Abstract
Synthetic Immunology, the development of synthetic systems capable of modulating and/or manipulating immunological functions, represents an emerging field of research with manifold possibilities. One focus of this area has been to create low molecular-weight synthetic species, called antibody-recruiting molecules (ARMs), which are capable of enhancing antibody binding to disease-relevant cells or viruses, thus leading to their immune-mediated clearance. This article provides a thorough discussion of contributions in this area, beginning with the history of small-molecule-based technologies for modulating antibody recognition, followed by a systematic review of the various applications of ARM-based strategies. Thus, we describe ARMs capable of targeting cancer, bacteria, and viral pathogens, along with some of the scientific discoveries that have resulted from their development. Research in this area underscores the many exciting possibilities at the interface of organic chemistry and immunobiology, and is positioned to advance both basic and clinical science in the years to come.
Keywords: Antibody-Recruiting Molecules (ARMs); synthetic; bifunctional molecules capable of inducing antibodies to bind disease-relevant proteins; cells, or organisms; Synthetic immunology; the creation of synthetic systems that perform complex immunological functions; Antibody; protein that is produced by B-cells which identifies and neutralizes disease associated objects; Immunomodulators; rationally-designed molecules that can affect components of the immune system; Immunotherapy; disease treatment that utilizes; induces; suppresses, or enhances components of the immune system; bifunctional molecule; a molecule that possesses two distinct binding targets; ternary complex; an assembly containing three distinct species; held together either through covalent or non-covalent bonds
Introduction
The introduction of cowpox (vaccinia) virus immunization by Edward Jenner in 1796 was a landmark moment in the history of medicine.(1) Not only did Jenner’s vaccination strategy ultimately lead to the eradication of smallpox, it made clear the extraordinary power of a person’s own immune system for warding off deadly illness. Subsequent advances, including the implementation of passive antibody therapy or “serum therapy” in the late 1800s,(2) and Milstein and Kohler’s report of the first engineered monoclonal antibody in the 1970s,(3) have paved the way for a new revolution in immunotherapeutics focused on monoclonal antibody-based drugs. The first member of this class, Muromonab-CD3 (anti-CD3 or OKT3),(3) was cleared by the FDA in 1986 for treating transplant rejection, and since that time, the number of antibody drugs has increased dramatically; 31 agents are currently approved for clinical use, and more than 300 are undergoing clinical trials.(4–7) Furthermore, in 2011 alone, antibody-based therapies grossed $44.6 billion worldwide and this number has been predicted to increase in the upcoming years.(6, 8, 9)
The surge in popularity of monoclonal antibodies can be easily understood in light of their many extraordinary properties. Antibody molecules are readily generated against a variety of disease-relevant targets, some of which have been conventionally considered “undruggable”.(10) Additionally, because antibodies often interact with their targets with excellent affinity and specificity, undesirable side-effects related to off-target binding are thought to be low relative to traditional small-molecule-based therapeutics. Finally, antibodies may elicit therapeutic responses by a variety of mechanisms including inhibition of protein function,(11, 12) targeting the delivery of cytotoxic drugs,(13–15) and triggering immune effector responses.(16–18)
There are two mechanisms by which antibodies can trigger immune-mediated cytotoxicity: engagement of plasma complement proteins and/or direct activation of immune effector cells. The former process, termed complement-dependent cytotoxicity (CDC), begins upon cell-surface immobilization of certain members of the complement protein family (e.g., C1q) by opsonizing antibodies. This event initiates a downstream proteolytic cascade culminating in direct cell lysis or recruitment of complement-receptor-expressing effector cells, ultimately leading to target cell clearance.(19) Alternatively, binding of the antibody’s crystallizable fragment (Fc) to Fc-receptors expressed on the surface of various immune cells can lead to receptor crosslinking, followed by target cell phagocytosis or the release of potent oxidizing agents and protein toxins (e.g., granzyme and perforin).(20) These processes are termed antibody-dependent cellular phagocytosis (ADCP), and/or antibody-dependent cellular cytotoxicity (ADCC), respectively.(19) Importantly, because Fc receptor-mediated cytotoxicity can enhance the processing and presentation of disease-relevant antigens, these cellular mechanisms have the capacity to give rise to long-lasting adaptive immunity.(21–24)
These advantages notwithstanding, antibody-based therapeutics suffer from certain limitations that arise primarily from their high molecular weights and peptide structures. For example, antibody administration can result in systemic inflammatory response syndrome, IgE-mediated acute anaphylactic reactions, serum sickness, and cytokine release syndrome, all of which have the potential to be life-threatening.(25) In addition, because antibody therapeutics contain “non-self” protein sequences, they can elicit host immune reactions, thus counteracting their efficacy.(25) Although the widespread “humanization” of antibody protein sequences has drastically reduced the potential for undesired immune responses, such reactions remain problematic clinically.(26, 27) Another limiting factor is that individuals can express different allelic variants of Fc receptors, which can directly impact the efficacy of “naked” monoclonal antibodies intended to act through interaction with these receptors.(28) Antibody-toxin conjugates, developed to address such difficulties, are associated with other drawbacks. These include premature drug release arising from antibody–drug linker instability, which can lead to toxic effects in normal tissues.(29) In addition, identifying optimal levels of drug conjugation can be difficult; low loadings may render treatment ineffective, while high loadings may lead to excess toxicity or a loss of antigen specificity.(30) Other limitations of antibody-based therapeutics include a lack of oral bioavailability, difficulties in standardization and characterization, and high costs.(25, 30–32)
Researchers have begun to investigate the development of low molecular weight species (“small molecules”) that possess the complex functional properties of antibodies and other biologics. Because small molecules are generally inexpensive to produce, optimizable for oral bioavailability,(29) and unlikely to cause unwanted allergic or immunogenic responses,(31) these agents may be able to address many of the limitations of biologics without compromising their advantages. These studies have motivated efforts in Synthetic Immunology, a field focused on developing synthetic systems and strategies for controlling and/or creating human immunity.(33, 34) Despite still being in its infancy, this research area has already given rise to a number of exciting strategies, with potential applications in both basic and biomedical science.
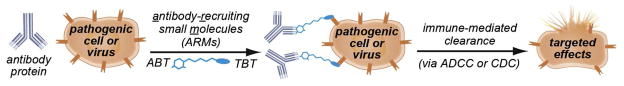
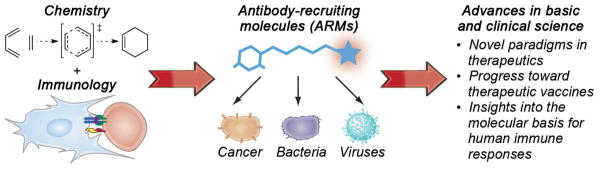
We focus here on antibody-recruiting small molecules (ARMs), which we define as synthetic, bifunctional molecules capable of inducing antibodies to bind disease-relevant proteins, cells, or organisms (Figure 1). Simultaneous association of ARMs with antibodies and surface-exposed receptors results in the formation of ternary complexes, which can elicit antibody-dependent immune effector responses. By convention, we define the two regions of ARMs as the target-binding terminus (TBT), which recognizes the disease-associated protein target (either present on cell/viral surfaces or free in solution), and the antibody-binding terminus (ABT), which associates with anti-hapten antibodies. Notably, we have chosen to limit the scope of this Review to technologies in which synthetic (i.e., non-recombinant), organic ligands are employed to control the immunological functions of antibody proteins; therefore, a variety of interesting and important systems, including antibody-drug conjugates,(35) antibody-based recombinant constructs (e.g., bispecific antibodies, diabodies, and others), (36–38) synthetic vaccines, (39) and ligand-templated supramolecular assemblies, (40–44) are not covered in detail herein.
Figure 1.
Antibody Recruiting Small Molecules (ARMs). ARMs are bifunctional small molecules that function by forming ternary complexes with disease-relevant targets and endogenous antibodies. Ternary complex assembly leads to the activation of immune effector functions, followed by immune-mediated cytotoxicity and/or clearance of disease-causing species
As early as the 1970’s, artificially engineered systems were shown to redirect antibody responses to the surfaces of cells that are ordinarily non-immunogenic. For example, liver, spleen, and red blood cells covalently modified with small molecule haptens, such as trinitrophenyl (TNP) groups, were shown to induce antibody-dependent cell-mediated immune responses.(45, 46) Furthermore, anti-DNP antibodies of IgG and IgM isotypes were shown to target liposomes labeled with dinitrophenyl (DNP) groups, leading to the induction of a complement-mediated cytotoxic response.(47) In all of these cases, antibody targeting and cytotoxicity were shown to be hapten-dependent; that is, antibodies were directed as a result of covalent DNP or TNP labeling. Although hapten labeling in these cases was non-specific, these early studies were critical in demonstrating that antibody-mediated immune responses could be templated by “non-native,” synthetic materials.
Subsequently, strategies utilizing rationally-designed bifunctional systems to redirect the immune response against disease-relevant targets began to emerge. For example, chimeric proteins consisting of IgG or IgM Fc domains fused to human CD4, the cell-surface receptor target of HIV gp120, were shown to bind both to the complement protein C1q and to Fc-receptors.(48–50) These “immunoadhesins” were further shown to enhance immune effector response selectively against HIV infected cells in the presence of peripheral blood mononuclear cells (PBMCs), while inflicting minimal background cytotoxicity on uninfected cells. These pioneering studies represent the first evidence that rationally-designed, bifunctional molecules could specifically target immune-mediated functions to pathogenic proteins.
Subsequent research efforts expanded on these findings by demonstrating that proteins derivatized with small-molecule haptens could also possess immunomodulatory properties. For example, soluble CD4 covalently modified with the dinitrophenyl (DNP) motif was shown to mediate the formation of a quaternary complex between gp120, anti-DNP antibodies, and soluble complement protein C1q.(51) Similarly, a dimeric Fab fragment (F(ab′)2) directed against the T-cell marker anti-thymocyte globulin (ATG), covalently labeled with fluorescein, was shown to induce selective cytotoxicity against T-cells in the presence anti-fluorescein antibodies and complement proteins.(52) Additionally, treatment of fluorescein-immunized mice with this molecule resulted in the clearance of peripheral ATG-expressing T-cells. Together these studies were critical in setting the stage for developing bifunctional ARMs.
