Abstract
Objective
To provide an overview of impulse oscillometry and its application to the evaluation of children with diseases of the airways.
Data Sources
Medline and PubMed search, limited to English language and human disease, with keywords forced oscillation, impulse oscillometry, and asthma.
Study Selections
The opinions of the authors were used to select studies for inclusion in this review.
Results
Impulse oscillometry is a noninvasive and rapid technique requiring only passive cooperation by the patient. Pressure oscillations are applied at the mouth to measure pulmonary resistance and reactance. It is employed by health care professionals to help diagnose pediatric pulmonary diseases such asthma and cystic fibrosis; assess therapeutic responses; and measure airway resistance during provocation testing.
Conclusions
Impulse oscillometry provides a rapid, noninvasive measure of airway impedance. It may be easily employed in the diagnosis and management of diseases of the airways in children.
INTRODUCTION
Asthma is reported to be the most common chronic respiratory disease in children, and its prevalence appears to be high worldwide.1 Accordingly, significant efforts have been made to develop noninvasive techniques that accurately measure lung function in children. Examples of such techniques include spirometry, plethysmography, interrupter technique (discussed later), tidal breathing measurements, multiple-breath inert gas washout technique, forced oscillation technique (FOT) and impulse oscillometry (IOS).2– 4 The forced oscillation technique is the general name for airway mechanic measurements using the noninvasive superimposition of pressure fluctuations on the airway over the subject’s normal, quiet, tidal breathing. More than 50 years ago, FOT was first determined by Dubois et al5 and has developed with regard to configuration, standardization, and application. Impulse oscillometry is one type of FOT. Other techniques of FOT use only one frequency or change the frequency “pseudo randomly.” Impulse oscillometry delivers a regular square wave of pressure 5 times per second, which has the advantage of generating a larger sample during measurements and emitting a continuous spectrum of frequencies that may provide more detailed characterization of respiratory function.6 Impulse oscillometry has been used in adults as well as in preschool children to identify lung dysfunction, such as in asthma.3,4 It is noninvasive, easy to perform, and requires only passive patient cooperation.3 IOS systems are finding increased use in the clinical diagnostic testing of patients with airway hyper-reactivity and airway obstruction. Impulse oscillometry also may be used during challenge and provocation testing. The IOS apparatus generates small pressure oscillations that are applied at the mouth and transmitted into the lungs, to help determine the impedance (Zrs) of the respiratory system.7 Pulmonary resistance and reactance are the key components of impedance8 and are measured and graphically displayed.
The purpose of this article is to provide a comprehensive overview of the application of IOS to the evaluation of respiratory disease in children. A complete search in Medline and PubMed was performed for articles on impulse oscillometry, in peer-reviewed journals, using the key words forced oscillation, impulse oscillometry, and asthma. The articles included in this study were based on the expert opinions of the authors.
MEASUREMENT
Measurement Parameters
The IOS apparatus generates pressure oscillations at the mouth that propagate via movement of the air column in the conducting airways, which is followed by distension and recoil of the elastic components of lung tissues and creation of backpressure. The pressure oscillations are applied at a fixed (square wave) frequency of 5 Hz, from which all other frequencies of interest are derived. Low-frequency signals (5 Hz) penetrate out to the lung periphery, whereas high-frequency signals (20 Hz) only reach the proximal airways. This is because of the physical properties of the size, shape, and tissue composition of the human chest. This concept is analogous to how loud music will cause a listener’s entire body to rumble with low frequency (bass) sounds, but not with higher-frequency tones. Airway resistance, especially at lower frequencies, is inversely correlated to age; younger children generally have higher airway resistance than older children and adults.9
Impulse oscillometry measures pulmonary impedance (Zrs) (Table 1), which comprises pulmonary resistance (energy required to propagate the pressure wave through the airways) and reactance (amount of recoil generated against that pressure wave).8 Resistance (R) is a well-recognized term, and is a measure of central and peripheral airway caliber; which includes the resistance of the oropharynx and larynx, the trachea, the large and small airways, the lung, and chest wall tissue.10 Impulse oscillometry measures the resistance (R) of the airways and lungs to differing frequencies, and is calculated from pressure and flow signals where pressure is in phase with flow (when the pressure wave enters the lungs unopposed by any recoil force). Reactance (X) is a complex concept and incorporates forces of inertia, which result from the movement of the air column in the conducting airways, and the elastic recoil properties of the lung tissue. It has been correlated at low frequencies with peripheral airway obstruction and is computed from pressure out of phase with flow.6 At low frequencies the lung passively distends, there is high compliance, little elastic recoil, and low reactance. As frequency increases, the amount of energy imparted into the pulmonary system increases, and the lungs go from passive distension to active stretch. Analogous to inflating a balloon, a small volume of air initially distends the balloon without generating any recoil. Yet, there is a distinct point where the amount of air inflating the balloon will cause it to stretch, will begin to generate resistance to further inflation, and this potential energy in the material can produce recoil. The frequency at which there is a transition in the lungs from passive distention to active stretch is the point when the inflation pressure and elastic recoil cancel out, resulting in a reactance equal to zero. This point is referred to as the resonant frequency and is dependent on the physical properties of chest size and tissue composition.11 The area of reactance (AX) is the area under the curve of reactance between 5Hz and resonant frequency and reflects a composite index for reactance.
