Abstract
Bacterial endosymbionts induce various effects on hosts and can dramatically impact host fitness and development. An example is provided by obligate, maternally-inherited Wolbachia, which infect a broad range of invertebrates. Wolbachia are capable of altering host reproduction, thereby promoting infection spread. Wolbachia also pose direct physiological costs and benefits to hosts, complicating their categorization as parasites or mutualists. This study examines for an effect of Wolbachia infection in intraspecific larval competition by Aedes albopictus mosquitoes, with the goal of examining for an impact of Wolbachia infection in mixed populations. Similar to prior work examining for an influence of Wolbachia infection on the fitness of A. albopictus in adults, the results presented here support the hypothesized impact of Wolbachia across all life stages, including immatures. The differential competitiveness of infected larvae detected in our experiments indicates that Wolbachia infected A. albopictus females are less competitive relative to uninfected females when competing under highly competitive conditions. In contrast, under low competitive pressures, infected females experience higher survivorship. Thus, Wolbachia infection shifts from parasitism to mutualism as a function of developmental conditions. Results are discussed in relation to the invasion and persistence of Wolbachia in A. albopictus populations.
The results are important to the evolution of stable Wolbachia symbioses, including Wolbachia invasion of an uninfected population. The resulting infection dynamics that occur in an infected population are discussed.
Keywords: Endosymbiont, Wolbachia, Intraspecific competition, Fitness, Development
1. Introduction
The present study investigates the larval stages of the Asian tiger mosquito Aedes albopictus (Skuse) (Diptera: Culicidae), individuals of which are naturally infected by two different Wolbachia strains (Sinkins et al., 1995). Previous studies on A. albopictus have shown that Wolbachia, in addition to altering host reproduction via CI, can augment fitness of infected adult females under some conditions (Dobson et al., 2004). In contrast, prior comparisons of infected and uninfected larval strains revealed relatively minor fitness effects in immatures (Gavotte et al., 2009; Islam and Dobson, 2006). However, the prior experiments examined competition within A. albopictus strains (i.e., separate pools of infected and uninfected larvae) and not direct competition between infected and uninfected larvae.
Symbionts can be powerful evolutionary motors (Cavalier-Smith, 1992; Corsaro et al., 1999; Searcy, 2003), with characteristics of both parasites and mutualists. On one hand, stimulation of immunity and diversion of resources by parasitic symbionts impart costs to the host (Fleury et al., 2000; Sachs and Wilcox, 2006), while symbionts operating as mutualists present relatively low virulence and may provide hosts with novel metabolic benefits (Dale and Moran, 2006; Stewart and Cavanaugh, 2006). Symbionts that are horizontally transmitted between individuals are commonly linked with high virulence (i.e., classical pathogenic infection among individuals). In contrast, vertical transmission (i.e., from parents to offspring) is more commonly associated with relatively benign (commensial) or beneficial (mutualistic) symbioses (Anderson and May, 1982; Lipsitch et al., 1995). Vertical transmission exclusively through females (maternal transmission) can result in an evolutionary paradigm of “reproductive parasitism” (Werren, 1997), in which endosymbionts evolve a capacity to manipulate host reproduction, promoting the invasion of host populations.
An extensively studied group of endosymbionts are α-proteobacteria identified as Wolbachia Hertig and Wolbach (Rickettsiales: Rickettsiaceae) (Bourtzis and Miller, 2003). This maternally inherited, obligatory intracellular bacterium is one of the most widespread invertebrate endosymbionts known, infecting >17% of arthropods (Werren, 1997) and many filarial nematode species (Bourtzis et al., 1998). The spread and persistence of Wolbachia in arthropod populations are often related to the manipulation of a host’s reproduction by the bacterium, including cytoplasmic incompatibility (CI) (O'Neill and Karr, 1990), male killing (Huigens et al., 2004; Jiggins et al., 2001), parthenogenesis (Stouthamer et al. 1990) or feminization (Bouchon et al., 1998; Rousset et al., 1992).
Prior research efforts have focused primarily on Wolbachia reproductive manipulation of hosts, including studies examining for additional Wolbachia effects on host fitness, generally during adult stages. A range of effects has been described including a moderate physiological cost (Fleury et al., 2000; Hoffmann et al., 1990; Olsen et al., 2001), an absence of cost (Fry et al., 2004; Montenegro et al., 2006) and benefits (Bandi et al., 2001; Dedeine et al., 2001; Dobson et al., 2004; Islam and Dobson, 2006; Vavre et al., 1999) for the host. By comparison, relatively few studies have examined Wolbachia effects in immature hosts (Gavotte et al., 2009; Harcombe and Hoffmann, 2004; Islam and Dobson, 2006).
