Abstract
BACKGROUND AND PURPOSE
Neuropeptide Y (NPY) and its receptors have been implicated in the control of emotional-affective processing, but the mechanism is unclear. While it is increasingly evident that stimulation of Y1 and inhibition of Y2 receptors produce prominent anxiolytic and antidepressant effects, the contribution of the individual NPY receptor subtypes in the acquisition and extinction of learned fear are unknown.
EXPERIMENTAL APPROACH
Here we performed Pavlovian fear conditioning and extinction in NPY knockout (KO) and in NPY receptor KO mice.
KEY RESULTS
NPY KO mice display a dramatically accelerated acquisition of conditioned fear. Deletion of Y1 receptors revealed only a moderately accelerated acquisition of conditioned fear, while lack of Y2 receptors was without any effect on fear learning. However, the strong phenotype seen in NPY KO mice was reproduced in mice lacking both Y1 and Y2 receptors. In addition, NPY KO mice showed excessive recall of conditioned fear and impaired fear extinction. This behaviour was replicated only after deletion of both Y1 and Y2 receptors. In Y1 receptor single KO mice, fear extinction was delayed and was unchanged in Y2 receptor KO mice. Deletion of NPY and particularly Y2 receptors resulted in a generalization of conditioned fear.
CONCLUSIONS AND IMPLICATIONS
Our data demonstrate that NPY delays the acquisition, reduces the expression of conditioned fear while promoting fear extinction. Although these effects appear to be primarily mediated by Y1 receptors, the pronounced phenotype of Y1Y2 receptor double KO mice suggests a synergistic role of Y2 receptors in fear acquisition and in fear extinction.
Keywords: neuropeptide Y, NPY, Y1 receptor, Y2 receptor, fear conditioning, fear extinction
Introduction
A high incidence of human anxiety disorders and limited treatment options pose a major challenge for health-care systems and a requirement for novel drug therapies (Wittchen and Jacobi, 2005; Wittchen et al., 2011). Neuropeptide systems are promising drug targets for the modulation of anxiety-related disorders. In particular, neuropeptide Y (NPY), a highly conserved 36-amino acid peptide that has been shown to be involved in the modulation of anxiety (Kask et al., 2002; Heilig, 2004). NPY and its receptors (Y1, Y2, Y4, Y5; receptor nomenclature follows Alexander et al., 2011) are concentrated in the limbic areas of the brain including the hippocampus and the amygdala (Tatemoto et al., 1982; Gustafson et al., 1986; Dumont et al., 1993; El Bahh et al., 2005; Stanic et al., 2011). In the amygdala, Y1 and Y2 receptors are expressed in the basolateral (BLA) and central (CEA) nucleus (Kopp et al., 2002; Stanic et al., 2006; 2011). Considerable evidence supports an important role of NPY in modulating anxiety-related behaviours in rodents. The anxiolytic action of NPY is predominantly mediated by stimulation of Y1 receptors in the BLA (Heilig et al., 1993; Heilig, 1995; Karlsson et al., 2007). Activation of presynaptic Y2 receptors increases anxiety-like responses (Nakajima et al., 1998; Sajdyk et al., 2002; Bacchi et al., 2006), whilst deletion of Y2 receptors results in reduced anxiety-like behaviour (Redrobe et al., 2003; Tschenett et al., 2003; Tasan et al., 2009; 2010). Recently, a possible role of NPY in models of learned fear has been suggested (Gutman et al., 2008; Fendt et al., 2009).
Cued fear conditioning is a simple form of associative learning predominantly mediated by the amygdala (LeDoux, 2000). Using fear-potentiated startle as a measure in rats, Broqua et al. (1995) showed that intracerebroventricular application of the Y1 receptor-preferring agonist Leu31Pro34NPY results in a reduction of fear expression. Similarly, Gutman et al. (2008) demonstrated that NPY inhibits the expression and facilitates the extinction of fear, presumably by acting on Y1 receptors in the BLA. Conversely, recent evidence suggests that NPY application into the amygdala may influence the expression of fear in mice independent of Y1 receptor activation (Fendt et al., 2009). While a role of NPY in fear expression and extinction is increasingly evident, the contributions of the NPY receptors involved are still unclear.
The aim of the present study was to characterize the role of endogenous NPY in acquisition, expression and extinction of conditioned fear, and to investigate the participation of individual NPY receptors in these processes. In order to achieve these aims, we employed Pavlovian fear conditioning and extinction in mice deficient in NPY, deficient in individual NPY receptors (Y1, Y2) or lacking both Y1 and Y2 receptors.
Methods
Animals
All animal care and experimental procedures complied with international laws and policies (Directive 2010/63/EU of the European parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes; Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Council NR, 2010) and were approved by the Austrian Ministry of Science. All effort was taken to minimize the number of animals used and their suffering. Experiments were performed on adult male mice (10–16 weeks old, weighing 25–30 g) maintained on a C57BL/6-129SvJ background. They were housed in groups of three to five under standard laboratory conditions (12 h/12 h light/dark cycle, lights being on at 07:00, food and water ad libitum). Generation of NPY and Y1, Y2 and Y1/Y2 receptor knockout (KO) mice has been described in detail previously (Sainsbury et al., 2002a,b; Karl et al., 2008). In brief, floxed, chimeric conditional KO mice (Y1lox/lox or Y2lox/lox) were crossed with oocyte-specific Cre-recombinase expressing C57BL/6 mice (Schwenk et al., 1995). Wildtype mice (WT) of the same mixed C57BL/6-129SvJ background were used as controls (Sainsbury et al., 2002a). Deletion of NPY, Y1 and Y2 receptor genes was confirmed in all mice used for the experimentation by PCR and agarose gel electrophoresis. Further characterization of receptor deletion was done in randomly selected mice by in situ hybridization and receptor autoradiography [using rat (125I)- Leu31, Pro34 PYY and rat (125I)-PYY3-36 as ligands for Y1 and Y2 receptors, respectively], as described in detail previously (Gobbi et al., 1998; Tasan et al., 2009).
Genotyping
Genotypes of the mice were monitored as described previously (Sainsbury et al., 2002a; Karl et al., 2008; Tasan et al., 2009). In brief, PCR was performed using the following primers for NPY oligo-NPY-F1 (5′ ATG GAA GTC AGA GGA TGC 3′), oligo-NPY-R1 (5′ TCA AAT GTT ATT CCC AGT CG 3′) and oligo-NPY-F2 (5′ GTT AAA CCT TCG ATT CCG ACC TC 3′) and oligo-NPY-R2 (5′ ATT CTA GGG TCT GGG ATG 3′), the Y1 receptor oligo-Y1-F (5′ TGG CAA AAC AGG TCC CTG 3′) and oligo-Y1-R (5′ CTA GCC AGT TGG TAA TGG 3′), the Y2 receptor oligo-Y2-F (5′ TTA ACA TCA GCT GGC CTA GC 3′), oligo-Y2-R1 (5′GGA AGT CAC CAA CTA GAA TGG 3′), oligo-Y2-R2 (5′AGC ATC CAG AGA AGT GCA AC 3′) with 40 cycles of 94°C for 45 s, 59°C for 45 s and 72°C for 45 s. DNA was loaded on a 2% agarose gel. Ethidium bromide-labelled bands were evaluated under UV light and monitored with a concomitantly run size marker. Using a combination of oligo-NPY-R1 and oligo-NPY-F1, oligo-Y1-R and oligo-Y1-F, oligo-Y2-F and oligo-Y2-R1 sequence corresponding to the intact NPY, Y1 or Y2 receptor genes could be detected (NPY WT: 200 bp, Y1 WT: 650 bp, Y2 WT mice: 330 bp), whereas oligo-NPY-R2 and oligo-NPY-F2, oligo-Y1-R and oligo-Y1-F, oligo-Y2-F and oligo-Y2-R1 were used to demonstrate the deletion of the NPY, Y1 receptor and Y2 receptor respectively (NPY KO mice: 200 bp, Y1 receptor KO mice: 520 bp, Y2 receptor KO mice: 250 bp) (for details see Sainsbury et al., 2002a,b; Karl et al., 2008).