Anti-hapten Antibodies Used in ARM Strategies
To date, ARMs have incorporated two types of functionality at the ABT: (1) small molecule ligands for “endogenous” antibodies, or (2). rationally-designed functional handles, which require delivery of pre-formed antibody-small molecule conjugates or pre-immunization for induction of selective antibody responses. Perhaps the most common targets in the first category include the galactosyl-(1–3)-galactose (α-Gal) carbohydrate epitope and the 2,4-dinitrophenyl (DNP) motif. Intriguingly, 2–8% of circulating antibodies in the bloodstream are believed to recognize the α-Gal trisaccharide,(80, 81) and these are believed to arise following exposure to this carbohydrate on the surfaces of cells derived from prokaryotes and non-primate eukaryotes.(82) Although DNP and other nitroarenes are not likely the products of biosynthesis,(83) unlike α-Gal, approximately 1% of circulating antibodies in the human bloodstream have been shown to recognize this epitope.(83, 84) Although the origin of anti-DNP antibodies is not known, one potential route of human inoculation involves exposure to DNP-containing dyes, preservatives, and/or pesticides,(85) which have been detected as environmental contaminants throughout the United States.(86, 87) An alternative hypothesis is that dietary ingestion of proteins and/or peptides containing nitroaromatic amino acids, formed in foods during the cooking process,(88) leads to adaptive immune responses against hapten-containing neo-epitopes,(89–91) perhaps in a TLR-independent manner.(92)
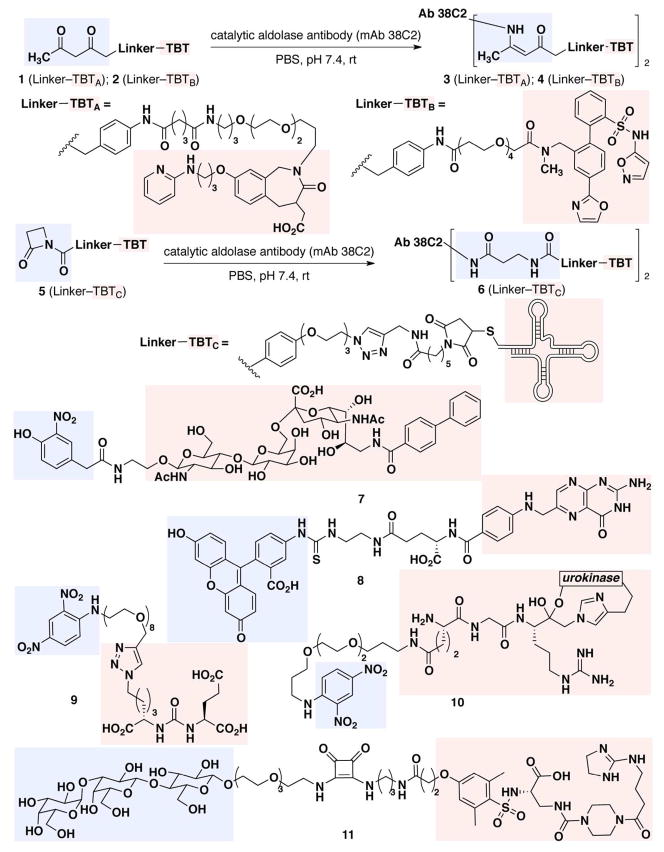
The second class of ABT that has been used in ARM strategies is composed of non-native antigens. Although such motifs by definition are not expected to bind pre-existing antibody proteins, humoral immune responses against these epitopes are readily induced by immunization with the hapten of interest conjugated to a carrier protein. One advantage of this approach is that haptens with useful chemical and/or physical properties can be chosen. For example, anti-fluorescein antibodies can easily be induced through immunization with protein conjugates of fluorescein isothiocyanate (FITC),(62, 93) and the haptens’ photophysical properties provide a convenient handle for binding and imaging studies. Another useful immunization-dependent strategy exploits the unique properties of catalytic aldolase antibodies, which can form covalent adducts with 1,3-diketones (Figure 2, 1 → 3; 2 → 4) or β-lactam (Figure 2, 5 → 6) functionalities.(65, 94–97) Antibodies specific for the 1,3-diketone functionality can either be administered passively or generated through reactive immunization with a 1,3-diketone-KLH conjugate.(98)
Figure 2.
Chemical structures of cancer-targeting ARMs. TBTs are highlighted in red boxes and ABTs in blue boxes
Applications of Antibody Recruiting Small Molecules in Disease Targeting
Advances in synthetic and biophysical chemistry have enabled researchers to construct bifunctional small molecules against a broad variety of structurally unrelated disease-relevant targets. Due to their modular nature, ARMs are able to form immuno-modulatory ternary complexes with various macromolecular species, simply as a function of the structure and recognition properties of the TBT. Thus, applications of the ARM strategy have included both cancer and infectious disease (Table 1), and suggest a number of additional possibilities for future therapeutic development.
Table 1.
Summary of the applications of antibody-recruiting small-molecules to disease targets
| Disease / Pathogen | Target | Antibody-binding Moiety | Molecule Type | In vitro response | In vivo model | Ab source |
|---|---|---|---|---|---|---|
| E. coli(53, 54) | mannose receptor | avidin | small molecule-protein conjugate | CDC, phagocytosis | - | commercial |
| E. coli(55) | mannose receptor | α-Gal | peptide | inhibition of agglutination | - | endogenous |
| Gram-positive bacteria(56, 57) | D-Ala-D-Ala | fluorescein | polymer | phagocytosis | - | commercial |
| HIV(58) | gp41 | α-Gal | peptide | viral inhibition | - | endogenous |
| HIV(59) | gp120 | Gal(α1–3)Gal | peptide | CDC, ADCC | - | endogenous |
| HIV(60) | gp120 | DNP | small molecule | CDC, viral inhibition | - | commercial |
| HIV(61) | CCR5 | β-lactam | mAb-small molecule conjugate | viral inhibition | - | monoclonal antibody |
| lung cancer(62–64) | folate receptor | fluorescein DNP, | small molecule | CDC, ADCC, phagocytosis | syngeneic female Balb/c | immunization |
| Karposi’s sarcoma, colon cancer, melanoma(65–67) | integrin receptors (αvβ3 and αvβ5) | 1,3-diketone | mAb-small molecule conjugate | CDC, ADCC | female nude and SCID xenografts | monoclonal antibody |
| prostate cancer(68) | ETA | 1,3-diketone | mAb-small molecule conjugate | opsonization | nude xenograft | monoclonal antibody |
| breast cancer, melanoma, osteosarcoma, Karposi’s sarcoma(69, 70) | αvβ5 | α-Gal | small molecule | CDC | - | endogenous |
| B cell lymphoma(71, 72) | CD22 | nitrophenol | small molecule, polymer | opsonization | - | commercial |
| colon cancer, melanoma(73) | integrin receptors (αvβ3 and αvβ5) | 1,3-diketone | small molecule | ADCC | syngeneic female BALB/c, and C57BL6 | reactive immunization |
| melanoma, ovarian adenocarcinoma(74) | integrin receptors (αvβ3 and αvβ5) and LHRH receptor | β-lactam | mAb-small molecule conjugate | opsonization | - | monoclonal antibody |
| prostate cancer(75) | PSMA | DNP | small molecule | ADCC | - | commercial |
| umbilical cord endothelial cells (HUVEC)(76) | VEGF | β-lactam | RNA aptamer | inhibition of cell migration | female athymic nude mice (pharmacokinetic) | monoclonal antibody |
| colon adenocarcinoma breast carcinoma(77), | VEGF and Ang2 | β-lactam (azetidinone) | mAb-small molecule conjugate | inhibition of receptor binding | female athymic nude mice | monoclonal antibody |
| prostate cancer(78) | PSMA | DNP | small molecule | ADCC | hu-PBL-NOD/SCID | immunization |
| colon adenocarcinoma, glioblastoma(79) | uPAR | DNP | small molecule-protein conjugate | ADCC, phagocytosis | - | commercial |
Cancer
Cancer is one of the leading causes of death worldwide, and is believed to be responsible for one in every four deaths in the United States.(99) Traditional treatment options for patients suffering with malignancies include surgical resection, direct irradiation, and cytotoxic chemotherapy, all of which are frequently associated with severe side-effects.(100–103) Recently, biologic agents and cellular immunotherapies have emerged as popular alternatives for cancer treatment, and due to their high specificity, these modalities have the potential to address many of the problems associated with traditional chemotherapies (e.g., off-target effects, etc.).(104) Indeed, with the inclusion of monoclonal antibodies into the repertoire, cancer therapeutics grossed $18.5 billion in sales in 2009 alone. Despite these successes, available anticancer agents remain inadequate for most patients suffering with cancer, and the demand for novel, targeted therapies is growing.(105) To this end, ARM technologies may represent promising alternatives for these patients, and have the potential both to complement, and improve upon, available anti-cancer modalities.
Barbas and colleagues were among the first investigators to demonstrate the benefits of combining small molecules with antibody proteins for cancer-relevant applications. These researchers have primarily exploited a “catalytic monoclonal antibody,” mAb 38C2, capable of reacting with the 1,3-diketone moiety to form an enaminone (Figure 2, 1 → 3; 2 → 4). mAb 38C2 has been conjugated to various β-diketone-containing small-molecule TBTs to generate “chemically programmed antibodies” capable of recognizing various cell-surface targets. For example, the conjugate cp38C2 (Figure 2, 3),(65–67, 73) targets the αvβ3 and αvβ5 integrins,(106, 107) cell-surface proteins that are highly overexpressed in a wide variety of cancers, including ovarian, cervical, breast, and melanoma.(108, 109) This construct has been shown to mediate CDC and ADCC against M21 cells, and it demonstrated remarkable efficacy in human M21 melanoma cell xenograft models (81% average reduction in tumor growth after 42 days).(67) Compound cp38C2 also can inhibit metastasis of M21 tumor cells in female SCID mice, more than doubling their median survival versus untreated controls.
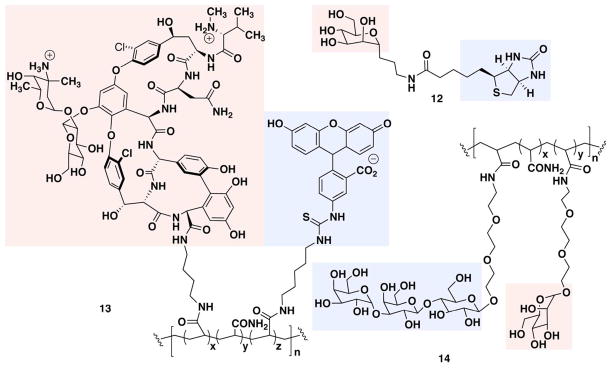
Figure 3.