Here, direct competition during larval stages (intra-specific, inter-strain competition) was monitored by rearing infected and uninfected A. albopictus larvae in shared containers. Measuring the Wolbachia infection status of emerging adults allowed assessment of relative advantages in larval competitiveness under conditions in which infected and uninfected individuals compete directly for the same resources. Repeating the experiment at two different densities allowed examination for Wolbachia effects under different competition levels. In addition to the Wolbachia infection status, the survivorship, wing size, sex ratio and developmental times were also measured. The results are discussed in relation to A. albopictus population dynamics and Wolbachia infection dynamics.
2. Materials and Methods
2.1. Mosquito strains
The UT strain was generated by tetracycline treatment and is free of Wolbachia (Dobson and Rattanadechakul, 2001). The aposymbiotic UT strain has been maintained in the absence of tetracycline for >20 generations. The IH strain is infected by Wolbachia with both natural wAlbA and wAlbB types and has been introgressed by repeated matings with UT males to reduce genetic differences between the two strains (Dobson et al., 2004). Mosquitoes were maintained as previously described (Dobson et al., 2001).
2.2. Rearing conditions and experimental setup
Mosquitoes were reared at 28ºC in identical cylindrical Mosquito Breeders (BioQuip, Rancho Dominguez, CA) holding 200 mL deionized water and food. Each container received 600 μL of a 20 mg/ml food solution (liver powder, ICN Biomedical, Aurora, OH) each week. Four replicates for each density (50 and 400 larvae) were made, containing a 1:1 ratio of IH (infected) and UT (uninfected) larvae, and reared under similar conditions. All larvae were hatched within a 1-hour time window and all larval cohorts were assembled simultaneously. Rearing conditions (50 and 400 larval densities) were based upon the results of previous experiments (Gavotte et al., 2009).
Two times a day, emerging adults were removed from each container by aspiration and killed by freezing. The sex was determined and the right wing of each individual was removed and measured using an ocular micrometer and stereo-dissecting microscope. Mosquitoes were stored in individual tubes at −20ºC for DNA extraction.
2.3. DNA extraction and PCR
DNA was extracted by homogenizing mosquitoes individually in 100 μl extraction buffer (10 mM Tris-HCl, 1 mM EDTA, 50 mM NaCl, pH 8.2) with a glass bead using a Mini-beadbeater (BioSpec Products, Inc., Bartlesville, OK). Homogenized samples were heated at 100ºC for 5 minutes and centrifuged at 14,000g for 5 minutes. DNA samples were stored at −20ºC and 2 μl of supernatant used for PCR reactions. PCR reactions were done as described previously (Dobson et al., 2004) using Wolbachia primers [81F: 5’-TGG TCC AAT AAG TGA TGA AGA AAC-3’ and 691R: 5’-AAA AAT TAA ACG ACT CTC CA-3’] (Zhou et al., 1998). DNA quality of all samples that were Wolbachia negative was confirmed using a mosquito-specific PCR primer set that amplifies a nuclear ribosomal protein L8 gene [RPL8f: 5’-CCT TAC AAG TTC AAC GTC CGC-3’ and RPL8r: 5’-CAG CAA CAA TTC CGA CCA TGG-3’] (Lan and Fallon, 1992).
2.4. Statistical analyses
Survivorship and sex ratio were used after arcsine transformations of proportions, and median dates were used for development time. All data sets were normally distributed (Kolmogorov-Smirnov normality test). One-way and two-way ANOVA were used to compare treatment, and interaction means and Chi-square tests were used to determine departures from 1:1 ratios (for sex ratio or infected individuals). These analyses were done using Statview 5.0.1 software (SAS Institute 1998). Contingency analysis of categorical emergence frequencies was performed using JMP 8.0 (SAS Institute 2008). A log-linear frequency analysis was used to determine whether observed survival frequencies differed significantly from expected frequencies if all competing larvae had an equal probability of survival. Expected frequencies of larvae were generated by assuming a 1:1 sex ratio among first instar larvae. The analysis was performed using Statistica (Softstat 1995).
3. Results
3.1. Survivorship
Immature survivorship to adult eclosion (mean proportion ± standard deviation = 0.753 ± 0.053 for 50 larvae and 0.246 ± 0.025 for 400 larvae) was significantly greater among larvae at the less restrictive initial density (one-way ANOVA on arcsine-transformed proportions: F1,10 = 197.0, p < 0.0001).