Behavioural experiments
Home cage activity
Motor activity of mice was assessed in their home cages for 72 h, as described in detail previously (Tasan et al., 2009). For homecage activity measurements, naïve mice were single-housed for 72 h in standard cages with food and water ad libitum. Briefly, movements were determined using an infrared sensor mounted on top of the cages (TSE LabMaster InfraMot, Bad Homburg, Germany). After a 24 h acclimatization period, cumulative activity was recorded during the subsequent 72 h. Our setting allowed concomitant testing of four KO mice and four controls. Cumulative activity counts per12 h period were analysed for the dark and light cycle separately and presented as a mean of three consecutive cycles.
Baseline activity in the fear-conditioning box
To validate reactive motor activity in an unfamiliar environment that is more relevant to testing conditions, the behaviour of naïve mice was investigated in a fear-conditioning box (similar to context A of the fear-conditioning experiments) for 15 min (corresponding to the testing time in acquisition and extinction trials) in the absence of any stimulus. Videotapes were analysed for motor activity by a pixel-based analysis software (http://topowatch.sourceforge.net/, TopoWatch v0.3) and verified manually by two different observers that were unaware of the genotype of the mice.
Determination of sensitivity threshold to the unconditioned stimulus (US)
Naïve mice were placed individually into the conditioning box. After a 3 min habituation period, a series of electric foot shocks of increasing current intensity was applied (0.1–0.9 mA, 2 s, increase in 0.1 mA steps every 30 s). The sensitivity threshold was defined as the current at which the mice displayed each sign of the US sensitivity response (flinching, running, jumping and vocalization).
Differential fear-conditioning paradigm
Naïve mice were used for fear conditioning. All fear-conditioning experiments were repeated with a different set of naïve mice, yielding the same significant results and are shown as a pooled analysis. In the fear-conditioning paradigm, an US, usually a mild electric foot shock is repetitively paired with a conditioned stimulus (CS), typically represented by a tone. After a few of these pairings, the CS alone can elicit a typical fear reaction. Subsequently, repetitive presentations of the CS in the absence of the US results in a gradual reduction of the learned fear response, a process called fear extinction. Fear conditioning was performed in context A consisting of a transparent acrylic rodent-conditioning chamber with a metal grid floor that was enclosed by a sound-attenuating chamber. Illumination was 80 lux and chambers were cleaned with 70% ethanol. Fear recall as well as fear extinction and extinction recall were performed in a different context consisting of a dimly illuminated (10 lux) chamber with black, smooth walls and the floor cleaned with 1% acetic acid (context B). On day 1 (context A) mice were subjected to a differential fear-conditioning paradigm in which one auditory stimulus served as a CS (CS+, 30 s white noise, 80 dB) because it was explicitly paired with a US, whereas the second auditory stimulus was not paired (CS−, 30 s, 3.5 kHz, 80 dB). All animals received 5 CS− and 5 CS+ in an alternating order, starting with a CS+. The US co-terminating with each CS+ consisted of a mild electric foot shock. The shock intensity was set to 0.7 mA (2 s), a threshold at which all strains showed a respective behavioural reaction in the sensitivity analysis. On days 2 and 3, fear recall and extinction training was performed in context B. After a 2 min habituation period, 5 CS− (30 s, inter-stimulus interval 5 s) were presented followed by 15 presentations of CS+ (30 s, inter-stimulus interval 5 s) or 40 CS+ for an extended extinction protocol. Extinction recall was tested on day 4 by presenting 5 CS+ in context B. To further investigate the generalization of conditioned fear, we used two stimuli that were more distant from each other, a visual (CS−, 30 s, house light, 50 lux) and an auditory stimulus (CS+, 30 s white noise, 80 dB). In order to use the light as a cue for fear conditioning, these experiments were performed in the dark with an infrared light source Monacor IR-28-plate LED infrared light, Austria) in a separate group of naïve mice. Behaviour was recorded by a video camera and scored offline by a pixel-based analysis software (http://topowatch.sourceforge.net/, TopoWatch v0.3). The parameters of the program were validated by comparison with a manual analysis by two independent observers. To control for unpredictable factors that might occur during fear conditioning, a CS only/no shock group was included in all experiments for the respective genotypes.
Statistical analysis
Data are presented as means ± SEM. They were analysed for normal distribution and equal variances using GraphPad Prism software (Prism 5 for Macintosh, GraphPad Software Inc., San Diego, CA, USA). All acquisition and extinction experiments as well as motor activity measurements were analysed by repeated two-way anova for time, genotype and interaction (time × genotype) with a Bonferroni post hoc test for selected comparisons. Kruskal Wallis with Dunn's multiple comparison tests were used for analysing US sensitivity threshold.
Results
Homecage activity
The different KO mouse lines (NPY and Y1, Y2 and Y1/Y2 receptor) were evaluated for baseline characteristics relevant to fear conditioning, such as home cage activity, reactive motor activity and the sensitivity threshold to the US. General home cage activity was significantly reduced in NPY KO mice (Figure 1B; genotype F(1/14)= 15.84, P < 0.01 and light/dark cycle F(1/14)= 52.59, P < 0.0001 with interaction F(1/14)= 16.75, P < 0.01) and Y1/Y2 receptor double KO mice (Figure 1D; genotype F(1/14)= 4.59, P < 0.05 and light/dark cycle F(1/14)= 65.88, P < 0.0001 with interaction F(1/14)= 6.17, P < 0.05) during the dark phase (Figure 1B and D; WT vs. NPY KO: t(14)= 5.71, P < 0.0001 and WT vs. Y1/Y2 receptor KO: t(14)= 3.23, P < 0.01; Bonferroni post hoc test), but not during the light phase of the light/dark cycle. However, there was no difference in home cage behaviour between Y1 or Y2 receptor single KO mice and WT controls (not shown; n= 8 mice/genotype).
Figure 1.
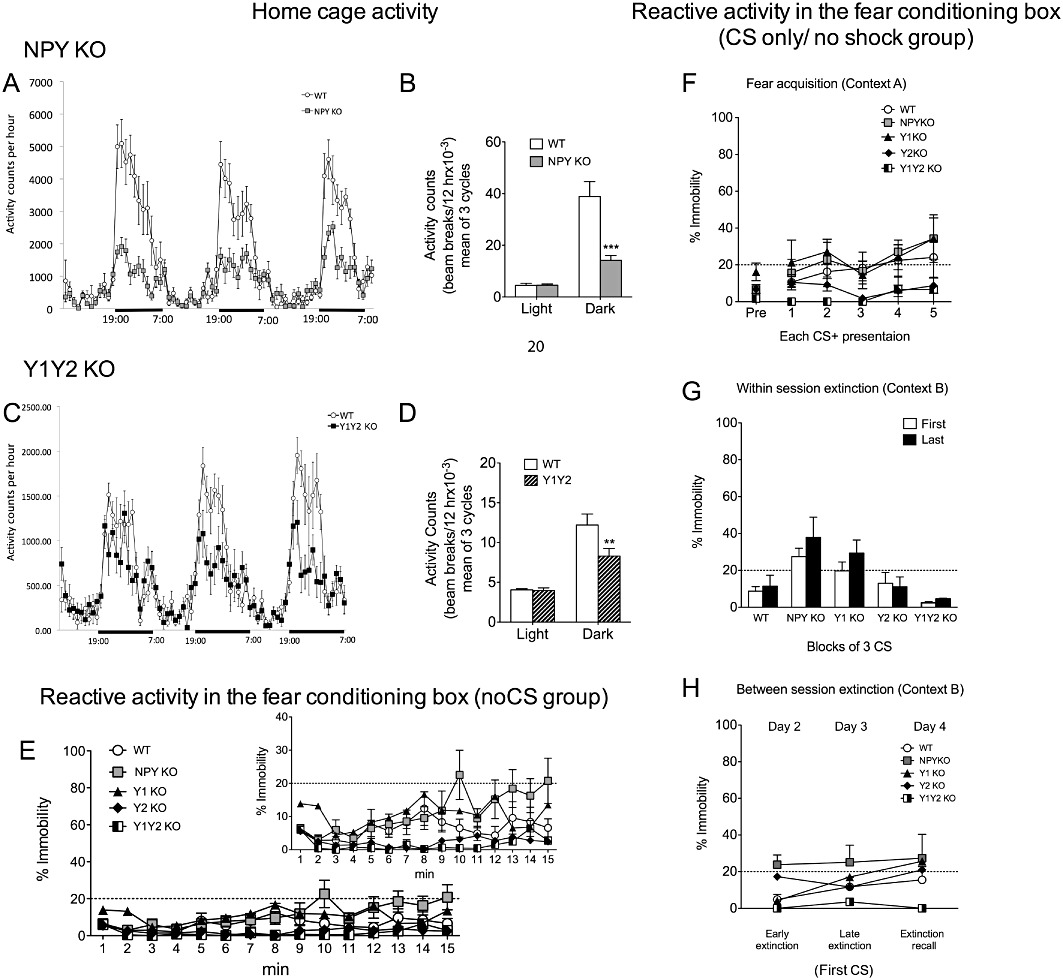
Home cage activity, reactive activity in the fear-conditioning box and CS only/no shock data of NPY KO, Y1 receptor KO, Y2 receptor KO and Y1/Y2 receptor double KO mice. (A) Home cage activity measurement in NPY KO mice and WT controls during three consecutive light/dark cycles; (B) quantification of cumulative activity demonstrates significantly decreased activity of NPY KO mice during the dark phase, but equal activity in the light phase of the light/dark cycle; (C) home cage activity determined during three consecutive light/dark cycles and (D) quantitative evaluation of cumulative activity demonstrating decreased activity of Y1/Y2 receptor double KO mice in the dark phase, but not in the light phase of the light/dark cycle; (E) baseline immobility during 15 min (comparable with the respective experimental time scales used in fear acquisition and extinction experiments) in context A of the fear-conditioning box without any stimulus (insert with altered scale more accurately displays differences of individual groups); (F) reactive motor activity of CSonly/no shock groups of the different genotypes during acquisition (G) within-session extinction period of CS only/no shock group (shown by comparison of the first three with the last three CS); and (H) between-session analysis of CS only/no shock group (shown by comparison of % immobility to the first CS on three individual extinction days). Dashed line in E–H indicates freezing threshold. Data shown are means ± SEM, repeated two-way anova with Bonferroni post hoc test (home cage n= 8 per group, reactive motor activities n= 5 per group), **P < 0.01, ***P < 0.001.