Bacteria-targeting ARMs, with TBTs in red boxes and ABTs in blue boxes
Subsequent studies from the Barbas group eliminated the requirement to use exogenous antibody proteins in their chemically-programmed antibody strategy. Using syngeneic murine colon cancer (CT26) and melanoma (B16) models, it was demonstrated that wild-type BALB/c mice, pre-immunized with a diketone hapten (JW-KLH), produced aldolase antibodies capable of forming covalent adducts with synthetic diketone groups. Once “programmed,” these animals were treated with either 1 or cRGD-dk, a cyclic-peptide-based integrin-targeting conjugate, and tumor-specific ADCC responses were observed, resulting in approximately 75% tumor reduction compared with vehicle control.(73)
Another useful function of small molecule conjugates with aldolase antibodies, termed “CovX-bodies,” is to improve the pharmacokinetic properties of therapeutic compounds. For example, mAb 38C2 conjugate 4 has been employed as a delivery vehicle for a metabolically-labile small-molecule inhibitor of endothelin A (ETA),(68) a receptor involved in neovascularization and implicated in cancer, renal failure, heart failure, and hypertension.(110–112) This conjugate exhibited efficacy in a murine xenograft model of human prostate cancer (PC-3) and demonstrated up to a 45% inhibition of tumor growth versus controls. This antibody-attachment strategy has also been employed to minimize toxic side-effects of the HIV entry inhibitor Aplaviroc,(61) and to stabilize peptide-based targeting agents such as VEGF(77) and angiogenesis inhibitors of thrombospondin-1.(113) Indeed, a CovX-body containing a peptide-based angiopoietin-2 inhibitor (CVX-060) is currently being evaluated in a Phase II clinical trial in patients with advanced renal cell carcinoma.(2, 114)
Recent work from the Barbas lab has taken the ARM concept into a variety of novel directions. For example, these authors have demonstrated that the catalytic antibody mAb 38C2 can be conjugated with bifunctional small molecules, enabling them to target two different surface macromolecules: the integrin receptors (αvβ3 and αvβ5) and the luteinizing hormone releasing hormone receptors (LHRH-R).(74) More recent investigations have shown that conjugating mAb 38C2 to ARC245 (5), an RNA aptamer capable of binding VEGF, can increase the ARC245 serum half-life from minutes to 21 hours.(76) Given the flexibility of available screening methods for identifying selective, high-affinity aptamers,(115, 116) this strategy has the potential to greatly accelerate the process of TBT discovery against numerous disease-relevant targets.
Reports from the Paulson and Bundle groups have disclosed a class of ARMs for targeting B-cell lymphomas through multivalent interactions with CD22,(71, 72) a cell-surface regulator of B-cell signaling overexpressed in malignant cells.(117) To this end, a bifunctional molecule (7) was constructed containing an o-nitro phenol element at the ABT to bind endogenous anti-nitrophenol (anti-NP) antibodies and a glycan sequence at the TBT for binding CD22. By complexing this construct with decavalent anti-NP IgM, these authors demonstrated an increase in binding avidity for CD22-expressing cells of approximately two orders of magnitude versus control conditions lacking anti-NP IgM. Follow-up studies demonstrated that further increases in TBT valency, through conjugation to a polymer support, led to 100-fold higher levels of anti-NP IgM recruitment to target B-cells versus unconjugated compounds. (72)
The Low group has developed ARMs targeting the folate receptor (FR), a cell-surface protein overexpressed in many cancers, by utilizing folic acid at the TBT and either fluorescein (8) or DNP at the ABT.(62–64) For example, when co-administered with IL-2, construct 8 enhanced median survival by 250% in wild-type female BALB/c mice grafted with FR positive M109 lung tumors and pre-immunized with BSA-FITC or KLH-FITC.(62) Co-administration of this fluorescein-folate construct with both IL-2 and IFN-γ enhanced survival by 300%. Follow-up studies suggested that anti-tumor effects resulted from ADCP and ADCC mechanisms,(63) and substitution of the fluorescein group with DNP did not diminish efficacy in these models.(64) Notably, FR-targeted ARMs were found to induce long-lasting immunity against FR-expressing tumors; re-challenge of mice previously treated with M109 tumor cells led to rejection of tumor without introduction of any additional ARM.(118) Although details relevant to the mechanism of this memory effect were not reported, depletion of both CD4+ and CD8+ T cells resulted in no protective immunity upon tumor re-challenge. These results suggest the intriguing possibility that ARMs can be utilized as therapeutic vaccines,(119) consistent with data reported for monoclonal antibody therapies.(120–123)
Seeking to exploit the widespread prevalence of endogenous anti-α-Gal antibodies in the human bloodstream,(69) Kiessling and coworkers synthesized bifunctional constructs containing known integrin-binding functionality at the TBT and the α-Gal trisaccharide motif at the ABT (11). These ARMs proved effective at inhibiting integrin-mediated cell adhesion, recruiting anti-α-Gal antibodies, and mediating complement-dependent cytotoxicity against various cancer cells using normal human serum as the sole source of anti-α-Gal antibodies and complement proteins.(70) Cytotoxicity studies comparing this ARM with a toxin-conjugate in which the α-Gal trisaccharide was replaced by doxorubicin, revealed the antibody-recruiting agent to be more selective; construct 11 only exhibited activity against cells expressing high levels of integrin, while the doxorubicin conjugate proved cytotoxic to cells expressing both high and low levels of integrin. Based on these findings, the authors concluded that non-linear increases in antibody binding avidities due to multivalent interactions enable the ARM-based agents to select for cells expressing more than a “threshold” level of target receptor.
Anticancer efforts in the Spiegel laboratory have focused on the development of antibody-recruiting small molecules directed against prostate cancer cells, called ARM-Ps.(75, 124) These bifunctional molecules contain a glutamate urea moiety at the TBT for targeting the prostate specific membrane antigen (PSMA). This surface-bound protein is overexpressed in most subtypes of prostate cancer cells,(125) as well as in the neovasculature of many solid tumors (e.g., glioblastoma multiforme,(126) bladder cancer,(127) gastric and colorectal cancer(128)). An ARM-P derivative containing 8 oxyethylene units in the linker, called ARM-P8 (9), was found to possess an optimal compromise between affinity to PSMA and ability to form ternary complex. Follow-up studies demonstrated that ARM-P8 could mediate ADCC against PSMA-expressing prostate cancer cells in the presence of anti-DNP antibodies and peripheral blood mononuclear cells (PBMCs). Interestingly, high concentrations of ARM-P8 were found to serve as auto-inhibitors of ternary complex formation, both in biophysical and cell viability assays. Analogous observations have been made previously in systems involving bifunctional molecules that template ternary linkages, and these observations support ternary complex formation as a necessary pre-condition for ARM-P8-mediated ADCC.(63, 71) Furthermore, the amount of ternary complex was shown to be directly dependent on the concentration of antibody, indicating that levels of anti-DNP antibody in serum can directly affect the efficacy of ARM-P8.(75)
DUPA, an ARM-P8 homolog, was recently evaluated in a humanized NOD/SCID mouse model. Thus, NOD/SCID animals transfused with human PBMCs and xenografted with either LNCaP (PSMA-positive) or PSMA-negative (PSMA-negative) tumors were immunized with DNP-KLH.(78) DUPA administration was found both to inhibit tumor growth and prolong survival in an antibody- and PSMA-dependent fashion. Animals that were not pre-immunized against DNP, or that were xenografted with PSMA-negative DU145 tumors, were unaffected by DUPA treatment. Taken together, these studies provide further support the potential utility of ARMs in clinical applications.
During the course of characterizing ARM-P8, researchers in the Spiegel laboratory observed that ARM-P derivatives with short linker regions displayed surprisingly strong PSMA-binding affinities. Follow-up biochemical, crystallographic, and computational studies, led to the serendipitous discovery of an arene-binding site on PSMA that can accommodate electron poor aromatic rings, such as DNP.(124) Although the interaction of the DNP moiety with the arene-binding site appears to involve only two amino acids, it affords a potency increase in PSMA binding of up to two orders of magnitude. Next-generation ARM-Ps that take advantage of this arene-binding site interaction are expected to show significant enhancements in PSMA binding affinity versus available derivatives.
More recently, researchers in the Spiegel laboratory synthesized an antibody-recruiting molecule called “ARM-U” (10), which targets the urokinase-type plasminogen activator receptor (uPAR).(79) uPAR is expressed on the surfaces of breast, colon, stomach, and bladder cancers,(129, 130) and has been used as a diagnostic marker for malignancy.(131–135) ARM-U has been shown to target uPAR at a the high affinity uPA binding site, recruit anti-DNP antibodies to uPAR-expressing A172 human glioblastoma cells, and ultimately mediate ADCP and ADCC in an antibody- and uPAR-specific manner. These studies underscore the generality of the ARM strategy for cancer treatment.
Infectious disease (Bacteria and Viruses)
The World Health Organization (WHO) has estimated that infectious agents (viruses, bacteria and parasites) are responsible for approximately 25% (15 million) of global deaths each year and are the predominant cause of mortality in developing nations.(136, 137) Vaccines are considered to be among the most successful strategies for fighting infectious disease,(138) and such strategies have been extremely successful in combating agents such as typhoid, cholera, rabies, measles, mumps, hepatitis B, rubella, tetanus and polio. Despite these achievements, current vaccination strategies are limited by difficulties in production,(139) variable levels of immunostimulation, and high costs. (140) (141, 142)
Although traditional small-molecule-based antibacterial and antiviral therapeutics have proven extremely successful, their utility has been hampered by surges of rapid resistance.(143, 144) Monoclonal antibody-based therapies have demonstrated substantial preclinical success in treating various infectious diseases;(145–148) however, despite their therapeutic promise, only a small percent of antibodies currently in development are indicated for infectious disease treatment.(148) To date, only one antibody-based antiviral agent (against respiratory syncytial virus, Palivizumab) has obtained FDA approval, and there are no clinically-approved monoclonal antibodies that target bacterial pathogens.(149, 150) Thus, there is a critical need to develop new therapeutic strategies targeting infectious agents.
Bacteria
The therapeutic arsenal against bacterial infection has largely consisted of natural products and synthetic small molecules. Conventional antibiotics act by targeting vital bacterial functions such as cell wall synthesis, protein synthesis, RNA transcription, and DNA replication. Because many bacterial species share common essential targets, these agents are often harmful to native flora as well as pathogenic microbes, and can increase host susceptibility to certain infections.(151, 152) Furthermore, the emergence of organisms that are resistant to many, if not all, available agents has proven increasingly problematic. Indeed, there has been significant recent interest in the development of monoclonal antibodies for treating drug-resistant bacteria, in part because these agents could exploit mechanisms distinct from conventional antibiotics, making them less likely to induce cross-resistance.(147) Despite this, out of 13 mAbs currently in clinical development for treating bacterial infection, none have demonstrated significant efficacy.(147) Antibacterial therapeutics with novel mechanisms of action would therefore be highly desirable.(153–155)
A variety of ARM-based antibacterial strategies have been evaluated. The first example of such an approach was reported by Bednarski and colleagues and employed a rationally designed, bifunctional molecule capable of directing anti-avidin antibodies to E. coli (Figure 3, 12).(53, 54) Biotin was conjugated to the C-glycoside of mannose, a known ligand for bacterial mannose receptors, and this construct was shown to recruit anti-avidin antibodies to the surface of E. coli in a manner dependent on the presence of conjugate, avidin and antibodies. These researchers further demonstrated that complexes between avidin, antibody and ARM could mediate complement- and macrophage-dependent cytotoxicity in a manner competable by α-mannopyranoside. Interestingly, the inherent multivalency of avidin significantly enhanced the millimolar binding affinity of the C-glycoside ligand to the mannose receptor.