3.2. Sex ratio
Despite the initial 1:1 sex ratio commonly noted for A. albopictus (Gavotte et al., 2009; Lounibos and Escher, 2008; Tseng, 2004), females survived better than males resulting in a female-biased sex ratio (58.3% female) in the low-density treatment (χ2 = 4.53; Df = 1; p < 0.05). In contrast, proportionally fewer females than expected (42.9%) emerged from the 400 larvae per container treatment (χ2 = 10.77; Df = 1; p < 0.005).
3.3. Wolbachia infection
Wolbachia infection status was determined by PCR for representative subsamples of the emerging individuals. Individuals were randomly selected (separately for the low- and high-density treatments). Two subsamples (representing ~25% and 10% of survivors) yielded the same proportions of infected and uninfected females and males following Chi-square analysis (χ2 = 0.74; Df = 3; p > 0.5 for 50 larvae and χ2 = 4.73; Df = 3; p > 0.1 for 400 larvae). Thus, analyses were subsequently performed on the pooled subsamples representing 196 individuals.
Although the cohorts were initial assembled using equal numbers of infected and uninfected females and males, the four categories (i.e., infected females; uninfected females; infected males; uninfected males) were not equally represented among survivors of competition at low (F3, 12 = 3.579, p < 0.05) or high (F3, 12 = 16.278, p < 0.0001) densities (Figure 1). Contingency analysis indicated that more infected females than expected emerged from the 50 larval density treatment while the reverse was true from the 400 larval density treatment (Chi-square likelihood ratio = 18.04, Df = 1, N = 94, p < 0.0001). For males, approximately equal numbers of infected and uninfected individuals emerged from the 50 larval density treatment whereas significantly fewer infected males than expected survived competition in the 400 larval density treatment (Chi-square likelihood ratio = 7.28, Df = 1, N = 101, p = 0.007). These departures from uniform emergence among categories are represented in Figure 2.
Figure 1. Emerging individuals for all experimental conditions.
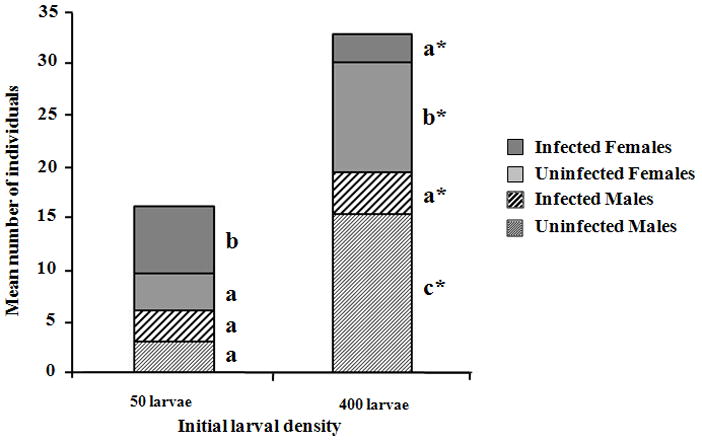
Relative numbers of emerging A. albopictus females and males infected and uninfected by Wolbachia at two initial larval densities.
Figure 2. Mean departure from uniform survival.
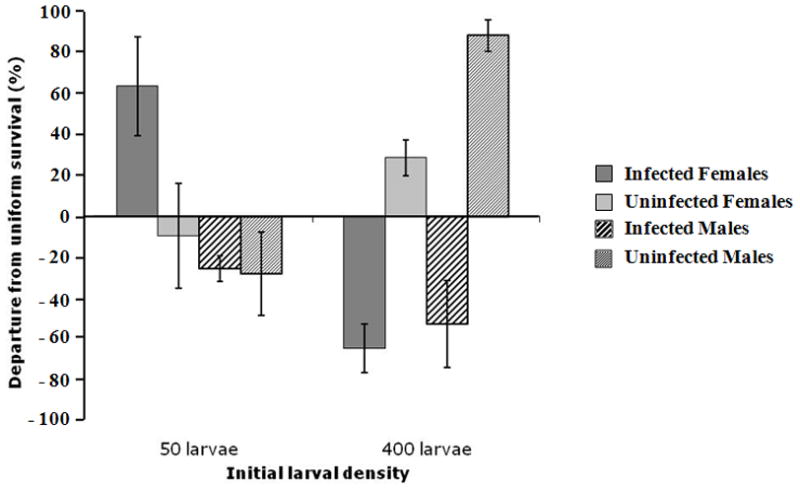
Mean departure ± SD (in %) from an equal emergence of A. albopictus competing at two larval densities. Expected ratios, based upon a theoretical distribution, should be 1:1:1:1 for uninfected females:uninfected males:infected females:infected males (equal numbers of infected and uninfected larvae and 1:1 sex ratio).