Reactive activity in the fear-conditioning box
To test for a possible confounding influence of reactive motor activity WT, NPY KO, Y1 KO, Y2 KO and Y1/Y2 receptor double KO mice were tested for their immobility times in the fear-conditioning box in the absence of any stimulus for the same time period as in acquisition and extinction trials (NoCS groups). As shown in Figure 1E, there was no significant difference in % of immobility between NPY KO, Y1 KO, Y2 KO, Y1/Y2 receptor double KO and WT controls as revealed by repeated two-way anova. Moreover a CS only/no shock group was included to control for an influence of altered CS perception. Two-way anova revealed an overall effect of genotype for extinction day 1 (Figure 1G; F(1/14)= 6.17, P < 0.01), but not for acquisition or between-session extinction (Figure 1F and H). Compared with WT controls, however, Bonferroni post hoc analysis did not show any significant difference of the individual genotypes in reactive motor activity when mice were exposed to the CS alone (Figure 1F–H).
Sensitivity threshold to US
We also investigated the sensitivity to the US by analysing the threshold of US-induced movements (flinching, running, jumping) and vocalization. As shown in Supporting Information Figure S1, NPY KO mice showed an increased threshold to all the US-induced behavioural responses investigated, while Y1, Y2 and Y1/Y2 receptor double KO mice responded similar to controls (Supporting Information Figure S1A–D; n= 8–9 mice/KO mouse strain, WT: n= 20; Kruskal Wallis test, flinching H(4)= 29.19, P < 0.0001, vocalization: H(4)= 34.26, P < 0.0001, running H(4)= 25.55, P < 0.0001 and jumping H(4)= 23.31, P < 0.0001).
Acquisition and recall of conditioned fear
In fear-conditioning experiments, NPY KO mice (n= 24) and age-matched WT controls (n= 25) showed similar baseline freezing levels on the acquisition day (Figure 2A; PreCS, day 1, context A). Acquisition of conditioned fear, however, was significantly accelerated in NPY KO mice (Figure 2A). Repeated two-way anova revealed an effect of genotype (F(1/47)= 57.60, P < 0.0001) and time (F(4/188)= 73.36, P < 0.0001) as well as interaction of genotype × time (F(4/188)= 7.49, P < 0.0001). Long-term fear memory, tested 24 h later in a different context (day 2, context B) was increased in NPY KO mice, as revealed by higher freezing levels to the CS+ (Figure 2B, two-way anova with Bonferroni post hoc test, genotype F(1/36)= 97.46, P < 0.0001 and CS F(1/36)= 7.02, P < 0.05 and interaction F(1/36)= 6.21, P < 0.05). To test whether NPY exerts a role in stimulus discrimination, we applied a differential auditory fear-conditioning paradigm, in which two different auditory stimuli were presented alternatingly: one (CS+) was paired with a US and a different CS (CS−) was not followed by a US. When tested 24 h later, WT mice were able to discriminate between CS+ and CS− (WT, CS+ vs. CS−: t(17)= 3.53, P < 0.01). In contrast, NPY KO mice also displayed an increased freezing response to the CS−, indicating a generalization of conditioned fear (Figure 2B; NPY KO, CS− vs. CS+: t(19)= 0.11, P= 0.92; and CS−, WT vs. NPY KO: t(36)= 4.70, P < 0.0001). To test whether this generalization of conditioned fear in NPY KO mice also extends to more distinct stimuli, we used a visual stimulus as CS− and an auditory stimulus as CS+ (Figure 3). Similar to WT, NPY KO mice were now able to distinguish between CS− and CS+ as revealed by repeated two-way anova (Figure 3B, genotype F(1/11)= 13.54, P < 0.01 and CS F(1/11)= 53.45, P < 0.0001 with no interaction F(1/11)= 2.06, P > 0.05 and Bonferroni post hoc test, CS+ vs. CS−, WT: t(11)= 4.33, P < 0.01 and NPYKO: t(11)= 5.96, P < 0.001). Compared with WT, freezing levels of NPY KO mice were increased to the CS+ but not to the CS− (Figure 3B; Bonferroni post test; WT vs. NPY KO, CS+: t(11)= 3.76, P < 0.01 and CS−: t(11)= 1.96, P > 0.05).
Figure 2.
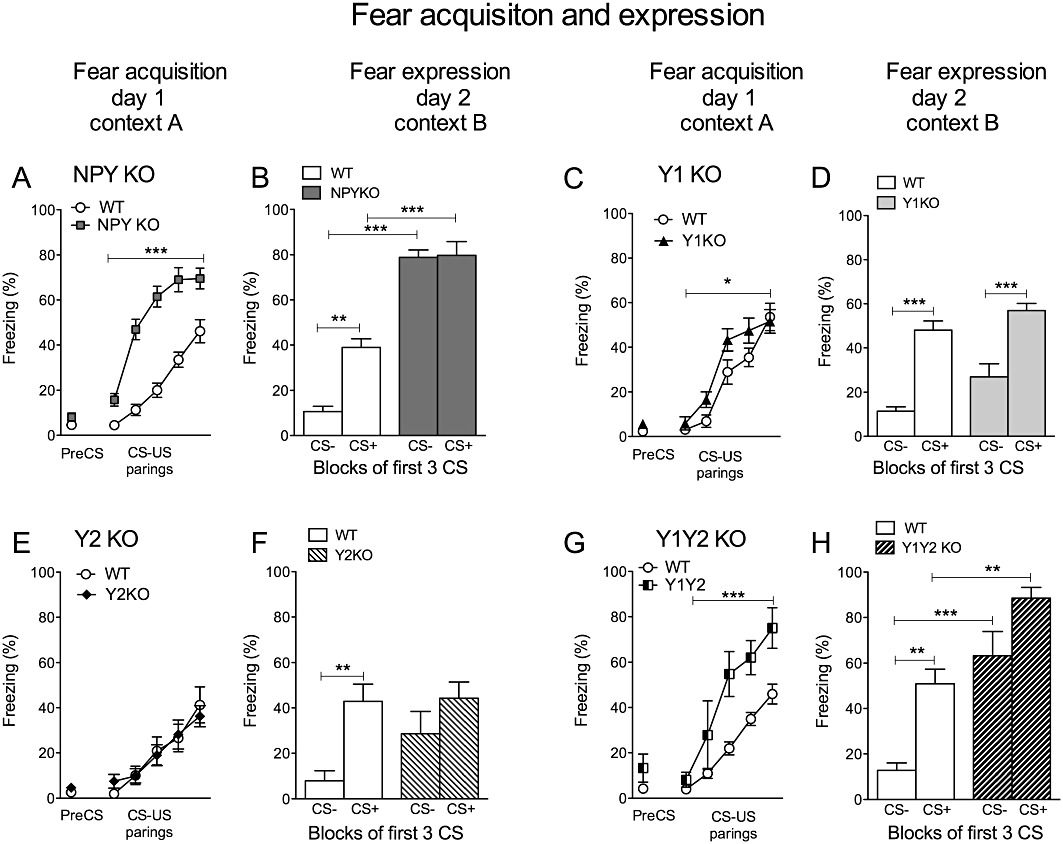
Acquisition and expression of conditioned fear. (A and B) Equal baseline freezing (PreCS) but significantly accelerated acquisition of fear was observed in NPY KO mice compared with WT controls (day 1, context A), histograms show increased expression/recall of fear indicated by higher % of freezing to the CS+ in NPY KO mice on day 2 in a different context (context B); stimulus discrimination was absent in NPY KO mice as demonstrated by similar % of freezing during CS− and CS+ presentations; (C and D) accelerated fear acquisition shown in Y1 receptor KO mice (day 1, context A), but similar % of freezing as WT controls after 24 h on day 2 in a different context (context B); (E and F) Y2 receptor KO mice show equal acquisition (day 1, context A) and expression (day 2, context B) of conditioned fear like WT, whereas in contrast to controls, Y2 receptor KO show impaired stimulus discrimination demonstrated by equal % of freezing to the CS+ and CS− on day 2; (G and H) Y1/Y2 receptor double KO mice demonstrate accelerated acquisition (day 1, context A) and increased expression (day 2, context B) of conditioned fear; compared with WT and Y1 receptor single KO mice, Y1Y2 double KO mice display increased % of freezing to CS−, indicating a generalization of conditioned fear. Data shown are means ± SEM, repeated two-way anova with Bonferroni post hoc test, *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 3.
