Abstract
The 5' region of BRCA1 contains multiple regulatory sequences flanking the two alternative promoters α and β and two alternative, non-coding exons, 1a and 1b. Aberrations within the 5' region BRCA1 (encompassing two alternative promoters α and β and exons 1a and 1b) may be associated with an increased risk of breast and ovarian cancer. In this study we screened 150 patients for polymorphism and mutations in this region of BRCA1. All probands came from familial breast and/or ovarian cancer that had been found to be mutation-negative in a previous search for founder mutations in BRCA1 (185delAG, C61G, 4153delA, 5382insC) or BRCA2 (6174delT, 9631delC). In our study we found several sequence alterations within the non-coding region of BRCA1 by using direct DNA sequencing and allele-specific PCR amplification. Three families with a polymorphic deletion in BRCA1 exon 1b (2223delAAAAA, Acc. U37574) were found. Moreover, two linked nucleotide substitutions (2642A>T, 2743T>C, Acc. U37574) in BRCA1 intron 1 were detected in 16 patients. In order to assess the functional significance of these two sequence variants, we constructed a reporter vector encoding firefly luciferase under the transcriptional and translational control of wild type and altered BRCA1 promoter region. The reporter assay was performed using a lung cancer cell line (NCI-H1299) and a breast cancer cell line (MCF7). We have demonstrated that the analysed sequence variants have no functional significance in our experimental system. However, we have found that the BRCA1 promoter has lower relative activity in the breast cancer cell line compared with the lung cancer cell line. Based on the results of our functional experiments we conclude that the polymorphic deletion 2223delAAAAA and two linked substitutions 2642A>T and 2743T>C do not significantly alter BRCA1 expression and are probably not disease-causing mutations.
Keywords: BRCA1 promoter, polymorphism, reporter assay, breast cancer
Introduction
The human BRCA1 gene is under the transcriptional control of two different promoters, α and β that drive the transcription of exon 1a and 1b, respectively [22]. At the RNA level each of the alternative first exons is linked by splicing with exon 2 [21]. However, the translational initiation site is the same for the two mRNA variants and is located in exon 2 [11]. The BRCA1 5'UTR region coded by exon 1b contains three additional ATG codons upstream of the major translation initiation site [21]. The promoter α is bidirectional and shared with the NBR2 gene [4,21]. BRCA1 contains multiple transcription factor binding sites identified in 5' flanking regions of exon 1a and exon 1b [17,18]. The different transcripts of the BRCA1 gene are present at different levels in various normal and tumour tissues and may have distinct biological functions [21]. Expression of transcripts α and β of the BRCA1 gene may be co-regulated by use of a dual promoter system. Moreover, the two mRNAs may differ in their stability or translational efficiency [21].
Germline mutations within the BRCA1 gene are responsible for familial cancer and reduced expression of the BRCA1 gene is frequently observed in sporadic breast [12,16,20] and ovarian tumours [23]. Various mechanisms such as methylation of the CpG islands within the promoter region [2,6,10,13], allelic deletion of the BRCA1 locus and sequence alterations identified outside the BRCA1 coding region, especially within the positive regulatory region (PRR) of the BRCA1 promoter, can modulate the level of BRCA1 expression [17,19]. There are also other mechanisms responsible for breast and ovarian cancer pathogenesis [5,15]. Expression patterns of BRCA1 mRNAs and differences in their translatability [14] and disruption of the DNA-protein complexes [18] may also contribute to breast/ovarian cancer susceptibility.
Our aim was to investigate the functional effect of sequence alterations within the BRCA1 promoter/5'UTR region using luciferase reporter gene assay.
Materials and methods
Patients
One hundred and fifty patients from families resident in Upper Silesia, Poland, were screened for deletions in the BRCA1 promoter/5'UTR region using genomic DNA extracted from peripheral blood lymphocytes using the phenol-chloroform method [7]. Each patient was selected after clinical genetic counselling in which they completed a detailed questionnaire, including family history, after signing an informed consent document. Each person selected for the study was diagnosed with breast and/or ovarian cancer and had a positive family history of breast and/or ovarian cancer.
