPVR, Alsoufi et al. reported no deaths.[16] Four patients required reoperation during the follow-up period, and most patients remained free from late complications including pulmonary vein stenosis (86%) and vena caval obstruction (97.8%).[16] Unfortunately, patients with repaired scimitar syndrome were much more likely to have postoperative pulmonary venous obstruction.
A recent, single-center review of 43 adult patients with isolated partial anomalous venous return (i.e., those without ASD or other anomalies) similarly found very good surgical outcomes in the 28 patients who required surgery, with no deaths reported, and few complications.[15] This study also noted that patients with a single anomalous pulmonary vein often had a benign course, not requiring surgery. Finally, the majority of patients who did require surgery had improvement in echocardiographic measurements of pulmonary artery pressures and right-sided heart function.[15]
Unfortunately, in patients with severe PAH and elevated pulmonary vascular resistance, reparative surgery is unlikely to alter disease course as the extensive vascular remodeling is unlikely to be reversible. In these patients, such as our second case, heart-lung transplant may be the only curative option. Unfortunately, the 10-year survival of heart-lung transplant remains only 30–40%, so the timing of transplant in order to optimize survival remains a difficult decision.[17] There is also increasing interest in various “treat-then-repair” strategies combining medical and surgical management, but little data currently exist.
Some patients may be candidates for catheter embolization of the anomalous vein(s), providing that there is some connection from the anomalous vein to the LA that can accommodate the venous drainage after the anomalous vein has been embolized. Forbess et al. reported significant clinical and hemodynamic improvement in two patients with PAPVR after catheter guided embolization.[18] In both patients, symptoms improved by two New York Heart Association classes with trace to no residual flow through the anomalous connection. Similar results were reported in another study of two patients who underwent catheter embolization.[19]
In both pediatric and adult patients there has been increasing interest in medical therapy for those in who surgical repair is high risk. While no prospective, controlled trials have been conducted, small retrospective and observational studies of patients with PAH secondary to congenital heart diseases, including PAPVR, or of patients who have shown clinical and hemodynamic improvements with prostaglandins, phosphodiesterase inhibitors, and bosentan, have been conducted.[17] Patients such as our first case, who present late in adult life with minor elevation in PA pressure and preserved exercise capacity, may be candidates for medical therapy with newly approved medications for PAH. However, clinical response to treatment and pulmonary hemodynamics should be monitored carefully in these patients, and surgery should be reconsidered if pulmonary hypertension progresses.
In conclusion, PAPVR is a rare congenital condition which is usually recognized in the pediatric population but may also be diagnosed during adulthood in patients who develop PAH, or in asymptomatic patients undergoing pulmonary vascular studies for other indications. The widespread use of more sophisticated diagnostic techniques such as CT pulmonary angiography, TEE, and cardiac MRI may be increasing the frequency with which this condition is diagnosed. The disease, as demonstrated by the cases discussed, can present with variable degrees of severity. In asymptomatic patients without signs of significant PAH or right-heart overload, no intervention may be necessary. However, these patients should be followed for signs of progressive pulmonary hypertension. For patients with mild-to-moderate pulmonary hypertension, surgical repair is usually safe and effective, although catheter guided and medical therapies may play an increasing role. Finally, in patients who have progress to severe pulmonary hypertension, lung or heart-lung transplantation may be necessary. Physicians who diagnose and treat adult patients with PAH should consider PAPVR as a potential etiology, particularly in those with a history of ASD or other congenital pulmonary vascular or heart diseases.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Senocak F, Ozme S, Bilgic A, Ozkutlu S, Ozer S, Saraçlar M. Partial anomalous pulmonary venous return.Evaluation of 51 cases. Jpn Heart J. 1994;35:43–50. doi: 10.1536/ihj.35.43. [DOI] [PubMed] [Google Scholar]