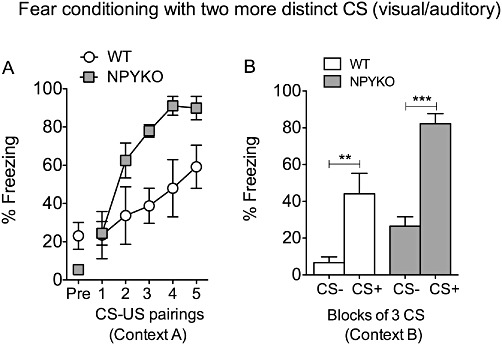
Differential fear conditioning in NPY KO mice using a visual (CS−) and an auditory (CS+) stimulus. (A) Accelerated acquisition of conditioned fear in NPY KO mice compared to WT, (B) both, WT and NPY KO mice display higher % freezing to CS+ compared to CS− at fear testing 24 h after acquisition, demonstrating discrimination of two more distinct CS. Values are means ± SEM, repeated two-way anova with Bonferroni post hoc test, **P < 0.01, ***P < 0.001.
Y1 receptor KO (n= 13) mice showed facilitated acquisition of conditioned fear (Figure 2C, repeated two-way anova, genotype: F(1/22)= 4.46, P < 0.05; time: F(4/88)= 46.45, P < 0.0001 but no interaction genotype × time: F(4/88)= 1.28, P > 0.05), whereas Y2 receptor KO mice (n= 18) acquired fear at the same rate as WT controls (Figure 2E, repeated two-way anova, no difference in genotype: F(1/33)= 0.01 and interaction genotype × time: F(4/132)= 0.61; time: F(4/132)= 27.84, P < 0.0001). Recall of fear, tested after 24 h, was similar in Y1 and Y2 receptor KO mice compared with WT controls (Figure 2D and F; Bonferroni post hoc test, CS+, WT vs. Y1 receptor KO: t(32)= 1.84; WT vs. Y2 receptor KO: t(52)= 0.13). Interestingly, only Y1/Y2 receptor double KO mice (n= 12) showed accelerated fear acquisition (Figure 2G; repeated two-way anova genotype: F(1/34)= 17.28, P < 0.001; time: F(4/136)= 40.51, P < 0.0001 and interaction genotype × time: F(4/136)= 2.84, P < 0.05) and increased fear expression on the retention day (Figure 2H, two-way anova; genotype F(1,18)= 23.34, P < 0.001 and CS F(1,18)= 44.96, P < 0.0001 but no interaction F(1,18)= 0.97 P > 0.05 with Bonferroni post hoc test, CS+, WT vs. Y1/Y2 receptor KO: t(18)= 4.05, P < 0.01), similar to NPY KO mice (Figure 2A and B). Further investigations into discriminative learning revealed that NPY KO, as well as Y2 receptor KO and Y1/Y2 receptor double KO, mice displayed increased freezing times to a CS−, indicating a generalization of conditioned fear (Figure 2F and H; Bonferroni post hoc tests, Y2 receptor KO, CS+ vs. CS−: t(32)= 1.64, P > 0.05 and CS−, WT vs. Y1/Y2 receptor KO: t(18)= 5.44, P < 0.0001).
Similar to CS+, acquisition of CS− induced freezing was strongly accelerated in NPY KO (Supplementary Figure S2A) and Y1/Y2 receptor double KO mice (Supplementary Figure S2D) as revealed by repeated two-way anova (NPY KO: genotype F(1/10)= 81.16, P < 0.0001 and time F(4/40)= 5.59, P < 0.01 but no interaction F(4/40)= 0.74, P > 0.05; Y1/Y2 receptor double KO mice: genotype F(1,9)= 26.73, P < 0.001 and time F(4/36)= 5.91, P < 0.001 but no interaction F(4/36)= 1.67, P > 0.05). In Y1 receptor KO mice (Supplementary Figure S2B) there was a moderate acceleration of CS− induced freezing (genotype F(1,9)= 6.89, P < 0.05 and time F(4/36)= 7.39, P < 0.001 and interaction F(4/36)= 3.11, P < 0.05) while there was no difference between WT and Y2 receptor KO mice (Supplementary Figure S2C; genotype F(1,13)= 0.04, P > 0.05 and time F(4/52)= 10.94, P < 0.0001 but no interaction F(4/52)= 0.86 P > 0.05).
Extinction of conditioned fear
Within-session extinction
Within-session extinction was determined by (i) the change of the freezing response over the course of consecutive CS presentations on extinction day 1; and (ii) by comparison of the first three CS with the last three CS on each extinction day. In NPY KO mice, within-session extinction, was significantly delayed, as revealed by repeated two-way anova of single CS presentations (Figure 4A; time, F(14/350)= 3.72, P < 0.0001 and genotype, F(1/25)= 54.45, P < 0.0001 with no interaction: F(14/350)= 1.01, P > 0.05) and by comparing the first three CS+ with the last three CS+ on extinction days 1 and 2 (Figure 4B and C, repeated two-way anova for day1: time, F(1/25)= 7.62, P < 0.05 and genotype, F(1/25)= 89.59, P < 0.0001 with no interaction: F(1/25)= 1.05, P > 0.05 and Bonferroni post hoc test, WT: t(24)= 2.63, P < 0.05 but NPY KO: t(26)= 1.25, P > 0.05 and day 2: time, F(1/25)= 13.86, P < 0.001 and genotype F(1/25)= 73.34, P < 0.0001 and no interaction F(1/25)= 0.09, P > 0.05, WT: t(24)= 2.79, P < 0.05; NPY KO: t(26)= 2.47, P < 0.05). Even an extended extinction protocol (40 CS/ day, 2 days) did not result in improved extinction learning in NPY KO mice (not shown).
Figure 4.