Screening for new sequence variants within BRCA1 promoter/5'UTR
Eighty-seven patients diagnosed with breast and/or ovarian cancer were selected for BRCA1 promoter/5'UTR (GenBank accession no. U37574) screening by direct DNA sequencing. All patients were mutation-negative for founder mutations in the BRCA1 gene (185delAG, 300T/G, 4153delA, 5382insC) and in the BRCA2 gene (6174delT, 9631delC) using ASA-PCR and RFLP PCRs analyses [8,9].
PCR amplification was performed using the following primers (forward/reverse, 5'→3'): fragment 1 G A C G C T T G G C T C T T T C T G T/TCTGGATCCTCCTCAAGCAC, fragment 2 G A G T G G A T T T C C G A A G C T G A/TCTGGACCTCCTCAAGCAC, fragment 3 G A T G G G A C C T T G T G G A A G A A/CGCGAAGAGCAGATAAATCC. All reactions were performed in 15 μl containing 1 μl 50-150 ng DNA, 1×PCR buffer II (50 mM KCl, 10 mM Tris-HCl pH 8.3), 1.5 mM MgCl, 50 pmol each primer and 1 U of AmpliTaq DNA Polymerase (Applied Biosystem). The PCR cycling conditions were: fragment 1, 94°C for 30 s, 64°C for 30 s, 72°C for 30 s; fragment 2, 94°C for 30 s, 59°C for 30 s, 72°C for 30 s; fragment 3, 94°C for 30 s, 58°C for 30 s, 72°C for 30 s. An initial denaturation for 5 min. at 95°C and a final extension at 72°C for 7 min. were also included in each PCR reaction. All reactions were carried out for 35 cycles on a GeneAmp™ PCR System 2400 (Applied Biosystem) thermal cycler. PCR products were separated in a 2% agarose gel, 1×TBE, stained with ethidium bromide and analysed using Scion Image Beta 4.02 Win Software (Scion Corporation, USA). Amplified products were purified before sequencing with exonuclease I and shrimp alkaline phosphatase according to the manufacturer's protocol (Amersham Life Science). Both strands were sequenced using the following primers 5'→3': fragment 1 (forward) GACGCTTGGCTCTTTCTGTC, fragment 2 (forward) GTAAGGCGTTGTGAACCCTG and fragment 3 (forward) GGAGACAGGATTTTGTGGGA, fragment 1 (reverse) CAGCCTCCTGAGTAGCTGGA fragment 2 (reverse) TCTGGATCCTCCTCAAGCAC fragment 3 (reverse) CGCGAAGAGCAGATAAATCC. Samples were sequenced with BigDye Terminator Cycle Sequencing Kit v3.1 (Applied Biosystem) and analysed with the ABI Prism 377 DNA automated sequencer (Applied Biosystem).
Allele-specific PCR amplification was used to estimate the frequency of the polymorphic deletion 2223delAAAAA in an additional sixty-three patients of breast/ovarian cancer patients fulfilling the above criteria. The following primers were used: (5'→3')TTTAAAAACGTCGGCTGGTC (forward), TCCCACAAAATCCTGTCTCC (reverse) and CAGCCGGTGTGTTTTGTTTT (reverse) with 'touch-down' PCR: denaturation at 95°C for 5 min. followed by 10 cycles of 94°C 20 s, 65°C 25 s (at this point temperature decreased 0.7°C per cycle), 72°C 30 s and followed by 30 cycles at 94°C 20 s, 58°C 25 s, 72°C 30 s ending with a final extension of 72°C for 7 min. and a quick chill to 4°C. PCR reactions were performed in 15 μl reactions containing 1 μl 50-150 ng DNA, 1×PCR buffer II (50 mM KCl, 10 mM Tris-HCl pH 8.3), 1.5 mM MgCl, 50 pmol each primer and 1 U of AmpliTaq DNA Polymerase (Applied Biosystem). The PCR product was amplified using a Mastercycler ep® gradient S (Eppendorf) thermal cycler. The amplified products were separated in 2% agarose gel. The expected size of the PCR fragment containing a polymorphic deletion within exon 1b was 261 bp.