Table 1.
Impulse Oscillometry Terminology
| Impedance (Xrs) | A calculation of the total force needed to propagate a pressure wave through the pulmonary system, comprising resistance and reactance |
| Resistance (R) | Energy required to propagate a pressure wave through the airways; to pass through the bronchi and bronchioles, and to distend the lung parenchyma. Resistance is determined when a pressure wave is unopposed by airway recoil and is in phase with airflow. |
| Reactance (X) | Energy generated by the recoil of the lungs after distention by a pressure wave out of phase with airflow |
| Area of reactance (AX or XA) | Area under the curve between the reactance values for 5Hz and the resonance frequency |
| Coefficient of variability (CV) | Statistical determinant of the trial-to-trial variability serving as an index of reproducibility |
| Coherence | An estimate of the quality of impedance measurements. Provides an index of discrepancy between input and measured signals |
| Compliance | An indicator of the ability of the lung tissue to distend in response to the pressure wave |
| Frequency-independent change | When resistance values do not vary at different frequencies. If overall resistance is increased, this may be indicative of proximal obstruction. |
| Frequency-dependent change | When resistance varies with frequency more than age-dependant normal values. This may be indicative of distal obstruction. |
| Resonance frequency | The frequency at which the lung tissue moves from passive distention to active stretch in response to the force of the pressure wave signal; graphically when reactance is zero. |
Impulse oscillometry measures impedance over a range of frequencies (5–20 Hz). Resistance (R) and reactance (X) when measured at 5 Hz, for example, are designated as R5 and X5, respectively. Lower-frequency oscillations, such as 5 Hz, generally travel farther to the lung periphery and provide indices of the entire pulmonary system. Therefore, when either proximal or distal airway obstruction occurs, R5 and X5 may be increased. Higher-frequency oscillations, such as 20 Hz, transmit signals more proximally and provide information primarily concerning the central airways. Thus, central airways obstruction will be reflected by an increased R20.
By extension therefore, disease isolated to the distal airways will increase R5 to a greater extent than the R20. This is because the increase in the proximal resistance (measured by the R20) makes up only a small percentage of the total change in the system’s resistance (measured by R5). This is referred to as frequency-dependant change and for resistance is routinely measured as a differential change (R5–R20). Conversely, disease isolated to the proximal system will be reflected as an equivalent increase in R5 and R20 and is referred to as frequency-independent change. In general, baseline frequency-dependent changes in resistance are inversely proportional to age; the younger the child, the greater R5–R20.
Coherence is a correlation between airflow and pressure and is considered to reflect the reliability of a given IOS trial. If there is a mismatch between the airflow into the lungs and the amplitude of the reflective pressure wave, coherence will be low. For 30 seconds of testing (which generates 120 samples of data), acceptable coherence values are 0.6 or greater at 5 Hz and 0.8 or greater at 10 Hz3,12; however, a clearly established cutoff of acceptable values has not been established in preschool children.8 Testing inaccuracies and low coherence may result from an inability of the subject to relax, irregular breathing or hyperventilation, a leak around the mouthpiece, inadequate seal with the nose clip, improper bracing of the cheeks, vocalization, swallowing, coughing, temporary airway closure by closing of the glottis, or the tongue obstructing air flow into the mouth. If the duration of measurement is longer, the acceptable coherence value is reduced. The IOS apparatus calculates the coefficient of variability (CV), which is an indicator of trial-to-trial variability and serves as an index of test reproducibility. Studies have determined day-to-day and weekly CV in children to be 16% and 17%, respectively.13 This degree of variability suggests that getting similar repeated measures is not difficult. When a bronchodilator is used to evaluate airway hyperreactivity, the response should be interpreted in light of the pretest and posttest CV to be certain that the bronchodilator response is at least twice the baseline CV.13
In children, normal lung reference values are based on standing height, which appears to be the strongest independent variable.14 Most studies suggest no effect of sex, age, race, ethnicity, and body surface area on the respiratory indices measured by IOS,12,15 but further establishment of reference values is warranted. A bronchodilator response of 20% to 40% in R5 and 15% to 30% in R10 suggestive of reversible obstruction has been reported in children,3,8 whereas a 40% to 50% change in AX has been determined in adults.6,10
Measurement Acquisition
The IOS apparatus consists of a measuring head, a resistor, a pneumotachograph, pressure and flow transducers, and a computer (Fig 1A). The measuring head, connected to one arm of a y-adapter, contains a loudspeaker that generates pressure oscillations. On the lower arm of the y-adapter, a pneumotachograph is connected. The transducer attached to the pneumotachograph measures total pressure and flow, a summation of the pressure and flow of the tidal breathing, and that of the superimposed oscillatory signals. The system is calibrated through several full strokes of a single volume (3 L) of air at different flow rates, which are validated and verified for human measurements with a reference resistance device (2.0 cmH2O/L/sec) supplied by the manufacturer.