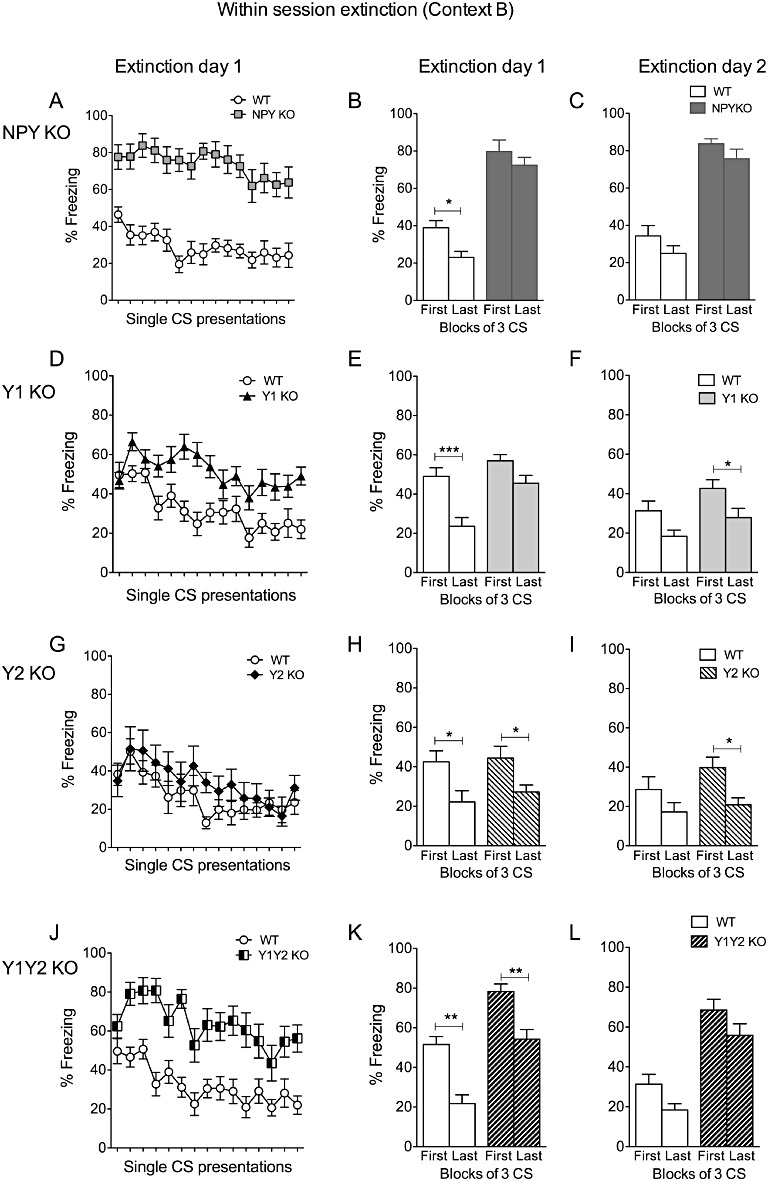
Within-session extinction of conditioned fear in NPY, Y1, Y2 and Y1/Y2 receptor double KO mice. (A–C) Histograms (right panels) show impaired fear extinction of NPY KO mice demonstrated by equal % of freezing to the first three and last three CS of extinction sessions on day 2 and day 3, (D–F) compared to WT, Y1 receptor KO mice show significantly higher % of freezing to the last three CS on extinction day 2 but not on extinction day 3, indicating a delayed extinction process, (G–I) Y2 receptor KO mice demonstrate equal % of freezing as WT controls on both extinction days, (J–L) Y1/Y2 receptor double KO mice display increased freezing to the first and last three CS on both extinction days. Data shown are means ± SEM, repeated two-way anova with Bonferroni post hoc test for expression of conditioned fear, *P < 0.05, **P < 0.01, ***P < 0.001.
As shown in Figure 4, Y1 receptor KO mice revealed significantly delayed extinction of conditioned fear (Figure 4D–F). This response was mainly reflected by delayed within-session extinction, as shown for the single CS presentations (Figure 4D; repeated two-way anova; time, F(14/308)= 4.49, P < 0.0001 and genotype, F(1/22)= 18.22, P < 0.001 with interaction F(14/308)= 2.10, P < 0.05) and by comparison of the first with the last 3 CS+ on individual extinction days (Figure 4E and F; day1: time, F(1/22)= 18.75, P < 0.001 and genotype, F(1/22)= 15.55, P < 0.001 with no interaction F(1/22)= 2.67, P > 0.05 and Bonferroni post hoc test, WT: t(22)= 4.05, P < 0.01; Y1 receptor KO: t(22)= 1.99, P > 0.05 and Figure 4F for day 2: time, F(1/22)= 13.82, P < 0.01 and genotype, F(1/22)= 4.47, P < 0.05 with no interaction F(1/22)= 0.06, P > 0.05 and WT: t(22)= 2.35, P > 0.05; Y1 receptor KO: t(22)= 2.93, P < 0.05). In contrast, the extinction process in Y2 receptor KO mice did not significantly differ from that in WT controls (Figure 4G, single CS presentations; time, F(14/252)= 6.42, P < 0.0001 and genotype, F(1/18)= 0.99, P > 0.05 with no interaction: F(14/252)= 0.91, P > 0.05). However, as observed in Y1 receptor KO mice, Y1/Y2 receptor double KO mice revealed intact, but delayed within-session extinction as shown by single CS presentations (Figure 4J, repeated two-way anova for time, F(14/294)= 6.17, P < 0.0001 and genotype, F(1/21)= 26.34, P < 0.0001 with no interaction: F(14/294)= 1.33, P > 0.05) and comparison of the first with the last three CS+ (Figure 4K, day1: time, F(1/21)= 35.92, P < 0.0001 and genotype, F(1/21)= 21.05, P < 0.001 with no interaction F(1/21)= 0.11, P > 0.05 and Bonferroni post hoc test, WT: t(21)= 4.38, P < 0.001; Y1/Y2 receptor KO: t(21)= 4.09, P < 0.01 and Figure 4L for day 2: time, F(1/21)= 12.02, P < 0.01 and genotype, F(1/21)= 21.61, P < 0.0001 with no interaction F(1/21)= 0.05, P > 0.05 and Bonferroni post hoc test, WT: t(21)= 2.56, P < 0.05; Y1/Y2 receptor KO: t(21)= 2.34, P > 0.05).
Between-session extinction
Between-session extinction was measured by the freezing response to the first CS over the course of the three different extinction days (Figure 5). NPY KO mice did not only display significantly impaired within-session extinction (Figure 4A–C), but also impaired between-session extinction, shown by comparing % freezing upon the first CS+ on three consecutive days (Figure 5A, repeated two-way anova, genotype: F(2/50)= 71.71, P < 0.0001; time: F(1/25)= 3.49, P < 0.05 and interaction genotype × time: F(2/50)= 4.92, P < 0.05).
Figure 5.
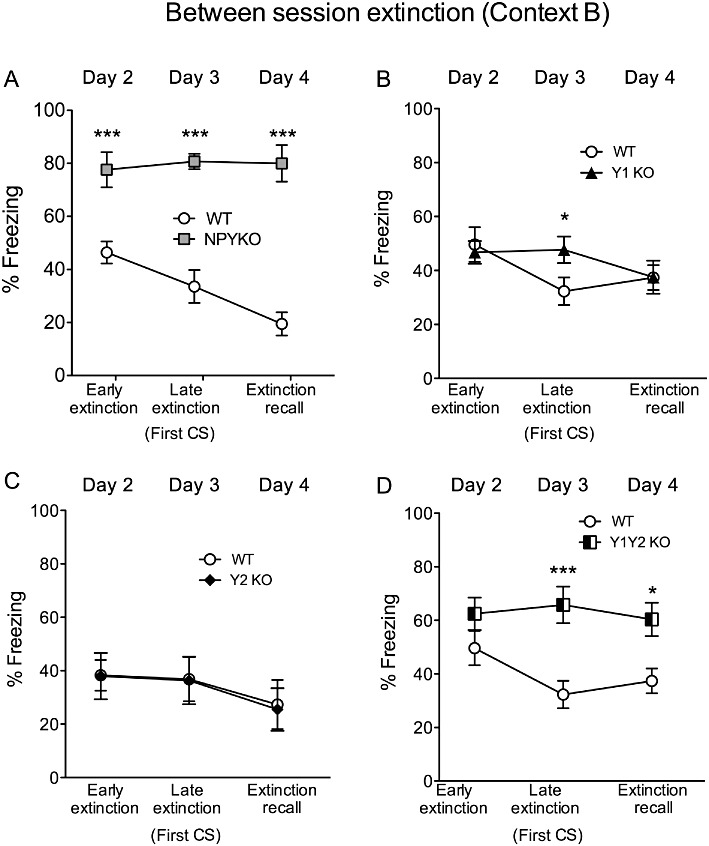
Between-session extinction is impaired in NPY KO and Y1/Y2 receptor double KO mice. (A) Impaired extinction in NPY KO mice shown by higher % of freezing to the first CS of day 2 (early extinction), day 3 (late extinction) and day 4 (extinction recall); (B) a delayed extinction was observed for Y1 receptor KO mice evidenced by increased % of freezing to the first CS on day 2 but not on day 3 (extinction recall), (C) no change in % of freezing between WT and Y2 receptor KO mice on all three consecutive extinction days, (D) increased % of freezing of Y1/Y2 receptor KO mice is demonstrated on days 3 and 4, indicating impaired between-session extinction. Data shown are means ± SEM, repeated two-way anova with Bonferroni post hoc test, *P < 0.05, ***P < 0.001.