- 2.Kiseleva IP, Malsagov GU. Differential diagnosis of anomalous pulmonary venous return.A clinical-roentgenological study. Cor Vasa. 1984;26:140–6. [PubMed] [Google Scholar]
- 3.Ho VB, Bakalov VK, Cooley M, Van PL, Hood MN, Burklow TR, et al. Major vascular anomalies in Turner syndrome: prevalence and magnetic resonance angiographic features. Circulation. 2004;110:1694–700. doi: 10.1161/01.CIR.0000142290.35842.B0. [DOI] [PubMed] [Google Scholar]
- 4.Healey JE., Jr An anatomic survey of anomalous pulmonary veins: their clinical significance. J Thorac Surg. 1952;23:433–44. [PubMed] [Google Scholar]
- 5.Haramati LB, Moche IE, Rivera VT, Patel PV, Heyneman L, McAdams HP, et al. Computed tomography of partial anomalous pulmonary venous connection in adults. J Comput Assist Tomogr. 2003;27:743–9. doi: 10.1097/00004728-200309000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Ho ML, Bhalla S, Bierhals A, Gutierrez F. MDCT of partial anomalous pulmonary venous return (PAPVR) in adults. J Thorac Imaging. 2009;24:89–95. doi: 10.1097/RTI.0b013e318194c942. [DOI] [PubMed] [Google Scholar]
- 7.Fraser RS, Paré PD. 4th ed. Philadelphia: W.B. Saunders; 1999. Fraser and Paré's diagnosis of diseases of the chest; pp. 637–75. [Google Scholar]
- 8.Diller GP, Gatzoulis MA. Pulmonary vascular disease in adults with congenital heart disease. Circulation. 2007;115:1039–50. doi: 10.1161/CIRCULATIONAHA.105.592386. [DOI] [PubMed] [Google Scholar]
- 9.Wong ML, McCrindle BW, Mota C, Smallhorn JF. Echocardiographic evaluation of partial anomalous pulmonary venous drainage. J Am Coll Cardiol. 1995;26:503–7. doi: 10.1016/0735-1097(95)80029-g. [DOI] [PubMed] [Google Scholar]
- 10.Ammash NM, Seward JB, Warnes CA, Connolly HM, O’Leary PW, Danielson GK. Partial anomalous pulmonary venous connection: diagnosis by transesophageal echocardiography. J Am Coll Cardiol. 1997;29:1351–8. doi: 10.1016/s0735-1097(97)82758-1. [DOI] [PubMed] [Google Scholar]
- 11.Petersen SE, Voigtlander T, Kreitner KF, Kalden P, Wittlinger T, Scharhag J, et al. Quantification of shunt volumes in congenital heart diseases using a breath-hold MR phase contrast technique-comparison with oximetry. Int J Cardiovasc Imaging. 2002;18:53–60. doi: 10.1023/a:1014394626363. [DOI] [PubMed] [Google Scholar]
- 12.Debl K, Djavidani B, Buchner S, Heinicke N, Poschenrieder F, Feuerbach S, et al. Quantification of left-to-right shunting in adult congenital heart disease: phase-contrast cine MRI compared with invasive oximetry. Br J Radiol. 2009;82:386–91. doi: 10.1259/bjr/18500608. [DOI] [PubMed] [Google Scholar]
- 13.Prasad SK, Soukias N, Hornung T, Khan M, Pennell DJ, Gatzoulis MA, et al. Role of magnetic resonance angiography in the diagnosis of major aortopulmonary collateral arteries and partial anomalous pulmonary venous drainage. Circulation. 2004;109:207–14. doi: 10.1161/01.CIR.0000107842.29467.C5. [DOI] [PubMed] [Google Scholar]
- 14.Toyoshima M, Sato A, Fukumoto Y, Taniguchi M, Imokawa S, Takayama S, et al. Partial anomalous pulmonary venous return showing anomalous venous return to the azygos vein. Intern Med. 1992;31:1112–6. doi: 10.2169/internalmedicine.31.1112. [DOI] [PubMed] [Google Scholar]
- 15.Majdalany DS, Phillips SD, Dearani JA, Connolly HM, Warnes CA. Isolated partial anomalous pulmonary venous connections in adults: Twenty-year experience. Congenit Heart Dis. 2010;5:537–45. doi: 10.1111/j.1747-0803.2010.00458.x. [DOI] [PubMed] [Google Scholar]
- 16.Alsoufi B, Cai S, Van Arsdell GS, Williams WG, Caldarone CA, Coles JG. Outcomes after surgical treatment of children with partial anomalous pulmonary venous connection. Ann Thorac Surg. 2007;84:2020–6. doi: 10.1016/j.athoracsur.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 17.Gatzoulis MA, Alonso-Gonzalez R, Beghetti M. Pulmonary arterial hypertension in paediatric and adult patients with congenital heart disease. Eur Respir Rev. 2009;18:154–61. doi: 10.1183/09059180.00003309. [DOI] [PubMed] [Google Scholar]
- 18.Forbess LW, O’Laughlin MP, Harrison JK. Partially anomalous pulmonary venous connection: Demonstration of dual drainage allowing nonsurgical correction. Cathet Cardiovasc Diagn. 1998;44:330–5. doi: 10.1002/(sici)1097-0304(199807)44:3<330::aid-ccd19>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 19.Dahnert I, Riede FT, Kostelka M. Partial anomalous pulmonary venous drainage of the left upper pulmonary vein – catheter interventional treatment is sometimes possible. Clin Res Cardiol. 2007;96:511–3. doi: 10.1007/s00392-007-0518-8. [DOI] [PubMed] [Google Scholar]