Functional analyses of region BRCA1 promoter/5'UTR
Plasmids
The 1.5 kb DNA region, encompassing minimal BRCA1 promoter (promoter α), alternative promoter (β), exons 1a, 1b and intron 1, in wild type and in polymorphic forms, was amplified using the primers 5'→3': TTTTGCTAGCCTTTATGGCAAACTCAGGTAG and TTTTAAGCTTTCTGTTCCAATGAACTTTAAC. The downstream and upstream primers were designed to include NheI and HindIII sites, respectively (underlined). The amplified products were digested with NheI and HindIII and ligated into pGL3-Basic reporter vector (Promega) digested with NheI and HindIII. The pGL3-BRCA1-prom plasmids contain the firefly luciferase gene (luc) under the transcriptional and translational control of either the wild type or polymorphic BRCA1 promoter/5'UTR region (Figure 2). The identity of BRCA1 promoter constructs was confirmed by sequencing. The inserts' nucleotide sequences were compared with the sequence U37574 from GeneBank.
Figure 2.
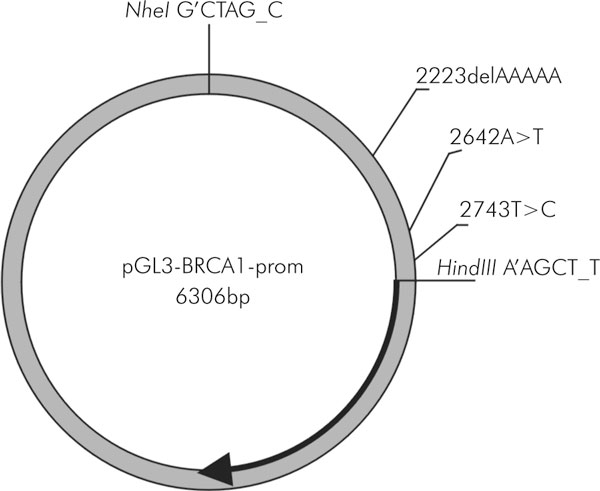
The pGL3 - BRCA1 - prom plasmid map. The localization of sequence alterations and the recognition sequences of the restriction enzymes used for the cloning are shown. The thick arrow represents the coding region of the firefly luciferase gene (luc)
Cell culture, transfection and luciferase assay
Functional analysis of the BRCA1 promoter/5'UTR region was carried out in human non-small cell lung cancer cell line NCI-H1299 and breast cancer cell line MCF7 (obtained from ATCC). Cells were cultured in DMEM or RPMI-1640 medium (respectively) supplemented with 10% foetal calf serum and kept at 37°C and 5% CO2. Cell cultures were plated in 12-well culture dishes at 24 hours prior to transfection. At 40% confluence, lung cancer NCI-H1299 and breast cancer MCF7 cells were transfected with 0.5 μg DNA of wild type or polymorphic reporter vectors using FuGene6 (Roche) transfection reagent according to the manufacturer's instructions. pRL-TK reporter vector coding for Renilla luciferase (Promega) was co-transfected with pGL3-BRCA1-prom plasmids and served as an internal control. The cells were incubated with FuGene6-DNA for 24 hours. Subsequently the cells were washed in phosphatate buffered saline, lysed in 250 μl 1× Passive Lysis Buffer and 20 μl of the cell lysates were assayed using the Dual-Luciferase Reporter Assay System (Promega). Firefly luciferase activity was divided by Renilla luciferase activity, which yielded normalized firefly luciferase activity. This normalization helps to minimize the confounding influence of differences in cell number, transfection efficiency, etc. Each pGL3-BRCA1-prom plasmid version was transfected to the three wells of the 12-well plate. The mean and standard deviation from three luciferase measurements were calculated and the statistical significance of the differences were estimated using the t-test.
Results and discussion
One hundred and fifty patients with breast and/or ovarian cancer from Upper Silesia in Poland were screened for sequence alterations in the 5' region of BRCA1.
The polymorphic deletion 2223delAAAAA (according to the GeneBank Acc. U37574) within BRCA1 exon 1b was detected in three families (2%). Two linked nucleotide sequence alterations within the BRCA1 intron 1 2642A>T, 2743T>C (Acc. U37574) were also detected in 16 patients (18.4%) (Figure 1).
Figure 1.