As shown in Figure 5, however, between-session extinction was not affected in Y1 KO mice (Figure 5B). Similarly, also in Y2 receptor KO mice between-session extinction did not differ from that in WT controls (Figure 5C, repeated two-way anova, time F(2/34)= 3.93, P < 0.05; genotype: F(1/17)= 0.01, P > 0.05 and interaction genotype × time: F(2/34)= 0.02, P > 0.05). However, as in NPY KO mice, in Y1/Y2 receptor double KO mice between-session extinction and extinction recall were significantly impaired (Figure 5D, repeated two-way anova, genotype: F(1/21)= 12.83, P < 0.01; time: F(2/42)= 1.48, P > 0.05 and interaction of genotype × time: F(2/42)= 2.37, P > 0.05).
Discussion
Results obtained in our study clearly demonstrate that NPY as well as Y1 and Y2 receptors are crucially involved in learned fear. NPY KO mice display facilitated acquisition, increased expression/recall and impaired extinction of conditioned fear. Moreover, although Y1 receptor KO mice displayed moderate changes in acquisition and delayed extinction, Y1/Y2 receptor double KO mice exhibited strongly accelerated fear acquisition and severe extinction deficits. The lack of Y2 receptors on its own, however, did not result in altered amygdala-dependent fear learning.
Brain areas and major projections involved in associative fear learning have been extensively investigated (LeDoux, 2000; Pape and Pare, 2010). The amygdala exerts a key role in associative plasticity and fear learning. Interestingly, the amygdaloid complex contains significant concentrations of different neuropeptides and neuropeptide receptors (Stanic et al., 2011). Thus, in the BLA, NPY is expressed in a specific class of GABA-ergic interneurons (McDonald and Pearson, 1989). NPY, released from amygdala interneurons may inhibit glutamatergic projection neurons resulting in decreased BLA output and consequently in reduced anxiety. Similarly, NPY may reduce glutamatergic excitation in the BLA also during fear conditioning and thereby inhibit synaptic plasticity and the acquisition of fear memories.
Role of NPY in fear acquisition
In the present study, NPY KO mice showed facilitated acquisition of conditioned fear (Figure 3A, Table 1). Lack of NPY may reduce the inhibitory tonus in the BLA during fear conditioning, an effect that is presumably dependent on Y1 receptors located on pyramidal neurons in the BLA (Giesbrecht et al., 2010).
Table 1.
Summary of effects of NPY and Y receptor deletion on fear learning
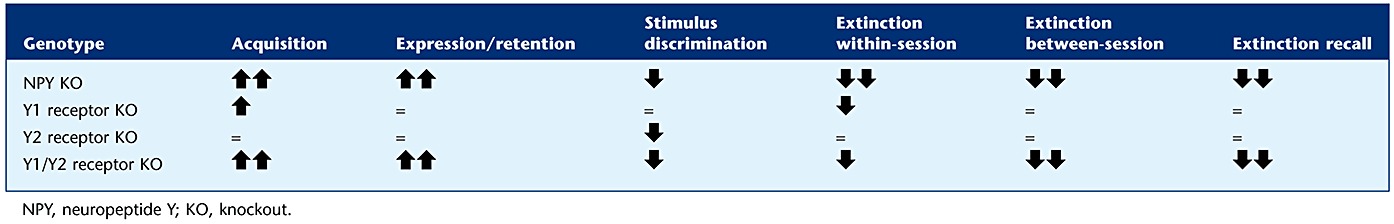 |
Both, Y1 and Y2 receptors are highly expressed in the BLA and CEA (Kopp et al., 2002; Stanic et al., 2006; 2011). Recently, generally higher freezing levels were observed in Y1 receptor KO mice during fear conditioning (Fendt et al., 2009). Similarly, we demonstrated facilitated fear conditioning in Y1 receptor KO mice (Figure 3C, Table 1), suggesting that NPY inhibits fear conditioning by acting on postsynaptic Y1 receptors. Recent evidence, however, indicates that intra-amygdala application of NPY also causes reduced fear expression in Y1 receptor KO mice, suggesting the involvement of different Y receptors in fear conditioning (Fendt et al., 2009). In addition to Y1 receptors, Y2 and Y5 receptors are also expressed in the amygdaloid complex, while expression of Y4 receptors are primarily restricted to specific brain stem nuclei (Wolak et al., 2003; Stanic et al., 2006; Tasan et al., 2009).
Our study revealed that the deletion of Y2 receptors by itself does not modify cued fear conditioning (Figure 3E and F, Table 1). On the other hand, Y1/Y2 receptor double KO mice exhibited exaggerated fear acquisition compared with Y1 receptor KO mice, strongly suggesting a dominant role of Y2 receptor deletion on fear learning while the contribution of Y1 receptors may be only of only minor significance. In contrast to Y1 receptors, activation of Y2 receptors presynaptically inhibits transmitter release (NPY, glutamate or GABA) (Colmers et al., 1991) and decreases long-term potentiation and synaptic plasticity (Sorensen et al., 2008; 2009). Site-specific injection of Y2 receptor preferring agonists (Sajdyk et al., 2002) and local deletion of Y2 receptors (Tasan et al., 2010), however, suggest an anxiogenic role of Y2 receptors in the BLA and CEA due to a Y2 receptor-mediated inhibition of NPY release. Thus, it is conceivable that in Y2 receptor KO mice, an accelerated fear learning may be masked by a concomitant anxiolytic effect mediated by increased release of NPY acting on postsynaptic Y1 receptors. In Y1/Y2 receptor double KO mice, however, deletion of postsynaptic Y1 receptors may reduce the inhibitory tone of NPY on pyramidal neurons and concomitant deletion of presynaptic Y2 receptors (located on glutamatergic neurons) may facilitate glutamate release and thereby reinforce synaptic plasticity.
Role of NPY in fear expression
Recently, Gutman et al. (2008) demonstrated that infusion of NPY into the BLA inhibits the expression of fear-potentiated startle responses. This observation is in accordance with our current experiments revealing an increased fear expression in NPY KO mice (Figure 3B, Table 1). In addition, we provide evidence that only combined Y1 and Y2 receptor deletion recapitulates increased fear expression observed in NPY KO mice. This finding is supported by the recent studies of Fendt et al. (2009) demonstrating reduced conditioned freezing after infusion of NPY, but not after infusion of the Y1 receptor preferring agonists Y-28 {Des-AA11–18[Cys7,21, D-Lys9 (Ac), D-His26, Pro34]-NPY} or Y-36 [(D-Arg25,D-His26)-NPY].
Role of NPY in fear generalization
The amygdala mediates predominantly immediate fear reactions upon discrete cues (Hitchcock and Davis, 1987; 1991; LeDoux et al., 1988), whereas the bed nucleus of the stria terminalis may be involved in a long-term response to diffuse stimuli (Walker and Davis, 1997; Walker et al., 2003). By using a differential fear-conditioning paradigm, we investigated the ability to discriminate between two different stimuli, one that was explicitly paired with the US (CS+) and a second one that was not paired (CS−). When CS+ and CS− were represented by two different auditory stimuli, NPY KO mice displayed an increased freezing response to the CS−, whereas both WT and NPY KO mice were able to differentiate between two completely different stimuli, such as a visual and an auditory stimulus. The inability to distinguish between two similar stimuli indicates a generalization of conditioned fear and a possible involvement of the bed nucleus of the stria terminalis. This generalized fear response was also observed in Y2 receptor KO and in Y1/Y2 receptor double KO mice, but not after Y1 receptor deletion alone, indicating an important role of Y2 receptors in conditioned fear stimulus discrimination (Figure 3, Table 1). Generalization of conditioned fear also increases with time, when memory traces become independent of hippocampal processing (Biedenkapp and Rudy, 2007; Wiltgen and Silva, 2007). On the other hand, hippocampal lesions after fear conditioning disrupt context discrimination for recent memories, whereas remote memories, that usually employ predominantly cortical areas, are not affected (Wang et al., 2009). Our results therefore indicate that NPY, and in particular, Y2 receptors that are highly expressed in the hippocampus, may be crucial for the accurate retention of recent fear memories.