Figure 1.
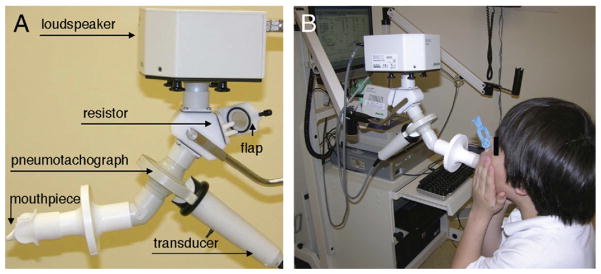
Components of the impulse oscillometry apparatus. A, A pressure signal generated in the loudspeaker reaching both the (terminal) resistor proximally and the pneumotachography more distally is transmitted into the airway through the mouthpiece. Pressure and flow signals from tidal breathing and pressure oscillations pass though the pneumotachograph and are measured by the transducer. B, Eight-year-old boy performing IOS. Patient is using a nose-clip, supporting the cheeks, and making a tight seal with the lips at the mouthpiece of the IOS apparatus. The results of IOS testing are graphically displayed on the computer monitor.
In the use of the device, the child should first be familiarized with the testing procedure; and then informed to breathe normally during the test itself. This helps prevent measurement inaccuracies.16 Measurements are then made by a skilled technician with the child in a relaxed sitting position with an effort toward a stable and constant posture. The head is held in a neutral or slightly extended position while avoiding flexion.13,17 Crossing of the legs should be avoided, because this maneuver contracts the abdominal musculature and may lead to diminished resting end-expiratory pulmonary volumes. During testing, the subject makes a tight seal with the lips around and tongue below an appropriate disposable mouthpiece, which is attached to the open end of the pneumotachograph (Fig 1B). A bacterial filter is placed between the mouthpiece and the pneumotachograph for hygiene purposes.
Measurements of lung function also may be performed on individuals on mechanical ventilation. In such a situation, the endotracheal tube is connected to the pneumotachograph through an adapter, and mathematical adjustments are used to account for the functional differences of the endotracheal tube.18
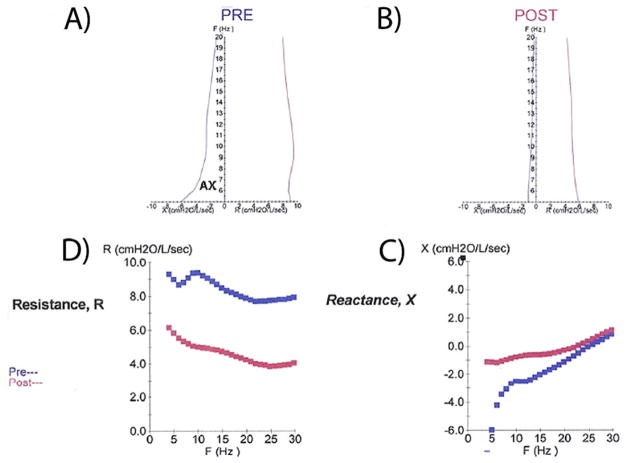
To compensate for the compliance of the cheeks, which expand when pressure oscillations are applied and limit the transmission of air into the lungs, the cheeks must be firmly supported by placing the subject’s or the examiner’s hands directly over them19 (Fig 1B). A nasal clip is placed to occlude the nares to prevent nasal breathing and inhibit the applied pressure oscillations from escaping through the nares. The measurements begin with a short sampling period to determine compliance of the subject, followed by collection of data. Generally, a 30-second interval of testing is performed, which produces a total of 120 impulses from which the mean values of reactance and resistance is calculated at frequencies from 5 to 20 Hz. Assuming the coherence is acceptable based on the aforementioned criteria, and the tracings show uninterrupted breathing, the trial value is saved. If the coherence is low or there is repeated coughing, swallowing, vocalization, or breath holding, the trial is rejected. The average of three to five measurements are obtained, analyzed, and graphically displayed. A representative example is shown in Figure 2. If airway hyperreactivity is being assessed, a bronchodilator is administered, and a similar number of measurements in the same fashion are then taken. Pre and post bronchodilator response for resistance, R, and reactance, X, may be jointly (Fig 2A, B) or separately displayed (Fig 2C, D).