Wang and coworkers expanded this concept by developing bifunctional polymers capable of redirecting endogenous anti-α-Gal antibodies to E. coli.(55) Using chemo-enzymatic synthesis, these researchers constructed a polymeric ARM derivative containing poly-mannose as the TBT and poly-α-Gal as the ABT (14). These bifunctional polymers were shown to bind both E. coli mannose receptors and endogenous anti-α-Gal antibodies from human serum using competition ELISA experiments.
More recently, Whitesides and coworkers(56, 57) developed ARMs that target pathogenic bacteria by utilizing the potent antibiotic vancomycin. Polyvalent polymers containing fluorescein at the ABT and vancomycin at the TBT were synthesized (13) and shown to redirect anti-fluorescein antibodies to the surface of various Gram-positive bacteria (S. epidermidis, S. pneumoniae, and S. aureus). Using fluorescence microscopy and flow cytometry, the authors demonstrated that the antibody-recruiting polymer could mediate phagocytosis of opsonized bacteria in the presence of anti-fluorescein antibodies.
Viruses
Although vaccine-based strategies have been extremely successful against various viral diseases, a significant number of viral pathogens that have proven refractory to such approaches (e.g., HIV, herpes simplex viruses, etc) still remain.(156, 157) For the most part, available antiviral agents function by inhibiting enzymes such as reverse transcriptase, polymerase, protease, integrase, primase, and neuraminidase,(158) and their utility is limited by resistance development,(159, 160) low efficacy,(161) and the high rate of spontaneous mutation inherent to the viral lifecycle.(162) Monoclonal antibody therapies targeting viruses have experienced only modest success, and only a single such agent, which targets respiratory syncytial virus (RSV), has been approved for clinical use.(150) Novel technologies with the potential to harness the endogenous immune response in killing viral pathogens could be profoundly useful in the fight against viral diseases.
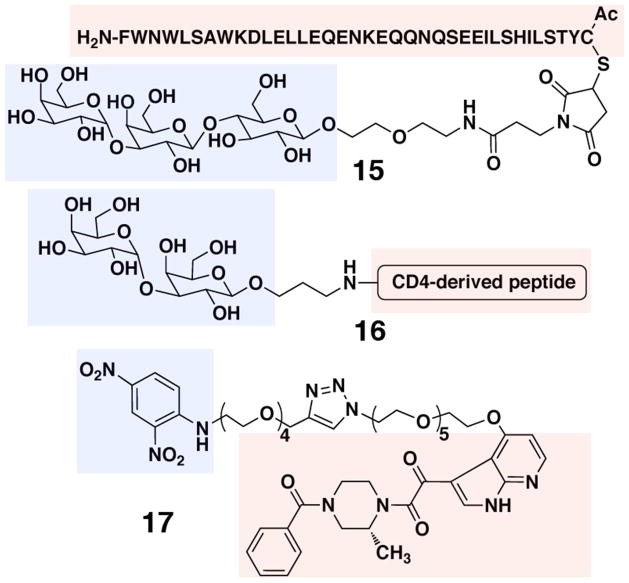
An early example of an ARM-based antiviral strategy was described by Wang and colleagues (Figure 4).(58) Using chemo-enzymatic synthesis, these researchers prepared a bifunctional molecule designed to redirect endogenous anti-α-Gal antibodies to HIV. This agent incorporated the α-Gal trisaccharide epitope at the ABT and was linked to the 36-amino acid gp41 fusion inhibitory peptide, T-20, at the TBT (15). The authors subsequently demonstrated that functionalization of T-20 had minimal effects on its ability to inhibit virus fusion, and that the bifunctional glycopeptide could bind anti-α-Gal IgG and IgM antibodies from human serum.
Figure 4.
Virus-targeting ARMs, with TBTs in red boxes and ABTs in blue boxes
More recently, Valhne, et al. developed a series bifunctional glycopeptides capable of mediating immune responses against HIV-infected cells.(59) These constructs were derived by chemically linking the α-Gal disaccharide to a series of 15-mer oligopeptides derived from the gp120-binding region of CD4 (16). Using ELISA and immunofluorescence microscopy, the authors then showed that these bifunctional glycopeptides could redirect endogenous α-gal antibodies from human serum to both immobilized and cell-surface-expressed gp120. Additional assays then demonstrated that the presence of human antibodies enhanced the fusion inhibitory activity of the peptide by 10% versus control conditions. Interestingly, an additional 5–15% enhancement in inhibition was observed when complement-preserved human serum was used in HIV infectivity assays, which was attributed to the direct cytolytic action of complement proteins on HIV-infected cells. Glycopeptide-derived ARMs were also shown to mediate immune responses against chronically HIVIIIB/LAV-infected ACH2 cells in the presence of human serum and isolated natural killer (NK) cells via an ADCC mechanism, although some analogs proved cytotoxic even in the absence of NK cells.
The Spiegel laboratory has recently developed a non-peptidic ARM, called ARM-H (antibody recruiting molecule targeting HIV).(60) This bifunctional small molecule incorporates a derivative of the known small-molecule fusion inhibitor BMS-378806 at the TBT,(163) along with the DNP motif at the ABT (17). ARM-H-mediated formation of ternary complex with anti-DNP antibodies and HIV-1 Env-expressing cells was shown to induce complement-dependent destruction of these cells. Furthermore, ARM-H can bind gp120 competitively with CD4, and also inhibit the entry of HIV-1 virus into human T-cells. Thus, ARM-H has the potential to interfere with the survival of HIV through multiple complementary mechanisms.
In general, by converting virulence factors (e.g. lectins, gp120) into recognition elements for immune-mediated destruction, ARMs have the potential to target various infectious pathogens. Although still in their infancy, such ARMs could serve as promising alternatives or adjuncts to available immunotherapies, antibiotics and antiviral agents.
Outlook
The strategies detailed herein underscore the promise of ARM technology for a range of therapeutically relevant contexts. Despite significant progress in this arena, certain obstacles remain in advancing this strategy into the clinic. For example, the different ABT types mentioned above are each likely to be associated with unique advantages and disadvantages. Although approaches that exploit endogenous antibodies are anticipated to be the most straightforward to implement in practice, their utility will likely vary between patients as a function of antibody concentrations, affinities, isotypes and sub-isotypes distributions, and other factors. Comprehensive, population-wide investigations into the prevalence and properties of known endogenous antibodies, as well as the identification of such species with entirely new binding specificities, will be critical for clinical applications. Pre-immunization strategies could afford greater control over these parameters, but would involve additional operational complexity, which may also carry increased risks of side-effects. Finally, pre-conjugated antibody-small molecule species can be constructed using a single antibody isotype (e.g., IgG1, a potent inducer of ADCC), however, such agents would likely carry many of the same limitations of available immunotherapies (e.g., dosing via injection, immunogenicity, etc.). Overall, the optimal ABT will likely depend on the specific patient and/or disease process being targeted.
While most reported TBT motifs interact with relatively well-characterized ligand–receptor systems, discovery strategies to enable unbiased targeting of disease-associated surface proteins could greatly extend the applicability of the ARM approach. To this end, modern techniques in rational ligand design,(164) and high-throughput chemistry(165) and biology(166) are likely to prove enabling, and recent strategies for the discovery of novel antibody-binding carbohydrate motifs using array technologies,(167–169) the systematic identification of antibody biomarkers for both healthy and disease states(170) and the identification of compounds capable of modulating protein-protein interactions,(171–174) provide cause for optimism along these lines. Finally, novel chemical scaffolds (e.g., for targeting multiple receptors at once),(71, 72, 74, 175) and assembly strategies (e.g., in vivo bioorthogonal chemistry),(176, 177) have the potential to facilitate ARM optimization. For example, improving receptor-binding profiles, decreasing molecular weight, enhancing oral bioavailability, all could serve to broaden the clinical utility of ARM agents.
By exploiting an emerging chemical understanding of complex biological systems, future efforts to rationally modulate human immunological functions have the potential to augment our ability to prevent, diagnose and treat human disease.(33) ARM-based strategies represent an important step in this direction, bridging mechanistic features of biologic agents with a detailed understanding of small molecule structure and function (Figure 5). Next-generation immunomodulators have the potential to move beyond the ARMs, enabling precise control over immune responses, and contributing to an understanding of the molecular events underlying human disease at the resolution of atoms and molecules.
Figure 5.
The development of ARMs emerged from the confluence of many disparate fields of study, including synthetic organic chemistry and immunology. Although still in early stages of development, ARMs have the potential to contribute significantly to basic and clinical sciences.
Acknowledgments
The authors apologize to many wonderful colleagues whose work we were unable to cite due to space limitations. Work in our laboratory on ARMs is supported by the National Institute of Health through the NIH New Innovator Award number 1DP2OD002913-01.