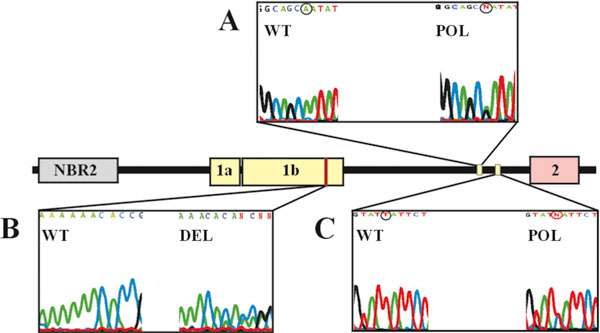
Schematic representation of the 5' region of the BRCA1 gene and location of sequence alterations found in this gene fragment -1a and 1b represent two alternative first exons, 2 represents the exon 2 with the translation start site.
The functional impact of the most frequent sequence variants within the 5' region of BRCA1 was analysed by the Dual-Luciferase Reporter Assay System (Promega) in the lung cancer cell line (NCI-H1299) and breast cancer cell line (MCF-7). Normalized firefly luciferase activity was significantly lower in the MCF7 cell line compared with the NCI-H1299 cell line (Figure 3).
Figure 3.
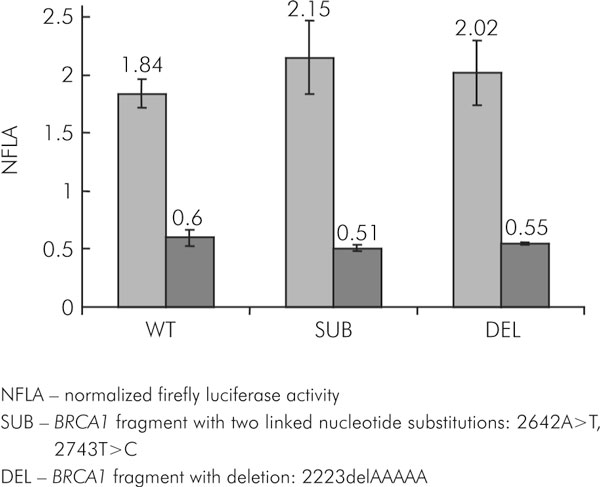
The normalized activity of the reporter gene (luc) under transcriptional and translational control of the wild-type and polymorphic forms BRCA1 promoter/5'UTR region in NCI-H1299 (grey) and MCF7 (dark grey) cell lines
All tested variants of the BRCA1 promoter/5'UTR induced expression of the reporter gene at levels very similar to the wild-type sequence in NCI-H1299 cell line (2.15 ± 0.32 and 2.02 ± 0.27 versus 1.84 ± 0.123; P = 0.19, t-test; Figure 3). The polymorphic sequences slightly reduced expression of reporter luc gene compared with wild-type BRCA1 promoter/5'UTR in MCF7 cell line (0.51 ± 0.024 and 0.55 ± 0.012 versus 0.6 ± 0.07); however, the differences were not significant (P = 0.089, P = 0.263, t-test, respectively; Figure 3). The evidence from this study suggests that the first polymorphism under investigation could only slightly modulate the levels of expression of the BRCA1 gene while the second polymorphism (deletion of 5 nucleotides) did not demonstrate any change in functional activity in the experimental system used in this study.
The 5' region of BRCA1 contains multiple functional domains which may regulate translation, transcription and alternative splicing of BRCA1 [21,22]. In this study we analysed the BRCA1 5' region and detected several sequence alterations. We cloned the region of interest of the BRCA1 gene into the reporter plasmid in order to find out if the sequence alterations modulate the expression of the reporter gene. The deletion of five adenines is a major sequence change. It is located in exon 1b, which is an alternative exon present in the mRNA mostly in cancer cells [21,14]. It may change the secondary structure of the 5'UTR of the alternative mRNA, influencing translation efficiency. However, we did not detect its influence on the reporter gene in either of the two cancer cell lines that we used for the assay. The two linked substitutions are located at the 3' end of intron 1. In principle, they may modulate the efficiency or accuracy of RNA splicing. However, in the case of these linked sequence changes, we observed no influence on reporter gene expression. Therefore, we conclude that the sequence alterations have no major functional impact on BRCA1 expression. However, we cannot rule out the possibility that they change regulation of BRCA1 expression in normal cells or when present in their proper chromatin environment. Interestingly, we noticed that the activity of the reporter gene controlled by BRCA1 promoter is significantly lower in the MCF7 breast cancer cell line compared with the lung cancer cell line (Fig. 3). Normalized firefly luciferase activity can be regarded as a ratio of experimental promoter activity (BRCA1 in this case) to the control promoter activity (thymidine kinase gene promoter from herpes simplex virus). This ratio is significantly lower in MCF7 cells. This is consistent with the observation of decreased BRCA1 protein level in breast cancer cell lines and primary breast carcinomas associated with the increased expression of negative regulators of BRCA1 promoter [1]. Methylation of the BRCA1 promoter region also decreases the expression level of the protein [3], but this mechanisms is found in a small number of BRCA1 negative breast cancer specimens [1].