Figure 2.
Representative graphical report of IOS testing. A and B, Composite plot developed by Goldman10 illustrates both resistance, R, and reactance, X, as a function of Freq (Hz) before bronchodilator (A) and postbronchodilator (B). AX is the area under the curve of reactance. C and D, The same curves displayed separately as pre and post R (C) and X (D).
COMPARISON OF LUNG PATTERNS USING IOS AND SPIROMETRY IN PATHOLOGIC CONDITIONS
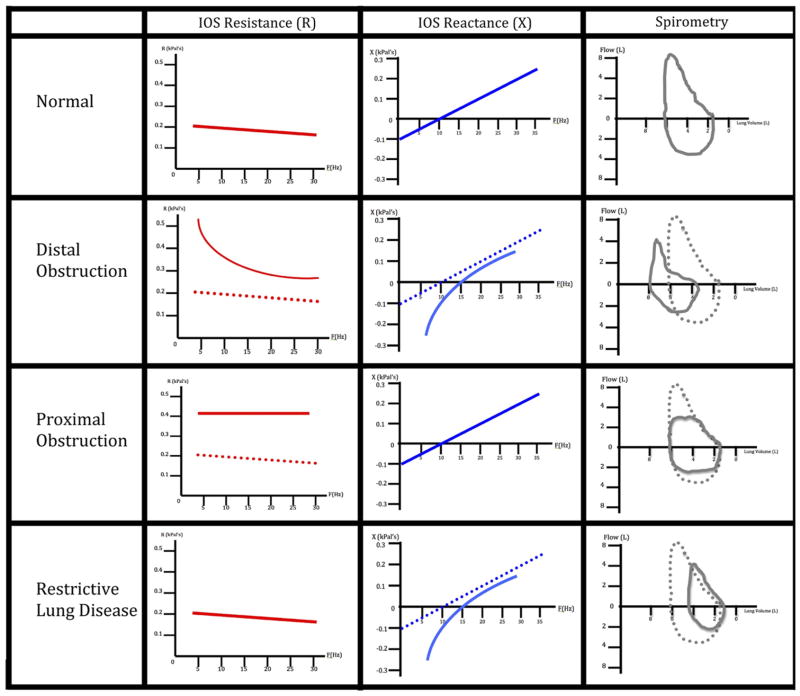
Figure 3 illustrates different patterns of abnormalities that may be identified using IOS in comparison with those patterns seen with spirometry. As mentioned, distal obstructive diseases, such as asthma and chronic bronchitis, result in a frequency-dependant increase in resistance (high R5–R20) because the pressure signal wave propagating out to the lung periphery (R5) encounters greater resistance than the more proximal higher-frequency (R20) impulse. Distal obstruction also results in a decrease in reactance because the signal returning from the lung periphery to the sensor also has to navigate these same narrowed airways. Proximal obstruction leads to frequency-independent elevations in resistance and should have little to no effect on reactance, because the capacity of the lung to recoil and the signal to return to the sensor are relatively unaffected. Restrictive lung diseases will result in a decrease in reactance, because the elastic recoil, and therefore the ability of the lung parenchyma to reflect the signal back, will be decreased. Restrictive processes should not affect resistance because the airway diameter is unchanged.
Figure 3.
Representative graphs of IOS and spirometry in patients with normal, obstructive, and restrictive lung disease. Tracings of lung resistance and reactance in comparison with spirometric flow-volume loop for prototypical patients with normal lung function, distal obstruction, proximal obstruction, and restrictive lung disease. Dotted lines indicate the normal tracing, whereas solid lines show pathological tracings.