References
- 1.Riedel S. Edward Jenner and the history of smallpox and vaccination. Proceedings (Baylor University Medical Center) 2005;18:21–25. doi: 10.1080/08998280.2005.11928028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casadevall A, Dadachova E, Pirofski LA. Passive antibody therapy for infectious diseases. Nature reviews Microbiology. 2004;2:695–703. doi: 10.1038/nrmicro974. [DOI] [PubMed] [Google Scholar]
- 3.Elbakri A, Nelson PN, Abu Odeh RO. The state of antibody therapy. Hum Immunol. 2010;71:1243–1250. doi: 10.1016/j.humimm.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Ledford H. ‘Biosimilar’ drugs poised to penetrate market. Nature. 2010;468:18–19. doi: 10.1038/468018a. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal S. What’s fueling the biotech engine-2008. Nat Biotechnol. 2009;27:987–993. doi: 10.1038/nbt1109-987. [DOI] [PubMed] [Google Scholar]
- 6.Reichert JM. Antibody-based therapeutics to watch in 2011. MAbs. 2011;3:76–99. doi: 10.4161/mabs.3.1.13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FDA Approved Drug Products. U.S. Food and Drug Administration; 2012. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. [Google Scholar]
- 8.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forecasting BRM. Antibody Drugs: Technologies and Global Markets. BCC Research Market Forecasting; 2011. BIO016H. [Google Scholar]
- 10.Visintin M, Melchionna T, Cannistraci I, Cattaneo A. In vivo selection of intrabodies specifically targeting protein-protein interactions: A general platform for an “undruggable” class of disease targets. Journal of Biotechnology. 2008;135:1–15. doi: 10.1016/j.jbiotec.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Sunada H, Magun BE, Mendelsohn J, MacLeod CL. Monoclonal antibody against epidermal growth factor receptor is internalized without stimulating receptor phosphorylation. Proc Natl Acad Sci U S A. 1986;83:3825–3829. doi: 10.1073/pnas.83.11.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li S, Schmitz KR, Jeffrey PD, Wiltzius JJW, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–311. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Capala J, Bouchelouche K. Molecular imaging of HER2-positive breast cancer: a step toward an individualized ‘image and treat’ strategy. Curr Opin Oncol. 2010;22:559–566. doi: 10.1097/CCO.0b013e32833f8c3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francisco JA, Cerveny CG, Meyer DL, Mixan BJ, Klussman K, Chace DF, Rejniak SX, Gordon KA, DeBlanc R, Toki BE, Law C-L, Doronina SO, Siegall CB, Senter PD, Wahl AF. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood. 2003;102:1458–1465. doi: 10.1182/blood-2003-01-0039. [DOI] [PubMed] [Google Scholar]
- 15.Alley SC, Okeley NM, Senter PD. Antibody-drug conjugates: Targeted drug delivery for cancer. Curr Opin Chem Biol. 2010;14:529–537. doi: 10.1016/j.cbpa.2010.06.170. [DOI] [PubMed] [Google Scholar]
- 16.Clynes R, Takechi Y, Moroi Y, Houghton A, Ravetch JV. Fc receptors are required in passive and active immunity to melanoma. Proc Natl Acad Sci U S A. 1998;95:652–656. doi: 10.1073/pnas.95.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 18.Sampson JH, Crotty LE, Lee S, Archer GE, Ashley DM, Wikstrand CJ, Hale LP, Small C, Dranoff G, Friedman AH, Friedman HS, Bigner DD. Unarmed, tumor-specific monoclonal antibody effectively treats brain tumors. Proc Natl Acad Sci U S A. 2000;97:7503–7508. doi: 10.1073/pnas.130166597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy KP, Travers P, Walport M, Janeway C. Janeway’s Immunobiology 2008 [Google Scholar]
- 20.Nimmerjahn F, Ravetch JV. Fcγ receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 21.Dhodapkar KM, Krasovsky J, Williamson B, Dhodapkar MV. Antitumor monoclonal antibodies enhance cross-presentation of cellular antigens and the generation of myeloma-specific killer T cells by dendritic cells. J Exp Med. 2002;195:125–133. doi: 10.1084/jem.20011097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akiyama K, Ebihara S, Yada A, Matsumura K, Aiba S, Nukiwa T, Takai T. Targeting Apoptotic Tumor Cells to Fcγ Provides Efficient and Versatile Vaccination Against Tumors by Dendritic Cells. J Immunol. 2003;170:1641–1648. doi: 10.4049/jimmunol.170.4.1641. [DOI] [PubMed] [Google Scholar]
- 23.Cioca DP, Deak E, Cioca F, Paunescu V. Monoclonal Antibodies Targeted Against Melanoma and Ovarian Tumors Enhance Dendritic Cell-Mediated Cross-Presentation of Tumor-Associated Antigens and Efficiently Cross-Prime CD8+ T Cells. J Immunother. 2005;29:41–52. doi: 10.1097/01.cji.0000175496.51594.8b. [DOI] [PubMed] [Google Scholar]
- 24.Murillo O, Dubrot J, Palazon A, Arina A, Azpilikueta A, Alfaro C, Solano S, Ochoa MC, Berasain C, Gabari I, Perez-Gracia JL, Berraondo P, Hervas-Stubbs S, Melero I. In vivo depletion of DC impairs the anti-tumor effect of agonistic anti-CD137 mAb. Eur J Immunol. 2009;39:2424–2436. doi: 10.1002/eji.200838958. [DOI] [PubMed] [Google Scholar]
- 25.Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJT. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9:325–338. doi: 10.1038/nrd3003. [DOI] [PubMed] [Google Scholar]
- 26.Getts DR, Getts MT, McCarthy DP, Chastain EM, Miller SD. Have we overestimated the benefit of human(ized) antibodies? MAbs. 2010;2:682–694. doi: 10.4161/mabs.2.6.13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harding FA, Stickler MM, Razo J, DuBridge R. The immunogenicity of humanized and fully human antibodies: Residual immunogenicity resides in the CDR regions. mAbs. 2010;2:256–265. doi: 10.4161/mabs.2.3.11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siberil S, Dutertre CA, Fridman WH, Teillaud JL. FcgammaR: The key to optimize therapeutic antibodies? Crit Rev Oncol Hematol. 2007;62:26–33. doi: 10.1016/j.critrevonc.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Wu A, Senter P. Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol. 2005;23:1137–1146. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- 30.Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002;2:750–763. doi: 10.1038/nrc903. [DOI] [PubMed] [Google Scholar]
- 31.Imai K, Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer. 2006;6:714–727. doi: 10.1038/nrc1913. [DOI] [PubMed] [Google Scholar]
- 32.Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nat Med. 2005;11:S63–S68. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- 33.Spiegel DA. Grand Challenge Commentary: Synthetic immunology to engineer human immunity. Nat Chem Biol. 2010;6:871–872. doi: 10.1038/nchembio.477. [DOI] [PubMed] [Google Scholar]
- 34.Kodadek T. Development of antibody surrogates for the treatment of cancers and autoimmune disease. Curr Opin Chem Biol. 2010;14:721–727. doi: 10.1016/j.cbpa.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ducry L, Stump B. Antibody-Drug Conjugates: Linking Cytotoxic Payloads to Monoclonal Antibodies. Bioconjugate Chemistry. 2010;21:5–13. doi: 10.1021/bc9002019. [DOI] [PubMed] [Google Scholar]
- 36.Müller D, Kontermann RE. Bispecific antibodies for cancer immunotherapy: Current perspectives. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2010;24:89–98. doi: 10.2165/11530960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Baeuerle PA, Reinhardt C. Bispecific T-Cell Engaging Antibodies for Cancer Therapy. Cancer Res. 2009;69:4941–4944. doi: 10.1158/0008-5472.CAN-09-0547. [DOI] [PubMed] [Google Scholar]
- 38.Xiao J, Chen R, Pawlicki MA, Tolbert TJ. Targeting a Homogeneously Glycosylated Antibody Fc To Bind Cancer Cells Using a Synthetic Receptor Ligand. J Am Chem Soc. 2009 doi: 10.1021/ja9045179. 090903092048010. [DOI] [PubMed] [Google Scholar]
- 39.Morelli L, Poletti L, Lay L. Carbohydrates and Immunology: Synthetic Oligosaccharide Antigens for Vaccine Formulation. European Journal of Organic Chemistry. 2011;2011:5723–5777. [Google Scholar]
- 40.Li Q, So CR, Fegan A, Cody V, Sarikaya M, Vallera DA, Wagner CR. Chemically Self-Assembled Antibody Nanorings (CSANs): Design and Characterization of an Anti-CD3 IgM Biomimetic. J Am Chem Soc. 2010 doi: 10.1021/ja107153a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Q, Hapka D, Chen H, Vallera DA, Wagner CR. Self-Assembly of Antibodies by Chemical Induction. Angewandte Chemie-International Edition In English. 2008:NA–NA. doi: 10.1002/anie.200803507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitov PI, Mulvey GL, Griener TP, Lipinski T, Solomon D, Paszkiewicz E, Jacobson JM, Sadowska JM, Suzuki M, Yamamura KI, Armstrong GD, Bundle DR. In vivo supramolecular templating enhances the activity of multivalent ligands: a potential therapeutic against the Escherichia coli O157 AB5 toxins. Proc Natl Acad Sci U S A. 2008;105:16837–16842. doi: 10.1073/pnas.0804919105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitov PI, Lipinski T, Paszkiewicz E, Solomon D, Sadowska JM, Grant GA, Mulvey GL, Kitova EN, Klassen JS, Armstrong GD, Bundle DR. An Entropically Efficient Supramolecular Inhibition Strategy for Shiga Toxins. Angewandte Chemie-International Edition In English. 2008;47:672–676. doi: 10.1002/anie.200704064. [DOI] [PubMed] [Google Scholar]
- 44.Carlson JCT, Kanter A, Thuduppathy GR, Cody V, Pineda PE, Mcivor RS, Wagner CR. Designing Protein Dimerizers: The Importance of Ligand Conformational Equilibria. J Am Chem Soc. 2003;125:1501–1507. doi: 10.1021/ja026264y. [DOI] [PubMed] [Google Scholar]
- 45.Shearer GM. Cell mediated cytotoxicity to trinitrophenyl-modified syngeneic lymphocytes. Eur J Immunol. 1974;4:527–533. doi: 10.1002/eji.1830040802. [DOI] [PubMed] [Google Scholar]
- 46.Nelson DL, Poplack DG, Holiman BJ, Henkart PA. ADCC against human erythrocyte target cells: role of the anti-target cell antibodies in determining lymphocyte killer activity. Clin Exp Immunol. 1979;35:447–453. [PMC free article] [PubMed] [Google Scholar]
- 47.Six HR, Uemura K, Kinsky SC. Effect of immunoglobulin class and affinity on the initiation of complement-dependent damage to liposomal model membranes sensitized with dinitrophenylated phospholipids. Biochemistry. 1973;12:4003–4011. doi: 10.1021/bi00744a034. [DOI] [PubMed] [Google Scholar]