The categorical factors (K factors) included in a log-linear model that was used to compare emergence frequencies included: initial larval density, Wolbachia infection status, sex, and survival status (i.e., “lived” versus “died”) until eclosion. Categories for replicates were omitted from the model because they explained none of the significance either alone or via interactions. The best initial model contained all three-way interactions (Pearson’s Chi-square: χ2 = 0.0086, Df = 1, p = 0.926). Initial larval density (χ2 = 163.7, Df = 4, p < 0.0001), infection status (χ2 = 25.3, Df = 6, p < 0.0001) and sex (χ2 = 11.5, Df = 4, p < 0.05) but not survival status (χ2 = 0.01, Df = 1, p = 0.920) significantly explained occurrence frequencies when all interactions were held at zero. Among significant two-way interactions resulting from tests of marginal association, density x survival status produced a significant effect (χ2 = 8.57, Df = 1, p < 0.01) because survival was higher than expected at the lower density but the reverse was true at the higher density. Additionally, Wolbachia infection status x survival status was significant (χ2 = 4.20, Df = 1, p < 0.05) because fewer infected individuals survived than expected (although the bulk of these were reared at 50 larval densities) whereas more uninfected mosquitoes survived (mostly from the 400 larval densities). Likewise, among three-way interactions, density x sex x survival status was significant (χ2 = 7.58, Df = 1, p < 0.001) as was infection status x sex x survival status (χ2 = 3.95, Df = 1, p < 0.05); whereas infected females were “overrepresented” among low-density survivors, uninfected males were most numerous among high-density survivors.
3.4. Wing length
Wing length, measured on the 196 individuals analyzed for infection status, (i.e., vertical axis in Figure 3) differed significantly between females and males (F1,24 = 148.3, p < 0.0001) but not between the two densities (F1,24 = 0.825, p = 0.373; no difference between LD and HD pairs in Figure 3) or as a function of infection status (F1,24 = 0.099, p = 0.756; no difference between closed and open symbols in Figure 3). The correlations between mean male wing length and median emergence date (r = −0.045, n = 16, p > 0.05) and between mean female wing length and median emergence date (r = 0.354, n = 16, p > 0.05) were not significant.
Figure 3. Relation between wing length and developmental time.
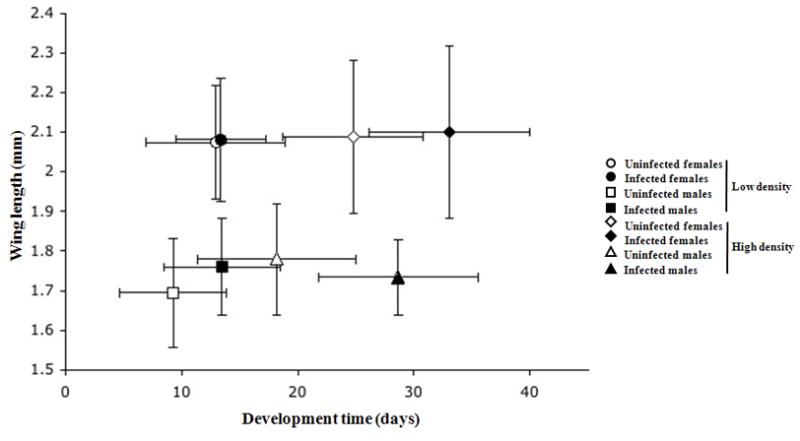
Mean ± SD of wing length (in mm) and median ± SD development time (in days) for A. albopictus females and males, infected or uninfected by Wolbachia, reared at low (LD; 50 larvae) or high (HD; 400 larvae) initial larval densities.
3.5. Development time
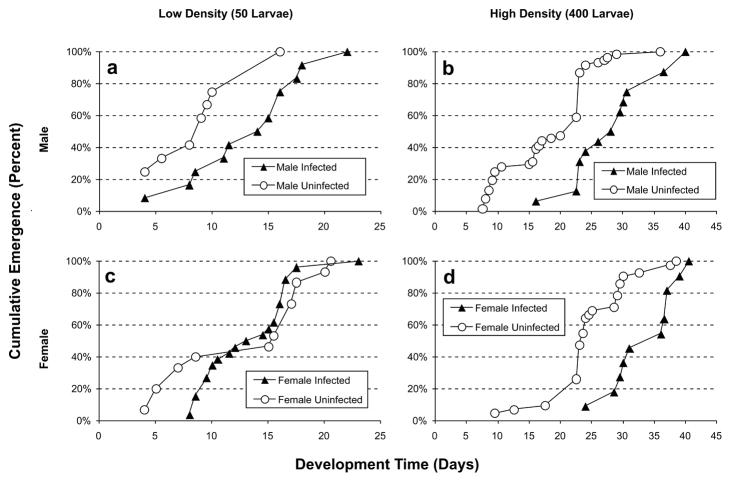
The time until emergence by the median adult was significantly influenced by initial larval density (F1,24 = 106.2, p < 0.0001), infection status (F1,24 = 16.0, p < 0.001), and sex (F1,24 = 6.19, p < 0.05) (i.e., horizontal axis in Figure 3). Both females and males emerged significantly earlier in the lower density treatments, relative to the higher density treatments. In all treatments, males emerged significantly earlier than females. Uninfected males emerged earlier on average than infected males at both densities (Figure 4a and 4b). Uninfected and infected females emerged at the same time on average at low density (Figure 4a). Uninfected females emerged earlier on average than infected at high density (Figure 4b). The latter exceptional females explain the only significant interaction term (i.e., density × infection status, F1,24 = 4.45, p < 0.05).