Role of NPY in fear extinction
Infusion of NPY into the ventricles promoted within-session as well as between-session extinction of conditioned fear in a fear-potentiated startle paradigm (Gutman et al., 2008). In addition, pharmacological blockade of Y1 receptors in the BLA inhibited extinction, suggesting a facilitating role of endogenous NPY on fear extinction by acting on Y1 receptors in the BLA. Similarly, Fendt et al. (2009) reported facilitated within-session extinction of conditioned freezing after infusion of NPY into the amygdala in mice. In our study, NPY KO mice did not show extinction of conditioned fear, even when extending the extinction trials to 40 presentations per day for 2 days. NPY in the BLA may be crucial for coordinating different excitatory inputs involved in fear extinction. On the other hand, the increased anxiety-like behaviour of NPY KO mice (Bannon et al., 2000; Karl et al., 2008) and the tendency towards a generalization of fearful stimuli observed in our study may interfere with the acquisition of extinction memory.
Interestingly, Y1 receptor KO mice display intact, but significantly delayed extinction, whereas Y2 receptor KO mice did not behave differently in this respect from control mice (Figures 4 and 5, Table 1). In Y1/Y2 receptor double KO mice, however, within-session extinction was significantly delayed, while between-session extinction was entirely absent, suggesting a crucial role of Y2 receptors in the consolidation of fear extinction. Besides the involvement of NPY and in particular of Y1 receptors in the extinction of conditioned fear, our data also suggest a crucial role of Y2 receptors in promoting the consolidation of extinction memory and/or increase of basal anxiety/attention levels.
Data obtained using germ-line KO mice may be viewed with caution as global gene deletion and developmental alterations could produce a complex phenotype limiting unambiguous conclusions on the role of NPY in fear conditioning. However, because NPY KO mice exhibit a decreased sensitivity to the US, the observed accelerated fear conditioning and impaired extinction may be even under-estimated.
Furthermore NPY KO as well as Y1/Y2 receptor double KO mice displayed reduced home cage activity in the dark phase of the light/dark cycle, whereas it was equal to controls during the light phase. The computerized analysis software (Topowatch v0.3) used in this study had the advantage of generating objective, reproducible data. Separation of freezing and immobility behaviour by an automatic analysis system may not be satisfactory. We therefore validated the program, by adjusting the parameters of the software according to the manual analyses of two independent observers. Moreover, experiments were performed in the light phase, when the activity of NPY KO mice was similar to WT, and baseline freezing during the first 2 min habituation period was similar in NPY KO and WT controls. More importantly, we also assessed reactive immobility levels recorded in the fear-conditioning chamber in the absence of any stimulus for the same time period as in acquisition and extinction experiments. There was no difference between the different genotypes, indicating equal activity of these mice under test conditions. Altered acoustic or visual perception, as well as habituation or sensitization play an important role in fear conditioning and extinction and may substantially influence experimental findings. Compared with WT controls, there was no significant difference between genotypes in the CS only/no shock group. On the other hand, an apparent difference in reactive, CS-induced motor activity was seen between NPY KO and Y1/Y2 receptor double KO mice (Figure 1E and G). Despite this difference in reactive motor activity, their respective behaviours in fear conditioning experiments were very similar, further supporting the role of NPY in fear processing.
In conclusion, we have investigated the role of NPY and its Y1 and Y2 receptors in fear conditioning and extinction. We demonstrated a prominent role of NPY and in particular of Y2 receptors in fear acquisition and fear stimulus discrimination, while NPY and Y1 receptors were crucial for extinction of conditioned fear. Knock out of NPY resulted in facilitated acquisition, increased expression of fear and in impaired fear extinction. Importantly, only deletion of both Y1 and Y2 receptors duplicated this phenotype. Thus, the data indicate an involvement of both receptors in acquisition and expression of conditioned fear, as well as in fear extinction.
Acknowledgments
The authors declare that this work was funded by the Austrian Science fund (S10204 and P 22830-B18). We thank Elisabeth Gasser for technical assistance and Prof. Heide Hörtnagl for discussion.
Glossary
- BLA
basolateral amygdala
- CEA
central amygdala
- CS
conditioned stimulus
- CS−
conditioned stimulus that was not paired with an unconditioned stimulus
- CS+
conditioned stimulus that was paired with an unconditioned stimulus
- icv
intracerebroventricular
- KO
knockout
- NPY
neuropeptide Y
- US
unconditioned stimulus
Conflicts of Interest
There are no other personal financial holdings of any of the authors that could be perceived as constituting a potential conflict of interest. None of the authors has any past or present financial links including consultancies with manufacturers of material or devices described in the paper as well as links to the pharmaceutical industry or regulatory agencies or any other potential conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Sensitivity threshold to electric foot shocks. Evaluation of sensitivity to electric foot shocks demonstrates increased threshold to (A) shock induced flinching, (B) vocalization, (C) running and (D) jumping in NPY KO mice. Kruskal Wallis with Dunns post hoc test (KO mice: n =8 mice/group; WT: n = 32).
Figure S2 Freezing levels to the CS− during fear acquisition. Accelerated acquisition of CS− induced % of freezing in (A) NPY KO, (B) Y1 receptor KO and (D) Y1/Y2 receptor double KO but not in (C) Y2 receptor KO mice. In general CS− induced freezing levels were similar to respective CS+ induced freezing (Figure 2A, C, E and G). Values are means ± SEM, repeated two-way ANOVA with Bonferroni post hoc test, *P <0.05, ***P < 0.001.
Please note: Wiley–Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchi F, Mathe AA, Jimenez P, Stasi L, Arban R, Gerrard P, et al. Anxiolytic-like effect of the selective neuropeptide Y Y2 receptor antagonist BIIE0246 in the elevated plus-maze. Peptides. 2006;27:3202–3207. doi: 10.1016/j.peptides.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Bannon AW, Seda J, Carmouche M, Francis JM, Norman MH, Karbon B, et al. Behavioral characterization of neuropeptide Y knockout mice. Brain Res. 2000;868:79–87. doi: 10.1016/s0006-8993(00)02285-x. [DOI] [PubMed] [Google Scholar]
- Biedenkapp JC, Rudy JW. Context preexposure prevents forgetting of a contextual fear memory: implication for regional changes in brain activation patterns associated with recent and remote memory tests. Learn Mem. 2007;14:200–203. doi: 10.1101/lm.499407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broqua P, Wettstein JG, Rocher MN, Gauthier-Martin B, Junien JL. Behavioral effects of neuropeptide Y receptor agonists in the elevated plus-maze and fear-potentiated startle procedures. Behav Pharmacol. 1995;6:215–222. [PubMed] [Google Scholar]
- Colmers WF, Klapstein GJ, Fournier AS, Pierre S, Treherne KA. Presynaptic inhibition by neuropeptide Y in rat hippocampal slice in vitro is mediated by a Y2 receptor. Br J Pharmacol. 1991;102:41–44. doi: 10.1111/j.1476-5381.1991.tb12129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Council NR. Guide for the Care and Use of Laboratory Animals. 8th edition. Washington, DC: The National Academies Press; 2010. p. 248. [Google Scholar]
- Dumont Y, Fournier AS, Pierre S, Quirion R. Comparative characterization and autoradiographic distribution of neuropeptide Y receptor subtypes in the rat brain. J Neurosci. 1993;13:73–86. doi: 10.1523/JNEUROSCI.13-01-00073.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Bahh B, Balosso S, Hamilton T, Herzog H, Beck-Sickinger AG, Sperk G, et al. The anti-epileptic actions of neuropeptide Y in the hippocampus are mediated by Y and not Y receptors. Eur J Neurosci. 2005;22:1417–1430. doi: 10.1111/j.1460-9568.2005.04338.x. [DOI] [PubMed] [Google Scholar]