- 48.Capon DJ, Chamow SM, Mordenti J, Marsters SA, Gregory T, Mitsuya H, Byrn RA, Lucas C, Wurm FM, Groopman JE, et al. Designing CD4 immunoadhesins for AIDS therapy. Nature. 1989;337:525–531. doi: 10.1038/337525a0. [DOI] [PubMed] [Google Scholar]
- 49.Traunecker A, Schneider J, Kiefer H, Karjalainen K. Highly efficient neutralization of HIV with recombinant CD4-immunoglobulin molecules. Nature. 1989;339:68–70. doi: 10.1038/339068a0. [DOI] [PubMed] [Google Scholar]
- 50.Byrn RA, Mordenti J, Lucas C, Smith D, Marsters SA, Johnson JS, Cossum P, Chamow SM, Wurm FM, et al. Biological properties of a CD4 immunoadhesin. Nature. 1990;344:667–670. doi: 10.1038/344667a0. [DOI] [PubMed] [Google Scholar]
- 51.Shokat KM, Schultz PG. Redirecting the immune response: ligand-mediated immunogenicity. J Am Chem Soc. 1991;113:1861–1862. [Google Scholar]
- 52.Lussow AR, Buelow R, Fanget L, Peretto S, Gao L, Pouletty P. Redirecting circulating antibodies via ligand-hapten conjugates eliminates target cells in vivo. J Immunother Emphasis Tumor Immunol. 1996;19:257–265. doi: 10.1097/00002371-199607000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Bertozzi CR, Bednarski MD. Antibody targeting to bacterial cells using receptor-specific ligands. J Am Chem Soc. 1992;114:2242–2245. [Google Scholar]
- 54.Bertozzi CR, Bednarski MD. A receptor-mediated immune response using synthetic glycoconjugates. J Am Chem Soc. 1992;114:5543–5546. [Google Scholar]
- 55.Li J, Zacharek S, Chen X, Wang J, Zhang W, Janczuk A, Wang PG. Bacteria targeted by human natural antibodies using alpha -gal conjugated receptor-specific glycopolymers. Bioorg Med Chem. 1999;7:1549–1558. doi: 10.1016/s0968-0896(99)00099-1. [DOI] [PubMed] [Google Scholar]
- 56.Metallo SJ, Kane RS, Holmlin RE, Whitesides GM. Using Bifunctional Polymers Presenting Vancomycin and Fluorescein Groups To Direct Anti-Fluorescein Antibodies to Self-Assembled Monolayers Presenting D-Alanine-D-Alanine Groups. J Am Chem Soc. 2003;125:4534–4540. doi: 10.1021/ja030045a. [DOI] [PubMed] [Google Scholar]
- 57.Krishnamurthy VM, Quinton LJ, Estroff LA, Metallo SJ, Isaacs JM, Mizgerd JP, Whitesides GM. Promotion of opsonization by antibodies and phagocytosis of Gram-positive bacteria by a bifunctional polyacrylamide. Biomaterials. 2006;27:3663–3674. doi: 10.1016/j.biomaterials.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 58.Naicker KP, Li H, Heredia A, Song H, Wang LX. Design and synthesis of alpha Gal-conjugated peptide T20 as novel antiviral agent for HIV-immunotargeting. Org Biomol Chem. 2004;2:660–664. doi: 10.1039/b313844e. [DOI] [PubMed] [Google Scholar]
- 59.Perdomo MF, Levi M, Saellberg M, Vahlne A. Neutralization of HIV-1 by redirection of natural antibodies. Proc Natl Acad Sci U S A. 2008;105:12515–12520. doi: 10.1073/pnas.0805777105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parker CG, Domaoal RA, Anderson KS, Spiegel DA. An Antibody-Recruiting Small Molecule That Targets HIV gp120. J Am Chem Soc. 2009;131:16392–16394. doi: 10.1021/ja9057647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gavrilyuk J, Uehara H, Otsubo N, Hessell A, Burton DR, Barbas CF., 3rd Potent inhibition of HIV-1 entry with a chemically programmed antibody aided by an efficient organocatalytic synthesis. Chembiochem : A European journal of chemical biology. 2010;11:2113–2118. doi: 10.1002/cbic.201000432. [DOI] [PubMed] [Google Scholar]
- 62.Lu Y, Low PS. Folate targeting of haptens to cancer cell surfaces mediates immunotherapy of syngeneic murine tumors. Cancer Immunol Immunother. 2002;51:153–162. doi: 10.1007/s00262-002-0266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu Y, Sega E, Low Philip S. Folate receptor-targeted immunotherapy: induction of humoral and cellular immunity against hapten-decorated cancer cells. Int J Cancer. 2005;116:710–719. doi: 10.1002/ijc.21126. [DOI] [PubMed] [Google Scholar]
- 64.Lu Y, You F, Vlahov I, Westrick E, Fan M, Low PS, Leamon CP. Folate-Targeted Dinitrophenyl Hapten Immunotherapy: Effect of Linker Chemistry on Antitumor Activity and Allergic Potential. Mol Pharmaceutics. 2007;4:695–706. doi: 10.1021/mp070050b. [DOI] [PubMed] [Google Scholar]
- 65.Rader C, Sinha SC, Popkov M, Lerner RA, Barbas CF., III Chemically programmed monoclonal antibodies for cancer therapy: Adaptor immunotherapy based on a covalent antibody catalyst. Proc Natl Acad Sci U S A. 2003;100:5396–5400. doi: 10.1073/pnas.0931308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Chemical Adaptor Immunotherapy: Design, Synthesis, and Evaluation of Novel Integrin-Targeting Devices. J Med Chem. 2004;47:5630–5640. doi: 10.1021/jm049666k. [DOI] [PubMed] [Google Scholar]
- 67.Popkov M, Rader C, Gonzalez B, Sinha SC, Barbas CF., III Small molecule drug activity in melanoma models may be dramatically enhanced with an antibody effector. Int J Cancer. 2006;119:1194–1207. doi: 10.1002/ijc.21924. [DOI] [PubMed] [Google Scholar]
- 68.Doppalapudi V, Tryder N, Li L, Aja T, Griffith D, Liao FF, Roxas G, Ramprasad M, Bradshaw C, Barbas C. Chemically programmed antibodies: endothelin receptor targeting CovX-Bodies. Bioorg Med Chem Lett. 2007;17:501–506. doi: 10.1016/j.bmcl.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 69.Owen RM, Carlson CB, Xu J, Mowery P, Fasella E, Kiessling LL. Bifunctional ligands that target cells displaying the alpha vbeta 3 integrin. ChemBioChem. 2007;8:68–82. doi: 10.1002/cbic.200600339. [DOI] [PubMed] [Google Scholar]
- 70.Carlson CB, Mowery P, Owen RM, Dykhuizen EC, Kiessling LL. Selective tumor cell targeting using low-affinity, multivalent interactions. ACS Chem Biol. 2007;2:119–127. doi: 10.1021/cb6003788. [DOI] [PubMed] [Google Scholar]
- 71.O’Reilly MK, Collins BE, Han S, Liao L, Rillahan C, Kitov PI, Bundle DR, Paulson JC. Bifunctional CD22 Ligands Use Multimeric Immunoglobulins as Protein Scaffolds in Assembly of Immune Complexes on B Cells. J Am Chem Soc. 2008;130:7736–7745. doi: 10.1021/ja802008q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cui L, Kitov PI, Completo GC, Paulson JC, Bundle DR. Supramolecular Complexing of Membrane Siglec CD22 Mediated by a Polyvalent Heterobifunctional Ligand that Templates on IgM. Bioconjugate Chem. 2011;22:546–550. doi: 10.1021/bc100579d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Popkov M, Gonzalez B, Sinha SV, Barbas CF., III Instant immunity through chemically programmable vaccination and covalent self-assembly. Proc Natl Acad Sci U S A. 2009;106:4378–4383. doi: 10.1073/pnas.0900147106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gavrilyuk J, Wuellner U, Salahuddin S, Goswami R, Sinha S, Barbas C. An efficient chemical approach to bispecific antibodies and antibodies of high valency. Bioorg Med Chem Lett. 2009;19:3716–3720. doi: 10.1016/j.bmcl.2009.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murelli RP, Zhang AX, Michel J, Jorgensen WL, Spiegel DA. Chemical Control over Immune Recognition: A Class of Antibody-Recruiting Small Molecules that Target Prostate Cancer. J Am Chem Soc. 2009;131:17090–17092. doi: 10.1021/ja906844e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wuellner U, Gavrilyuk J, Barbas C. Expanding the concept of chemically programmable antibodies to RNA aptamers: chemically programmed biotherapeutics. Angew Chem Int Ed. 2010;49:5934–5937. doi: 10.1002/anie.201001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Doppalapudi VR, Huang J, Liu D, Jin P, Liu B, Li L, Desharnais J, Hagen C, Levin NJ, Shields MJ, Parish M, Murphy RE, Del Rosario J, Oates BD, Lai J-Y, Matin MJ, Ainekulu Z, Bhat A, Bradshaw CW, Woodnutt G, Lerner RA, Lappe RW. Chemical generation of bispecific antibodies. Proceedings of the National Academy of Sciences. 2010;107:22611–22616. doi: 10.1073/pnas.1016478108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dubrovska A, Kim C, Elliott J, Shen W, Kuo TH, Koo DI, Li C, Tuntland T, Chang J, Groessl T, Wu X, Gorney V, Ramirez Montagut T, Spiegel D, Cho C, Schultz P. A chemically induced vaccine strategy for prostate cancer. ACS Chem Biol. 2011;6:1223–1231. doi: 10.1021/cb200222s. [DOI] [PubMed] [Google Scholar]
- 79.Jakobsche CE, McEnaney PJ, Zhang AX, Spiegel DA. Reprogramming Urokinase into an Antibody-Recruiting Anticancer Agent. ACS Chem Biol. 2011 doi: 10.1021/cb200374e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Galili U, Rachmilewitz EA, Peleg A, Flechner I. A unique natural human IgG antibody with anti-alpha -galactosyl specificity. J Exp Med. 1984;160:1519–1531. doi: 10.1084/jem.160.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parker W, Bruno D, Holzknecht ZE, Platt JL. Characterization and affinity isolation of xenoreactive human natural antibodies. J Immunol. 1994;153:3791–3803. [PubMed] [Google Scholar]
- 82.Galili U, Clark MR, Shohet SB, Buehler J, Macher BA. Evolutionary relationship between the natural anti-Gal antibody and the Galalpha 1 -> 3Gal epitope in primates. Proc Natl Acad Sci U S A. 1987;84:1369–1373. doi: 10.1073/pnas.84.5.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ortega E, Kostovetzky M, Larralde C. Natural DNP-binding immunoglobulins and antibody multispecificity. Mol Immunol. 1984;21:883–888. doi: 10.1016/0161-5890(84)90143-3. [DOI] [PubMed] [Google Scholar]
- 84.Farah FS. Natural antibodies specific to the 2,4-dinitrophenyl group. Immunology. 1973;25:217–226. [PMC free article] [PubMed] [Google Scholar]
- 85.Ju K-S, Parales R. Nitroaromatic Compounds, from Synthesis to Biodegradation. Microbiology and molecular biology reviews. 2010;74:250. doi: 10.1128/MMBR.00006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.2,4-Dinitrophenol (CASRN 51-28-5) United States Environmental Protection Agency; http://www.epa.gov/ncea/iris/subst/0152.htm. [Google Scholar]
- 87.Harris MO, Cocoran JJ. Toxicological Profile for Dinitrophenols, Agency for Toxic Substances and Disease Registry. 1995 http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=729&tid=132. [PubMed]