Figure 4. Adult emergence kinetics.
Cumulative percentage emergence of males (uninfected and infected) from 50 (a) and 400 (b) initial larvae density experiments and females (uninfected and infected) from 50 (c) and 400 (d) initial larvae density experiments.
4. Discussion
Intraspecific competition during larval development is a major determinant of individual fitness in many insect species, perhaps especially for container-developing mosquitoes with restrictive habitat space and resource availability. Larval competition influences many adult phenotypic traits, including size, fecundity and adult longevity. Moreover, larval competition represents a major mortality factor for insects (Teng and Apperson, 2000), prior to reproduction. Likewise, predators, parasites and symbionts can dramatically alter the outcomes of competition between individuals and induce strong selection pressures.
Our results demonstrate differential impacts of Wolbachia infection upon host competitive capacities as a function of larval density. The competition response contrasts with previous experimental observations in which infected and uninfected strains, allowed to develop separately, exhibited no differences in survivorship among females or among males (Gavotte et al., 2009). Similar to the prior study, larvae reared at high density experienced significantly lower relative survivorship compared to the low density treatment (Gavotte et al., 2009). But a clear effect related to the Wolbachia infection status appears to slow development of infected individuals at both densities and for both sexes (Fig. 4). If the Wolbachia infection status has no effect upon competition (i.e., hypothesized neutral effect), the frequency of infection in emerging adults would be in proportions similar to their initial numbers. Here, we observed significantly more infected females emerging from the low density treatment. In contrast, Wolbachia infected larvae that developed more slowly appeared to be at a disadvantage in the high density treatment, emerging at significantly lower rates than their uninfected competitors (Figures 1 and 2).
The results suggest a sex-specific effect of Wolbachia infection in this sexually dimorphic species. At low density, infected females emerged at a higher than expected rate. Considering the possible evolutionary implications, a competitive advantage conferred on host females by Wolbachia is a direct advantage for the endosymbiont, which is maternally inherited (i.e., not transmitted by males). Wolbachia have been shown to provide measurable fitness advantages to A. albopictus adult females in terms of fecundity and longevity (Dobson et al., 2004). Considering the described competitive advantages from Wolbachia infection for larval females and apparent reproductive advantages for infected female adults, at least in A. albopictus, Wolbachia appear to be mutualistic rather than parasitic for females at low densities. This is consistent with an earlier experiment in which the infection invaded at a rate faster than expected (Dobson et al., 2002). No advantages were apparent for infected males (which are a dead end for Wolbachia infection) in the present or earlier studies (Dobson, 2007; Dutton and Sinkins, 2004; Gavotte et al., 2009).
No advantage due to infection was observed for female larvae in the high density treatment. Emerging infected adults were underrepresented compared to their uninfected counterparts. Although a cause was not determined here, a prolonged development time observed for infected larvae at high density could explain this difference. According to one hypothetical model: in conditions that are more food-limited, a delay of development decreases the probability that adequate food resources will be obtained to complete development. However, at lower density/competition levels, the cost of slower development is masked by relatively high food levels.
Three possible mechanisms that could explain the slower developmental rate of infected individuals in the high density treatment are (i) infected individuals display a reduced capacity to capture or utilize resources compared to uninfected individuals, (ii) there are physiological costs associated with Wolbachia infection and/or (iii) Wolbachia could be advantageous for larvae as observed for adults (Dobson et al., 2004) but this advantage could be offset under highly competitive conditions by a decrease of symbiont density as observed previously (Dutton and Sinkins, 2004). A behavioral perturbation or a decrease of larval mobility induced by Wolbachia infection would result in decreased capacity to capture resources. Such mobility modification by Wolbachia was previously observed in an adult wasp (Fleury et al., 2000). Endosymbionts can tax resources of infected hosts, necessitating increased levels of resources and thus a longer time to achieve host development.