NFLA - normalized firefly luciferase activity
SUB - BRCA1 fragment with two linked nucleotide substitutions: 2642A>T, 2743T>C
DEL - BRCA1 fragment with deletion: 2223delAAAAA
Acknowledgements
We thank Iwona Matuszczyk for excellent technical assistance.
Grant sponsor: State Committee for Scientific Research (KBN, Poland); Grant number 6P05A 14221 (E. Grzybowska)
References
- Baldassarre G, Battista S, Belletti B, Thakur S, Pentimalli F, Trapasso F, Fedele M, Pierantoni G, Croce CM, Fusco A. Negative regulation of BRCA1 gene expression by HMGA1 proteins accounts for the reduced BRCA1 protein levels in sporadic breast carcinoma. Mol Cell Biol. 2003;23:2225–2238. doi: 10.1128/MCB.23.7.2225-2238.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin RL, Nemeth E, Tran H, Shvartsman H, Cass I, Narod S, Karlan BY. BRCA1 promoter region hypermethylation in ovarian carcinoma: a population-based study. Cancer Res. 2000;60:5329–5333. [PubMed] [Google Scholar]
- Bianco T, Chenevix-Trench G, Walsh DC, Cooper JE, Dobrovic A. Tumour-specific distribution of BRCA1 promoter region methylation supports a pathogenetic role in breast and ovarian cancer. Carcinogenesis. 2000;21:147–151. doi: 10.1093/carcin/21.2.147. [DOI] [PubMed] [Google Scholar]
- Brown MA, Xu CF, Nicolai H, Griffiths B, Chambers JA, Black D, Solomon E. The 5' end of the BRCA1 gene lies within a duplicated region of human chromosome 17q21. Oncogene. 1996;12:2507–2513. [PubMed] [Google Scholar]
- Brown MA, Lo LJ, Catteau A, Xu CF, Lindeman GJ, Hodgson S, Solomon E. Germline BRCA1 promoter deletions in UK and Australian familial breast cancer patients: Identification of a novel deletion consistent with BRCA1:psiBRCA1 recombination. Hum Mutat. 2002;19:435–442. doi: 10.1002/humu.10055. [DOI] [PubMed] [Google Scholar]
- Dobrovic A, Simpfendorfer D. Methylation of the BRCA1 gene in sporadic breast cancer. Cancer Res. 1997;57:3347–3350. [PubMed] [Google Scholar]
- Grzybowska E, Hemminki K, Szeliga J, Chorazy M. Seasonal variation of aromatic DNA adducts in human lymphocytes and granulocytes. Carcinogenesis. 1993;14:2523–2526. doi: 10.1093/carcin/14.12.2523. [DOI] [PubMed] [Google Scholar]
- Grzybowska E, Zientek H, Jasinska A, Rusin M, Kozlowski P, Sobczak K, Sikorska A, Kwiatkowska E, Gorniak L, Kalinowska E, Utracka-Hutka B, Wloch J, Chmielik E, Krzyzosiak WJ. High frequency of recurrent mutations in BRCA1 and BRCA2 genes in Polish families with breast and ovarian cancer. Hum Mutat. 2000;16:482–490. doi: 10.1002/1098-1004(200012)16:6<482::AID-HUMU5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Grzybowska E, Sieminska M, Zientek H, Kalinowska E, Michalska J, Utracka-Hutka B, Rogozinska-Szczepka J, Kazmierczak-Maciejewska M. Germline mutations in the BRCA1 gene predisposing to breast and ovarian cancers in Upper Silesia population. Acta Biochem Pol. 2002;49:351–356. [PubMed] [Google Scholar]
- Mancini DN, Rodenhiser DI, Ainsworth PJ, O'Malley FP, Singh SM, Xing W, Archer TK. CpG methylation within the 5' regulatory region of the BRCA1 gene is tumor specific and includes a putative CREB binding site. Oncogene. 1998;16:1161–1169. doi: 10.1038/sj.onc.1201630. [DOI] [PubMed] [Google Scholar]
- Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- Ozcelik H, To MD, Couture J, Bull SB, Andrulis IL. Preferential allelic expression can lead to reduced expression of BRCA1 in sporadic breast cancers. Int J Cancer. 1998;77:1–6. doi: 10.1002/(SICI)1097-0215(19980703)77:1<1::AID-IJC1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Rice JC, Futscher BW. Transcriptional repression of BRCA1 by aberrant cytosine methylation, histone hypoacetylation and chromatin condensation of the BRCA1 promoter. Nucleic Acids Res. 2000;28:3233–3239. doi: 10.1093/nar/28.17.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobczak K, Krzyzosiak WJ. Structural determinants of BRCA1 translational regulation. J Biol Chem. 2002;277:17349–17358. doi: 10.1074/jbc.M109162200. [DOI] [PubMed] [Google Scholar]
- Signori E, Bagni C, Papa S, Primerano B, Rinaldi M, Amaldi F, Fazio VM. A somatic mutation in the 5'UTR of BRCA1 gene in sporadic breast cancer causes down-modulation of translation efficiency. Oncogene. 2001;20:4596–4600. doi: 10.1038/sj.onc.1204620. [DOI] [PubMed] [Google Scholar]
- Sourvinos G, Spandidos DA. Decreased BRCA1 expression levels may arrest the cell cycle through activation of p53 checkpoint in human sporadic breast tumors. Biochem Biophys Res Commun. 1998;245:75–80. doi: 10.1006/bbrc.1998.8379. [DOI] [PubMed] [Google Scholar]
- Suen TC, Goss PE. Transcription of BRCA1 is dependent on the formation of a specific protein-DNA complex on the minimal BRCA1 Bi-directional promoter. J Biol Chem. 1999;274:31297–31304. doi: 10.1074/jbc.274.44.31297. [DOI] [PubMed] [Google Scholar]
- Thakur S, Croce CM. Positive regulation of the BRCA1 promoter. J Biol Chem. 1999;274:8837–8843. doi: 10.1074/jbc.274.13.8837. [DOI] [PubMed] [Google Scholar]
- Thakur S, Nakamura T, Calin G, Russo A, Tamburrino JF, Shimizu M, Baldassarre G, Battista S, Fusco A, Wassell RP, Dubois G, Alder H, Croce CM. Regulation of BRCA1 transcription by specific single-stranded DNA binding factors. Mol Cell Biol. 2003;23:3774–3787. doi: 10.1128/MCB.23.11.3774-3787.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson ME, Jensen RA, Obermiller PS, Page DL, Holt JT. Decreased expression of BRCA1 accelerates growth and is often present during sporadic breast cancer progression. Nat Genet. 1995;9:444–450. doi: 10.1038/ng0495-444. [DOI] [PubMed] [Google Scholar]
- Xu CF, Brown MA, Chambers JA, Griffiths B, Nicolai H, Solomon E. Distinct transcription start sites generate two forms of BRCA1 mRNA. Hum Mol Genet. 1995;4:2259–2264. doi: 10.1093/hmg/4.12.2259. [DOI] [PubMed] [Google Scholar]
- Xu CF, Chambers JA, Solomon E. Complex regulation of the BRCA1 gene. J Biol Chem. 1997;272:20994–20997. doi: 10.1074/jbc.272.34.20994. [DOI] [PubMed] [Google Scholar]
- Zheng W, Luo F, Lu JJ, Baltayan A, Press MF, Zhang ZF, Pike MC. Reduction of BRCA1 expression in sporadic ovarian cancer. Gynecol Oncol. 2000;76:294–300. doi: 10.1006/gyno.1999.5664. [DOI] [PubMed] [Google Scholar]