- 88.Lauer K. Environmental nitrophenols and autoimmunity. Mol Immunol. 1990;27:697–698. doi: 10.1016/0161-5890(90)90013-p. [DOI] [PubMed] [Google Scholar]
- 89.Gauba V, Grünewald J, Gorney V, Deaton LM, Kang M, Bursulaya B, Ou W, Lerner RA, Schmedt C, Geierstanger BH, Schultz PG, Ramirez-Montagut T. Loss of CD4 T-cell-dependent tolerance to proteins with modified amino acids. Proceedings of the National Academy of Sciences. 2011;108:12821–12826. doi: 10.1073/pnas.1110042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grünewald J, Hunt GS, Dong L, Niessen F, Wen BG, Tsao M-L, Perera R, Kang M, Laffitte BA, Azarian S, Ruf W, Nasoff M, Lerner RA, Schultz PG, Smider VV. Mechanistic studies of the immunochemical termination of self-tolerance with unnatural amino acids. Proceedings of the National Academy of Sciences. 2009;106:4337–4342. doi: 10.1073/pnas.0900507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grünewald J, Tsao M-L, Perera R, Dong L, Niessen F, Wen BG, Kubitz DM, Smider VV, Ruf W, Nasoff M, Lerner RA, Schultz PG. Immunochemical termination of self-tolerance. Proceedings of the National Academy of Sciences. 2008;105:11276–11280. doi: 10.1073/pnas.0804157105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palm NW, Medzhitov R. Immunostimulatory activity of haptenated proteins. Proceedings of the National Academy of Sciences. 2009;106:4782–4787. doi: 10.1073/pnas.0809403105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cherukuri A, Durack G, Voss EW., Jr Evidence for hapten recognition in receptor-mediated intracellular uptake of a hapten-protein conjugate by murine macrophage. Mol Immunol. 1997;34:21–32. doi: 10.1016/s0161-5890(97)00010-2. [DOI] [PubMed] [Google Scholar]
- 94.Wagner J, Lerner RA, Barbas CF., III Efficient aldolase catalytic antibodies that use the enamine mechanism of natural enzymes. Science. 1995;270:1797–1800. doi: 10.1126/science.270.5243.1797. [DOI] [PubMed] [Google Scholar]
- 95.Barbas CF, Heine A, Zhong G, Hoffmann T, Gramatikova S, Bjrnestedt R, List B, Anderson J, Stura EA, Wilson IA, Lerner RA. Immune versus natural selection: antibody aldolases with enzymic rates but broader scope. Science. 1997;278:2085–2092. doi: 10.1126/science.278.5346.2085. [DOI] [PubMed] [Google Scholar]
- 96.Guo F, Das S, Mueller B, Barbas C, Lerner R, Sinha S. Breaking the one antibody-one target axiom. Proc Natl Acad Sci U S A. 2006;103:11009–11014. doi: 10.1073/pnas.0603822103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gavrilyuk J, Wuellner U, Barbas C. Beta-lactam-based approach for the chemical programming of aldolase antibody 38C2. Bioorg Med Chem Lett. 2009;19:1421–1424. doi: 10.1016/j.bmcl.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tanaka F, Barbas CF. Reactive immunization: a unique approach to catalytic antibodies. J Immunol Methods. 2002;269:67–79. doi: 10.1016/s0022-1759(02)00225-9. [DOI] [PubMed] [Google Scholar]
- 99.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 100.Chabner BA, Roberts TG. Chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5:65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- 101.Helleday T, Petermann E, Lundin C, Hodgson B, Sharma R. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- 102.Tennant D, Duran R, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer. 2010;10:267–277. doi: 10.1038/nrc2817. [DOI] [PubMed] [Google Scholar]
- 103.Zhang J, Yang P, Gray N. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer. 2009;9:28–39. doi: 10.1038/nrc2559. [DOI] [PubMed] [Google Scholar]
- 104.Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol. 2010;10:580–593. doi: 10.1038/nri2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aggarwal S. Targeted cancer therapies. Nat Rev Drug Discov. 2010;9:427–428. doi: 10.1038/nrd3186. [DOI] [PubMed] [Google Scholar]
- 106.Nemeth JA, Nakada MT, Trikha M, Lang Z, Gordon MS, Jayson GC, Corringham R, Prabhakar U, Davis HM, Beckman RA. Alpha-v Integrins as Therapeutic Targets in Oncology. Cancer Invest. 2007;25:632–646. doi: 10.1080/07357900701522638. [DOI] [PubMed] [Google Scholar]
- 107.Felding-Habermann B. Integrin adhesion receptors in tumor metastasis. Clin Exp Metastasis. 2003;20:203–213. doi: 10.1023/a:1022983000355. [DOI] [PubMed] [Google Scholar]
- 108.Chattopadhyay N, Chatterjee A. Studies on the expression of alpha(v)beta3 integrin receptors in non-malignant and malignant human cervical tumor tissues. J Exp Clin Cancer Res. 2001;20:269–275. [PubMed] [Google Scholar]
- 109.Wong NC, Mueller BM, Barbas CF, Ruminski P, Quaranta V, Lin EC, Smith JW. Alphav integrins mediate adhesion and migration of breast carcinoma cell lines. Clin Exp Metastasis. 1998;16:50–61. doi: 10.1023/a:1006512018609. [DOI] [PubMed] [Google Scholar]
- 110.Nelson J, Bagnato A, Battistini B, Nisen P. The endothelin axis: emerging role in cancer. Nat Rev Cancer. 2003;3:110–116. doi: 10.1038/nrc990. [DOI] [PubMed] [Google Scholar]
- 111.Remuzzi G, Perico N, Benigni A. New therapeutics that antagonize endothelin: promises and frustrations. Nat Rev Drug Discov. 2002;1:986–1001. doi: 10.1038/nrd962. [DOI] [PubMed] [Google Scholar]
- 112.Attin T, Camidge R, Newby DE, Webb DJ. Endothelin antagonism in pulmonary hypertension, heart failure, and beyond. Heart. 2005;91:825–831. doi: 10.1136/hrt.2004.053991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li LN, Leedom TA, Do J, Huang HH, Lai JY, Johnson K, Osothprarop TF, Rizzo JD, Doppalapudi VR, Bradshaw CW, Lappe RW, Woodnutt G, Levin NJ, Pirie-Shepherd SR. Antitumor Efficacy of a Thrombospondin 1 Mimetic CovX-Body. Translational oncology. 2011;4:239–247. doi: 10.1593/tlo.11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang H, Lai J-Y, Do J, Liu D, Li L, Del Rosario J, Doppalapudi VR, Pirie-Shepherd S, Levin N, Bradshaw C, Woodnutt G, Lappe R, Bhat A. Specifically Targeting Angiopoietin-2 Inhibits Angiogenesis, Tie2-Expressing Monocyte Infiltration, and Tumor Growth. Clinical Cancer Research. 2011;17:1001–1011. doi: 10.1158/1078-0432.CCR-10-2317. [DOI] [PubMed] [Google Scholar]
- 115.Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 116.Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 117.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 118.Lu Y, Sega E, Leamon C, Low P. Folate receptor-targeted immunotherapy of cancer: mechanism and therapeutic potential. Adv Drug Delivery Rev. 2004;56:1161–1176. doi: 10.1016/j.addr.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 119.Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009;157:220–233. doi: 10.1111/j.1476-5381.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ellenhorn JD, Schreiber H, Bluestone JA. Mechanism of tumor rejection in anti-CD3 monoclonal antibody-treated mice. J Immunol. 1990;144:2840–2846. [PubMed] [Google Scholar]
- 121.Runyon K, Lee K, Zuberek K, Collins M, Leonard JP, Dunussi Joannopoulos K. The combination of chemotherapy and systemic immunotherapy with soluble B7-immunoglobulin G leads to cure of murine leukemia and lymphoma and demonstration of tumor-specific memory responses. Blood. 2001;97:2420–2426. doi: 10.1182/blood.v97.8.2420. [DOI] [PubMed] [Google Scholar]
- 122.Ruf P, Lindhofer H. Induction of a long-lasting antitumor immunity by a trifunctional bispecific antibody. Blood. 2001;98:2526–2534. doi: 10.1182/blood.v98.8.2526. [DOI] [PubMed] [Google Scholar]
- 123.Morecki S, Lindhofer H, Yacovlev E, Gelfand Y, Ruf P, Slavin S. Induction of long-lasting antitumor immunity by concomitant cell therapy with allogeneic lymphocytes and trifunctional bispecific antibody. Exp Hematol. 2008;36:997–1003. doi: 10.1016/j.exphem.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 124.Zhang A, Murelli R, Barinka C, Michel J, Cocleaza A, Jorgensen W, Lubkowski J, Spiegel D. A remote arene-binding site on prostate specific membrane antigen revealed by antibody-recruiting small molecules. J Am Chem Soc. 2010;132:12711–12716. doi: 10.1021/ja104591m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Holmes EH, Greene TG, Tino WT, Boynton AL, Aldape HC, Misrock SL, Murphy GP. Analysis of glycosylation of prostate-specific membrane antigen derived from LNCaP cells, prostatic carcinoma tumors, and serum from prostate cancer patients. Prostate Suppl. 1996;7:25–29. [PubMed] [Google Scholar]
- 126.Wernicke AG, Edgar M, Lavi E, Liu H, Salerno P, Bander N, Gutin P. Prostate-specific membrane antigen as a potential novel vascular target for treatment of glioblastoma multiforme. Archives of pathology & laboratory medicine. 2011;135:1486–1489. doi: 10.5858/arpa.2010-0740-OA. [DOI] [PubMed] [Google Scholar]
- 127.Samplaski M, Heston W, Elson P, Magi Galluzzi C, Hansel D. Folate hydrolase (prostate-specific antigen) 1 expression in bladder cancer subtypes and associated tumor neovasculature. Mod Pathol. 2011;24:1521–1529. doi: 10.1038/modpathol.2011.112. [DOI] [PubMed] [Google Scholar]
- 128.Haffner M, Kronberger I, Ross J, Sheehan C, Zitt M, Mhlmann G, Ofner D, Zelger B, Ensinger C, Yang X, Geley S, Margreiter R, Bander N. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Hum Pathol. 2009;40:1754–1761. doi: 10.1016/j.humpath.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 129.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nature reviews Molecular cell biology. 2002;3:932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 130.Romer J, Nielsen B, Ploug M. The urokinase receptor as a potential target in cancer therapy. Current pharmaceutical design. 2004;10:2359–2376. doi: 10.2174/1381612043383962. [DOI] [PubMed] [Google Scholar]
- 131.Andreasen PA, Kjller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis: a review. International journal of cancer. 1997;72:1–22. doi: 10.1002/(sici)1097-0215(19970703)72:1<1::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 132.Duffy MJ, O’Grady P, Devaney D, O’Siorain L, Fennelly JJ, Lijnen HJ. Urokinase-plasminogen activator, a marker for aggressive breast carcinomas. Preliminary report, Cancer. 1988;62:531–533. doi: 10.1002/1097-0142(19880801)62:3<531::aid-cncr2820620315>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 133.Sier CF, Stephens R, Bizik J, Mariani A, Bassan M, Pedersen N, Frigerio L, Ferrari A, Dan K, Brnner N, Blasi F. The level of urokinase-type plasminogen activator receptor is increased in serum of ovarian cancer patients. Cancer Res. 1998;58:1843–1849. [PubMed] [Google Scholar]