We have no experimental data to support or reject any of these three hypotheses, nor any evidence that they are mutually exclusive. Whatever the correct hypothesis, under our experimental conditions, delayed individuals emerge at the same body size (wing length) as their speedier competitors, so there was no size advantage gained from delayed emergence. However, it is relevant to note that prior studies comparing infected and uninfected A. albopictus adults have observed greater longevity and fecundity of infected adult females relative to uninfected adult females (Dobson et al., 2002).
Natural A. albopictus populations are infected with Wolbachia (Kittayapong et al., 2000; Sinkins et al., 1995), and natural loss of Wolbachia infection appears not to occur (Kittayapong et al., 2002). Therefore, direct competition between infected and uninfected A. albopictus larvae would occur in the field only during the period of Wolbachia invasion. However, while the interaction investigated in the present experiment represents a transient event in natural host populations, it is expected to be an important parameter affecting the success or failure of a novel Wolbachia invasion. The present experiment cannot differentiate between (i) the benefits of Wolbachia infection as a condition for endosymbiont invasion or (ii) the mutualistic co-evolution of benefits following initial infection.
Relating our results to field situations is not straightforward because reports of natural mosquito larval densities are rare. Natural populations are rarely composed of identically aged individuals; both natural habitat complexity (Alto et al., 2005) and food resources (Lord, 1999) are poorly represented by our model system. Moreover, temperature, which determines endosymbiont density (Mouton et al., 2006), varies throughout larval development. Nonetheless, the present results can inform the effects of Wolbachia in natural A. albopictus populations. Females lay their eggs in temporary habitats generally filled by rainwater. Leakage and evaporation tend to reduce the water volume and increase the larval density whereas rainfall and dew tend to increase the water volume. Likewise, resources will decrease unless they are replaced (e.g., falling plant material). Both larval physical encounters and per capita resources will change as habitat volume changes. As a result, intra-specific competition is not constant throughout a mosquito’s larval development. However, our results demonstrate that Wolbachia infection can affect intraspecific competitiveness as a function of larval density and may interact with other environmental factors not investigated here.
Impacts upon host reproduction by Wolbachia have been widely assumed to explain the spread of these endosymbionts (Mercot and Charlat, 2004; Sinkins, 2004). These induced phenotypes probably contribute importantly to Wolbachia expansion, but Wolbachia impacts upon other host developmental stages should not be neglected. Cytoplasmic incompatibility could interact synergistically with the differences in development that are described here. Specifically, CI can result in reduced larval density, since incompatible embryos fail to develop, and the results presented here demonstrate that conditions of lower competition can favor infected females. This bias toward infected females, when combined with the high fecundity of Wolbachia-infected adults (Dobson et al., 2002), could play an important role in the invasion success of Wolbachia in A. albopictus populations.
Acknowledgments
We greatly appreciate the technical assistance provided by James Mains and statistical advice from Rhonda D. VanDyke. This research was supported by NIH/NIAID R01-AI067434 and R01-AI051533.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
LAURENT GAVOTTE, Email: laurent.gavotte@univ-montp2.fr.
DAVID R. MERCER, Email: randy.mercer@gmail.com.
JOHN J. STOECKLE, Email: John.Stoeckle@gordon.edu.
STEPHEN L. DOBSON, Email: sdobson@email.uky.edu.