- Fendt M, Burki H, Imobersteg S, Lingenhohl K, McAllister KH, Orain D, et al. Fear-reducing effects of intra-amygdala neuropeptide Y infusion in animal models of conditioned fear: an NPY Y1 receptor independent effect. Psychopharmacology (Berl) 2009;206:291–301. doi: 10.1007/s00213-009-1610-8. [DOI] [PubMed] [Google Scholar]
- Giesbrecht CJ, Mackay JP, Silveira HB, Urban JH, Colmers WF. Countervailing modulation of Ih by neuropeptide Y and corticotrophin-releasing factor in basolateral amygdala as a possible mechanism for their effects on stress-related behaviours. J Neurosci. 2010;30:16970–16982. doi: 10.1523/JNEUROSCI.2306-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi M, Gariboldi M, Piwko C, Hoyer D, Sperk G, Vezzani A. Distinct changes in peptide YY binding to, and mRNA levels of, Y1 and Y2 receptors in the rat hippocampus associated with kindling epileptogenesis. J Neurochem. 1998;70:1615–1622. doi: 10.1046/j.1471-4159.1998.70041615.x. [DOI] [PubMed] [Google Scholar]
- Gustafson EL, Card JP, Moore RY. Neuropeptide Y localization in the rat amygdaloid complex. J Comp Neurol. 1986;251:349–362. doi: 10.1002/cne.902510306. [DOI] [PubMed] [Google Scholar]
- Gutman AR, Yang Y, Ressler KJ, Davis M. The role of neuropeptide Y in the expression and extinction of fear-potentiated startle. J Neurosci. 2008;28:12682–12690. doi: 10.1523/JNEUROSCI.2305-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M. Antisense inhibition of neuropeptide Y (NPY)-Y1 receptor expression blocks the anxiolytic-like action of NPY in amygdala and paradoxically increases feeding. Regul Pept. 1995;59:201–205. doi: 10.1016/0167-0115(95)00103-i. [DOI] [PubMed] [Google Scholar]
- Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Brot M, Heinrichs SC, Menzaghi F, Koob GF, et al. Anxiolytic-like action of neuropeptide Y: mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacology. 1993;8:357–363. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM, Davis M. Fear-potentiated startle using an auditory conditioned stimulus: effect of lesions of the amygdala. Physiol Behav. 1987;39:403–408. doi: 10.1016/0031-9384(87)90242-3. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM, Davis M. Efferent pathway of the amygdala involved in conditioned fear as measured with the fear-potentiated startle paradigm. Behav Neurosci. 1991;105:826–842. doi: 10.1037//0735-7044.105.6.826. [DOI] [PubMed] [Google Scholar]
- Karl T, Duffy L, Herzog H. Behavioural profile of a new mouse model for NPY deficiency. Eur J Neurosci. 2008;28:173–180. doi: 10.1111/j.1460-9568.2008.06306.x. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Choe JS, Cameron HA, Thorsell A, Crawley JN, Holmes A, et al. The neuropeptide Y Y1 receptor subtype is necessary for the anxiolytic-like effects of neuropeptide Y, but not the antidepressant-like effects of fluoxetine, in mice. Psychopharmacology (Berl) 2007;195:547–557. doi: 10.1007/s00213-007-0945-2. [DOI] [PubMed] [Google Scholar]
- Kask A, Harro J, von Horsten S, Redrobe JP, Dumont Y, Quirion R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci Biobehav Rev. 2002;26:259–283. doi: 10.1016/s0149-7634(01)00066-5. [DOI] [PubMed] [Google Scholar]
- Kopp J, Xu ZQ, Zhang X, Pedrazzini T, Herzog H, Kresse A, et al. Expression of the neuropeptide Y Y1 receptor in the CNS of rat and of wild-type and Y1 receptor knock-out mice. Focus on immunohistochemical localization. Neuroscience. 2002;111:443–532. doi: 10.1016/s0306-4522(01)00463-8. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioural correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Pearson JC. Coexistence of GABA and peptide immunoreactivity in non-pyramidal neurons of the basolateral amygdala. Neurosci Lett. 1989;100:53–58. doi: 10.1016/0304-3940(89)90659-9. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Inui A, Asakawa A, Momose K, Ueno N, Teranishi A, et al. Neuropeptide Y produces anxiety via Y2-type receptors. Peptides. 1998;19:359–363. doi: 10.1016/s0196-9781(97)00298-2. [DOI] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redrobe JP, Dumont Y, Herzog H, Quirion R. Neuropeptide Y (NPY) Y2 receptors mediate behaviour in two animal models of anxiety: evidence from Y2 receptor knockout mice. Behav Brain Res. 2003;141:251–255. doi: 10.1016/s0166-4328(02)00374-1. [DOI] [PubMed] [Google Scholar]
- Sainsbury A, Schwarzer C, Couzens M, Fetissov S, Furtinger S, Jenkins A, et al. Important role of hypothalamic Y2 receptors in body weight regulation revealed in conditional knockout mice. Proc Natl Acad Sci U S A. 2002a;99:8938–8943. doi: 10.1073/pnas.132043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainsbury A, Schwarzer C, Couzens M, Jenkins A, Oakes SR, Ormandy CJ, et al. Y4 receptor knockout rescues fertility in ob/ob mice. Genes Dev. 2002b;16:1077–1088. doi: 10.1101/gad.979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Smiley DL, Gehlert DR. Neuropeptide Y-Y2 receptors mediate anxiety in the amygdala. Pharmacol Biochem Behav. 2002;71:419–423. doi: 10.1016/s0091-3057(01)00679-7. [DOI] [PubMed] [Google Scholar]
- Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen AT, Kanter-Schlifke I, Carli M, Balducci C, Noe F, During MJ, et al. NPY gene transfer in the hippocampus attenuates synaptic plasticity and learning. Hippocampus. 2008;18:564–574. doi: 10.1002/hipo.20415. [DOI] [PubMed] [Google Scholar]
- Sorensen AT, Nikitidou L, Ledri M, Lin EJ, During MJ, Kanter-Schlifke I, et al. Hippocampal NPY gene transfer attenuates seizures without affecting epilepsy-induced impairment of LTP. Exp Neurol. 2009;215:328–333. doi: 10.1016/j.expneurol.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanic D, Brumovsky P, Fetissov S, Shuster S, Herzog H, Hokfelt T. Characterization of neuropeptide Y2 receptor protein expression in the mouse brain. I. Distribution in cell bodies and nerve terminals. J Comp Neurol. 2006;499:357–390. doi: 10.1002/cne.21046. [DOI] [PubMed] [Google Scholar]
- Stanic D, Mulder J, Watanabe M, Hokfelt T. Characterization of NPY Y2 receptor protein expression in the mouse brain. II. Coexistence with NPY, the Y1 receptor, and other neurotransmitter-related molecules. J Comp Neurol. 2011;519:1219–1257. doi: 10.1002/cne.22608. [DOI] [PubMed] [Google Scholar]
- Tasan RO, Lin S, Hetzenauer A, Singewald N, Herzog H, Sperk G. Increased novelty-induced motor activity and reduced depression-like behavior in neuropeptide Y (NPY)-Y4 receptor knockout mice. Neuroscience. 2009;158:1717–1730. doi: 10.1016/j.neuroscience.2008.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasan RO, Nguyen NK, Weger S, Sartori SB, Singewald N, Heilbronn R, et al. The central and basolateral amygdala are critical sites of neuropeptide Y/Y2 receptor-mediated regulation of anxiety and depression. J Neurosci. 2010;30:6282–6290. doi: 10.1523/JNEUROSCI.0430-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y – a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- Tschenett A, Singewald N, Carli M, Balducci C, Salchner P, Vezzani A, et al. Reduced anxiety and improved stress coping ability in mice lacking NPY-Y2 receptors. Eur J Neurosci. 2003;18:143–148. doi: 10.1046/j.1460-9568.2003.02725.x. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Wang SH, Teixeira CM, Wheeler AL, Frankland PW. The precision of remote context memories does not require the hippocampus. Nat Neurosci. 2009;12:253–255. doi: 10.1038/nn.2263. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Silva AJ. Memory for context becomes less specific with time. Learn Mem. 2007;14:313–317. doi: 10.1101/lm.430907. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Jacobi F. Size and burden of mental disorders in Europe – a critical review and appraisal of 27 studies. Eur Neuropsychopharmacol. 2005;15:357–376. doi: 10.1016/j.euroneuro.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jonsson B, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:655–679. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Wolak ML, DeJoseph MR, Cator AD, Mokashi AS, Brownfield MS, Urban JH. Comparative distribution of neuropeptide Y Y1 and Y5 receptors in the rat brain by using immunohistochemistry. J Comp Neurol. 2003;464:285–311. doi: 10.1002/cne.10823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