- 134.Harbeck N, Kates R, Gauger K, Willems A, Kiechle M, Magdolen V, Schmitt M. Urokinase-type plasminogen activator (uPA) and its inhibitor PAI-I: novel tumor-derived factors with a high prognostic and predictive impact in breast cancer. Thrombosis and haemostasis. 2004;91:450–456. doi: 10.1160/TH03-12-0798. [DOI] [PubMed] [Google Scholar]
- 135.Konecny G, Untch M, Pihan A, Kimmig R, Gropp M, Stieber P, Hepp H, Slamon D, Pegram M. Association of urokinase-type plasminogen activator and its inhibitor with disease progression and prognosis in ovarian cancer. Clin Cancer Res. 2001;7:1743–1749. [PubMed] [Google Scholar]
- 136.Mathers C, Boerma T, MaFat D. The global burden of disease: 2004 update. World Health Organization; Geneva, Switzerland: 2008. p. 146. [Google Scholar]
- 137.Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol. 2011;11:865–872. doi: 10.1038/nri3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hilleman MR. Vaccines in historic evolution and perspective: a narrative of vaccine discoveries. Vaccine. 2000;18:1436–1447. doi: 10.1016/s0264-410x(99)00434-x. [DOI] [PubMed] [Google Scholar]
- 139.Ulmer JB, Valley U, Rappuoli R. Vaccine manufacturing: challenges and solutions. Nat Biotechnol. 2006;24:1377–1383. doi: 10.1038/nbt1261. [DOI] [PubMed] [Google Scholar]
- 140.Plotkin SA. Vaccines: the fourth century. Clin Vaccine Immunol. 2009;16:1709–1719. doi: 10.1128/CVI.00290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guy B. The perfect mix: recent progress in adjuvant research. Nature reviews Microbiology. 2007;5:505–517. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- 142.Sheridan C. The business of making vaccines. Nat Biotechnol. 2005;23:1359–1366. doi: 10.1038/nbt1105-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Palumbi SR. Humans as the world’s greatest evolutionary force. Science. 2001;293:1786–1790. doi: 10.1126/science.293.5536.1786. [DOI] [PubMed] [Google Scholar]
- 144.Razonable RR. Antiviral drugs for viruses other than human immunodeficiency virus. Mayo Clin Proc. 2011;86:1009–1026. doi: 10.4065/mcp.2011.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saylor C, Dadachova E, Casadevall A. Monoclonal antibody-based therapies for microbial diseases. Vaccine. 2009;27(Suppl 6):G38–46. doi: 10.1016/j.vaccine.2009.09.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nagy E, Giefing C, von Gabain A. Anti-infective antibodies: a novel tool to prevent and treat nosocomial diseases. Expert Rev Anti-Infe. 2008;6:21–30. doi: 10.1586/14787210.6.1.21. [DOI] [PubMed] [Google Scholar]
- 147.Bebbington C, Yarranton G. Antibodies for the treatment of bacterial infections: current experience and future prospects. Curr Opin Biotechnol. 2008;19:613–619. doi: 10.1016/j.copbio.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 148.ter Meulen J. Monoclonal antibodies for prophylaxis and therapy of infectious diseases. Expert Opin Emerg Dr. 2007;12:525–540. doi: 10.1517/14728214.12.4.525. [DOI] [PubMed] [Google Scholar]
- 149.Xiao X, Dimitrov DS. Monoclonal antibodies against viruses and bacteria: a survey of patents. Recent Pat Antiinfect Drug Discov. 2007;2:171–177. doi: 10.2174/157489107782497272. [DOI] [PubMed] [Google Scholar]
- 150.Jan t M. Monoclonal Antibodies in Infectious Diseases: Clinical Pipeline in 2011. Infectious Disease Clinics of North America. 2011;25:789–802. doi: 10.1016/j.idc.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 151.Sekirov I, Tam NM, Jogova M, Robertson ML, Li Y, Lupp C, Finlay BB. Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infect Immun. 2008;76:4726–4736. doi: 10.1128/IAI.00319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nature reviews Microbiology. 2011;9:233–243. doi: 10.1038/nrmicro2536. [DOI] [PubMed] [Google Scholar]
- 153.Walsh C. Molecular mechanisms that confer antibacterial drug resistance. Nature. 2000;406:775–781. doi: 10.1038/35021219. [DOI] [PubMed] [Google Scholar]
- 154.Nikaido H. Multidrug resistance in bacteria. Annu Rev Biochem. 2009;78:119–146. doi: 10.1146/annurev.biochem.78.082907.145923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cooper MA, Shlaes D. Fix the antibiotics pipeline. Nature. 2011;472:32. doi: 10.1038/472032a. [DOI] [PubMed] [Google Scholar]
- 156.Huang DB, Wu JJ, Tyring SK. A review of licensed viral vaccines, some of their safety concerns, and the advances in the development of investigational viral vaccines. J Infect. 2004;49:179–209. doi: 10.1016/j.jinf.2004.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Berzofsky JA, Ahlers JD, Janik J, Morris J, Oh S, Terabe M, Belyakov IM. Progress on new vaccine strategies against chronic viral infections. J Clin Invest. 2004;114:450–462. doi: 10.1172/JCI22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Carreiras F, Denoux Y, Staedel C, Lehmann M, Sichel F, Gauduchon P. Expression and localization of alpha v integrins and their ligand vitronectin in normal ovarian epithelium and in ovarian carcinoma. Gynecol Oncol. 1996;62:260–267. doi: 10.1006/gyno.1996.0225. [DOI] [PubMed] [Google Scholar]
- 159.Margeridon-Thermet S, Shafer RW. Comparison of the Mechanisms of Drug Resistance among HIV, Hepatitis B, and Hepatitis C. Viruses. 2010;2:2696–2739. doi: 10.3390/v2122696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Griffiths PD. A perspective on antiviral resistance. J Clin Virol. 2009;46:3–8. doi: 10.1016/j.jcv.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 161.De Clercq E. Strategies in the design of antiviral drugs. Nat Rev Drug Discov. 2002;1:13–25. doi: 10.1038/nrd703. [DOI] [PubMed] [Google Scholar]
- 162.Dessain SK, Adekar SP, Berry JD. Exploring the native human antibody repertoire to create antiviral therapeutics. Curr Top Microbiol Immunol. 2008;317:155–183. doi: 10.1007/978-3-540-72146-8_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang T, Zhang Z, Wallace OB, Deshpande M, Fang H, Yang Z, Zadjura LM, Tweedie DL, Huang S, Zhao F, Ranadive S, Robinson BS, Gong YF, Ricarrdi K, Spicer TP, Deminie C, Rose R, Wang HG, Blair WS, Shi PY, Lin PF, Colonno RJ, Meanwell NA. Discovery of 4-benzoyl-1-[(4-methoxy-1H- pyrrolo[2,3-b]pyridin-3-yl)oxoacetyl]-2- (R)-methylpiperazine (BMS-378806): a novel HIV-1 attachment inhibitor that interferes with CD4-gp120 interactions. J Med Chem. 2003;46:4236–4239. doi: 10.1021/jm034082o. [DOI] [PubMed] [Google Scholar]
- 164.Reddy AS, Pati SP, Kumar PP, Pradeep HN, Sastry GN. Virtual screening in drug discovery - A computational perspective. Curr Protein Pept Sci. 2007;8:329–351. doi: 10.2174/138920307781369427. [DOI] [PubMed] [Google Scholar]
- 165.Lange T, Lindell S. Recent advances in microwave-assisted combinatorial synthesis and library generation. Comb Chem High Throughput Screen. 2005;8:595–606. doi: 10.2174/138620705774575445. [DOI] [PubMed] [Google Scholar]
- 166.Osmond RIW, Das S, Crouch MF. Development of cell-based assays for cytokine receptor signaling, using an AlphaScreen SureFire assay format. Analytical Biochemistry. 2010;403:94–101. doi: 10.1016/j.ab.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 167.Oyelaran O, McShane LM, Dodd L, Gildersleeve JC. Profiling Human Serum Antibodies with a Carbohydrate Antigen Microarray. J Proteome Res. 2009;8:4301–4310. doi: 10.1021/pr900515y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Huflejt ME, Vuskovic M, Vasiliu D, Xu H, Obukhova P, Shilova N, Tuzikov A, Galanina O, Arun B, Lu K, Bovin N. Anti-carbohydrate antibodies of normal sera: Findings, surprises and challenges. Mol Immunol. 2009;46:3037–3049. doi: 10.1016/j.molimm.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 169.Chen W, Gu L, Zhang W, Motari E, Cai L, Styslinger TJ, Wang PG. L-Rhamnose antigen: a promising alternative to alpha -Gal for cancer immunotherapies. ACS Chem Biol. 2011;6:185–191. doi: 10.1021/cb100318z. [DOI] [PubMed] [Google Scholar]
- 170.Reddy MM, Wilson R, Wilson J, Connell S, Gocke A, Hynan L, German D, Kodadek T. Identification of Candidate IgG Biomarkers for Alzheimer’s Disease via Combinatorial Library Screening. Cell. 2011;144:132–142. doi: 10.1016/j.cell.2010.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sergeeva A, Kolonin MG, Molldrem JJ, Pasqualini R, Arap W. Display technologies: Application for the discovery of drug and gene delivery agents. Adv Drug Delivery Rev. 2006;58:1622–1654. doi: 10.1016/j.addr.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Udugamasooriya DG, Dineen SP, Brekken RA, Kodadek T. A Peptoid “Antibody Surrogate” That Antagonizes VEGF Receptor 2 Activity. J Am Chem Soc. 2008;130:5744–5752. doi: 10.1021/ja711193x. [DOI] [PubMed] [Google Scholar]
- 173.Buller F, Steiner M, Frey K, Mircsof D, Scheuermann J, Kalisch M, Buhlmann P, Supuran CT, Neri D. Selection of Carbonic Anhydrase IX Inhibitors from One Million DNA-Encoded Compounds. ACS Chem Biol. 2011;6:336–344. doi: 10.1021/cb1003477. [DOI] [PubMed] [Google Scholar]
- 174.Meireles LMC, Mustata G. Discovery of modulators of protein-protein interactions: current approaches and limitations. Curr Top Med Chem. 2011;11:248–257. doi: 10.2174/156802611794072632. [DOI] [PubMed] [Google Scholar]
- 175.Mammen M, Choi SK, Whitesides GM. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Edit. 1998;37:2755–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 176.Lim RKV, Lin Q. Bioorthogonal chemistry: recent progress and future directions. Chem Commun. 2010;46:1589–1600. doi: 10.1039/b925931g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sletten EM, Bertozzi CR. Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew Chem Int Edit. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]