References Cited
- Alto BW, et al. Habitat complexity and sex-dependent predation of mosquito larvae in containers. Oecologia. 2005;146:300–10. doi: 10.1007/s00442-005-0198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, May RM. Coevolution of hosts and parasites. Parasitology. 1982;85(Pt 2):411–26. doi: 10.1017/s0031182000055360. [DOI] [PubMed] [Google Scholar]
- Bandi C, et al. Wolbachia in filarial nematodes: evolutionary aspects and implications for the pathogenesis and treatment of filarial diseases. Vet Parasitol. 2001;98:215–38. doi: 10.1016/s0304-4017(01)00432-0. [DOI] [PubMed] [Google Scholar]
- Bouchon D, et al. Evidence for widespread Wolbachia infection in isopod crustaceans: molecular identification and host feminization. Proc Biol Sci. 1998;265:1081–90. doi: 10.1098/rspb.1998.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtzis K, et al. Rescuing Wolbachia have been overlooked. Nature. 1998;391:852–3. doi: 10.1038/36017. [DOI] [PubMed] [Google Scholar]
- Bourtzis K, Miller TA. Insect Symbiosis. CRC Press LLC; Boca Raton: 2003. [Google Scholar]
- Cavalier-Smith T. The number of symbiotic origins of organelles. Biosystems. 1992;28:91–106. doi: 10.1016/0303-2647(92)90011-m. discussion 107–8. [DOI] [PubMed] [Google Scholar]
- Corsaro D, et al. Intracellular life. Crit Rev Microbiol. 1999;25:39–79. doi: 10.1080/10408419991299167. [DOI] [PubMed] [Google Scholar]
- Dale C, Moran NA. Molecular interactions between bacterial symbionts and their hosts. Cell. 2006;126:453–65. doi: 10.1016/j.cell.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Dedeine F, et al. Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc Natl Acad Sci U S A. 2001;98:6247–52. doi: 10.1073/pnas.101304298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson SL. Transfection of Wolbachia pipientis into Drosophila Embryos. Curr Protoc Microbiol. 2007;Chapter 3(Unit3A 4) doi: 10.1002/9780471729259.mc03a04s05. [DOI] [PubMed] [Google Scholar]
- Dobson SL, et al. Wolbachia-induced cytoplasmic incompatibility in single- and superinfected Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2001;38:382–7. doi: 10.1603/0022-2585-38.3.382. [DOI] [PubMed] [Google Scholar]
- Dobson SL, et al. Mutualistic Wolbachia infection in Aedes albopictus: accelerating cytoplasmic drive. Genetics. 2002;160:1087–94. doi: 10.1093/genetics/160.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson SL, Rattanadechakul W. A novel technique for removing Wolbachia infections from Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2001;38:844–9. doi: 10.1603/0022-2585-38.6.844. [DOI] [PubMed] [Google Scholar]
- Dobson SL, et al. Fitness advantage and cytoplasmic incompatibility in Wolbachia single- and superinfected Aedes albopictus. Heredity. 2004;93:135–42. doi: 10.1038/sj.hdy.6800458. [DOI] [PubMed] [Google Scholar]
- Dutton TJ, Sinkins SP. Strain-specific quantification of Wolbachia density in Aedes albopictus and effects of larval rearing conditions. Insect Mol Biol. 2004;13:317–22. doi: 10.1111/j.0962-1075.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- Fleury F, et al. Physiological cost induced by the maternally-transmitted endosymbiont Wolbachia in the Drosophila parasitoid Leptopilina heterotoma. Parasitology. 2000;121(Pt 5):493–500. doi: 10.1017/s0031182099006599. [DOI] [PubMed] [Google Scholar]
- Fry AJ, et al. Variable fitness effects of Wolbachia infection in Drosophila melanogaster. Heredity. 2004;93:379–89. doi: 10.1038/sj.hdy.6800514. [DOI] [PubMed] [Google Scholar]
- Gavotte L, et al. Wolbachia infection and resource competition effects on immature Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2009;46:451–9. doi: 10.1603/033.046.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcombe W, Hoffmann AA. Wolbachia effects in Drosophila melanogaster: in search of fitness benefits. J Invertebr Pathol. 2004;87:45–50. doi: 10.1016/j.jip.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, et al. Factors affecting the distribution of cytoplasmic incompatibility in Drosophila simulans. Genetics. 1990;126:933–48. doi: 10.1093/genetics/126.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huigens ME, et al. Natural interspecific and intraspecific horizontal transfer of parthenogenesis-inducing Wolbachia in Trichogramma wasps. Proc Biol Sci. 2004;271:509–15. doi: 10.1098/rspb.2003.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MS, Dobson SL. Wolbachia effects on Aedes albopictus (Diptera: Culicidae) immature survivorship and development. J Med Entomol. 2006;43:689–95. doi: 10.1603/0022-2585(2006)43[689:weoaad]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Jiggins FM, et al. Two male-killing Wolbachia strains coexist within a population of the butterfly Acraea encedon. Heredity. 2001;86:161–6. doi: 10.1046/j.1365-2540.2001.00804.x. [DOI] [PubMed] [Google Scholar]
- Kittayapong P, et al. Distribution and diversity of Wolbachia infections in Southeast Asian mosquitoes (Diptera: Culicidae) J Med Entomol. 2000;37:340–5. doi: 10.1093/jmedent/37.3.340. [DOI] [PubMed] [Google Scholar]
- Kittayapong P, et al. Maternal transmission efficiency of Wolbachia superinfections in Aedes albopictus populations in Thailand. Am J Trop Med Hyg. 2002;66:103–7. doi: 10.4269/ajtmh.2002.66.103. [DOI] [PubMed] [Google Scholar]
- Lan Q, Fallon AM. Sequence analysis of a mosquito ribosomal protein rpL8 gene and its upstream regulatory region. Insect Mol Biol. 1992;1:71–80. doi: 10.1111/j.1365-2583.1993.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Lipsitch M, et al. The population dynamics of vertically and horizontally transmitted parasites. Proc Biol Sci. 1995;260:321–7. doi: 10.1098/rspb.1995.0099. [DOI] [PubMed] [Google Scholar]
- Lord CC. Density dependence in larval Aedes albopictus (Diptera: Culicidae) J Med Entomol. 1999;35:825–829. doi: 10.1093/jmedent/35.5.825. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, Escher RL. Sex ratios of mosquitoes from long-term censuses of Florida tree holes. J Am Mosq Control Assoc. 2008;24:11–5. doi: 10.2987/5656.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercot H, Charlat S. Wolbachia infections in Drosophila melanogaster and D. simulans: polymorphism and levels of cytoplasmic incompatibility. Genetica. 2004;120:51–9. doi: 10.1023/b:gene.0000017629.31383.8f. [DOI] [PubMed] [Google Scholar]
- Montenegro H, et al. Fitness effects of Wolbachia and Spiroplasma in Drosophila melanogaster. Genetica. 2006;127:207–15. doi: 10.1007/s10709-005-3766-4. [DOI] [PubMed] [Google Scholar]
- Mouton L, et al. Effect of temperature on Wolbachia density and impact on cytoplasmic incompatibility. Parasitology. 2006;132:49–56. doi: 10.1017/S0031182005008723. [DOI] [PubMed] [Google Scholar]
- O'Neill SL, Karr TL. Bidirectional incompatibility between conspecific populations of Drosophila simulans. Nature. 1990;348:178–80. doi: 10.1038/348178a0. [DOI] [PubMed] [Google Scholar]
- Olsen K, et al. A field cage test of the effects of the endosymbiont Wolbachia on Drosophila melanogaster. Heredity. 2001;86:731–7. doi: 10.1046/j.1365-2540.2001.00892.x. [DOI] [PubMed] [Google Scholar]
- Rousset F, et al. Wolbachia endosymbionts responsible for various alterations of sexuality in arthropods. Proc Biol Sci. 1992;250:91–8. doi: 10.1098/rspb.1992.0135. [DOI] [PubMed] [Google Scholar]
- Sachs JL, Wilcox TP. A shift to parasitism in the jellyfish symbiont Symbiodinium microadriaticum. Proc Biol Sci. 2006;273:425–9. doi: 10.1098/rspb.2005.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searcy DG. Metabolic integration during the evolutionary origin of mitochondria. Cell Res. 2003;13:229–38. doi: 10.1038/sj.cr.7290168. [DOI] [PubMed] [Google Scholar]
- Sinkins SP. Wolbachia and cytoplasmic incompatibility in mosquitoes. Insect Biochem Mol Biol. 2004;34:723–9. doi: 10.1016/j.ibmb.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Sinkins SP, et al. Wolbachia pipientis: bacterial density and unidirectional cytoplasmic incompatibility between infected populations of Aedes albopictus. Exp Parasitol. 1995;81:284–91. doi: 10.1006/expr.1995.1119. [DOI] [PubMed] [Google Scholar]
- Stewart FJ, Cavanaugh CM. Bacterial endosymbioses in Solemya (Mollusca: Bivalvia)--model systems for studies of symbiont-host adaptation. Antonie Van Leeuwenhoek. 2006;90:343–60. doi: 10.1007/s10482-006-9086-6. [DOI] [PubMed] [Google Scholar]
- Stouthamer R, et al. Antibiotics cause parthenogenetic Trichogramma (Hymenoptera/Trichogrammatidae) to revert to sex. Proc Natl Acad Sci U S A. 1990;87:2424–7. doi: 10.1073/pnas.87.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng HJ, Apperson CS. Development and survival of immature Aedes albopictus and Aedes triseriatus (Diptera: Culicidae) in the laboratory: effects of density, food, and competition on response to temperature. J Med Entomol. 2000;37:40–52. doi: 10.1603/0022-2585-37.1.40. [DOI] [PubMed] [Google Scholar]
- Tseng M. Sex-specific response of a mosquito to parasites and crowding. Proc Biol Sci. 2004;271(Suppl 4):S186–8. doi: 10.1098/rsbl.2003.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavre F, et al. Phylogenetic status of a fecundity-enhancing Wolbachia that does not induce thelytoky in Trichogramma. Insect Mol Biol. 1999;8:67–72. doi: 10.1046/j.1365-2583.1999.810067.x. [DOI] [PubMed] [Google Scholar]
- Werren JH. Biology of Wolbachia. Annu Rev Entomol. 1997;42:587–609. doi: 10.1146/annurev.ento.42.1.587. [DOI] [PubMed] [Google Scholar]
- Zhou W, et al. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc Biol Sci. 1998;265:509–15. doi: 10.1098/rspb.1998.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]