CLINICAL APPLICATIONS OF IOS
Impulse oscillometry is used to diagnose, evaluate disease severity, and assess therapeutic responses20 –22 in chronic lung diseases23 such as asthma3 and cystic fibrosis.24 Impulse oscillometry may help distinguish between asthma, chronic bronchitis, and emphysema based on differences in pulmonary resistance, frequency dependence of resistance, and pulmonary reactance.25 It also has been used to determine lung function in individuals with stable asthma and during provocation by methacholine.26 Thus, Pairon et al27 found a significant correlation between forced expiratory volume in 1 second (FEV1) measured by spirometry and the changes in airway resistance, resonant frequency, and frequency dependence of resistance as measured by IOS during methacholine challenge testing. In the emergency room setting, IOS may be used to evaluate lung function and assess response to treatment of acutely ill children with asthma, who may be unable to perform forced expiratory maneuvers.16 Relative to this use, correlations have been shown between FEV1 and forced vital capacity by spirometry and impedance and resistance by IOS in children with hyperactive airways.28 Of practical interest, an established Current Procedural Terminology reimbursement code exists for IOS testing that is completely separate from the code for spirometry. Impulse oscillometry also may be applied in epidemiological settings to screen for asthmatic children29 and to examine bronchial responsiveness to methacholine challenge test in active working adults exposed to occupational respiratory irritants and cigarette smoke.27
The ability to transport the IOS apparatus and measure effort-independent lung function parameters highlights the utility of IOS to assess respiratory dysfunction at the bedside in critically ill patients18 and determine optimal parameters for mechanical ventilation from the patient’s pulmonary resistance and elastance.30 In obstructive sleep apnea syndrome, IOS has been used to evaluate the degree of upper airway obstruction,31 determine the optimal continuous positive airway pressure required to treat the obstruction,32 and estimate the resolution of obstruction subsequent to surgical intervention.33
COMPARISON WITH OTHER TECHNIQUES
In addition to IOS, a number of techniques are routinely used to assess lung dysfunction. These include standard spirometry as mentioned,34 body plethysmography,35 and the interrupter technique,36 as well as tidal breathing measurements and multiple-breath inert gas washout technique. Standard spirometry continues to be the mainstay in the clinical assessment of lung function of school-aged children and adults. The technique is well known and easily performed, and standards of performance and interpretation have been documented.37 However, the effort and skill required to accurately perform spirometry often precludes its utility in preschool children. This may help explain the lack of studies reported in the literature for this age group.38 – 41 Compared with standard spirometry in the preschool population, IOS, which requires only passive cooperation, has been validated by published standards of performance, interpretation, and reference values, and hence is an excellent alternative.13,14 Furthermore, in a study comparing 4-year-old children with a clinical history of asthma with children without asthma, IOS detected significant postbronchodilator responses in areas of resistance and nearly significant in reactance within the asthmatic group. This distinction was not detected using conventional spirometry.3 Follow-up from this study in children with mild to moderate asthma revealed that AX showed continued long-term improvement from daily use of anti-inflammatory treatment with inhaled corticosteroids that was not detected by spirometry.42
Body plethysmography has been successfully employed to determine specific airway resistance in preschool children with asthma,35 assess the efficacy of therapeutic intervention,43,44 determine response to bronchodilators,45 follow bronchial challenge,46 and evaluate lung dysfunction in individuals with cystic fibrosis.47 During measurements, the child is seated inside a sealed cabin and breathes through a pneumotachograph, using a mouthpiece or a facemask and nose clips. Flow in the pulmonary airways and pressure variations in the sealed box are simultaneously determined by a flow and pressure transducer. Similar to IOS, body plethysmography is noninvasive, entails passive cooperation by the subject, and is conducted during spontaneous tidal breathing. However, plethysmography may be difficult for some subjects, because it entails sitting in a sealed cabin during testing. The size and importable nature of the equipment also limits its utility in many clinical settings.
The interrupter technique generates during transient (~100 millisecond) interruptions of airflow at the mouth, during which alveolar pressure equilibrates with mouth pressure. Dynamic changes in mouth pressure after an interruption during spontaneous breathing are measured and provide information regarding airway resistance and the viscoelastic and stress adaptation properties of the respiratory system.8 It is also noninvasive, may be conducted during spontaneous tidal breathing, and requires only passive cooperation by the subject, thus making it a valuable technique to determine lung function.48 Similar to IOS, the patient sits upright, supports their cheeks, wears a nose clip, and breathes through a mouthpiece or facemask during measurements. The facemask is connected to a flow meter and a pressure transducer, which determine airflow and pressure variations, respectively. Studies have shown IOS to be more sensitive in determining airway caliber in asthmatic children who underwent bronchodilator therapy49 and methacholine challenge testing.50
CLINICAL SCENARIOS
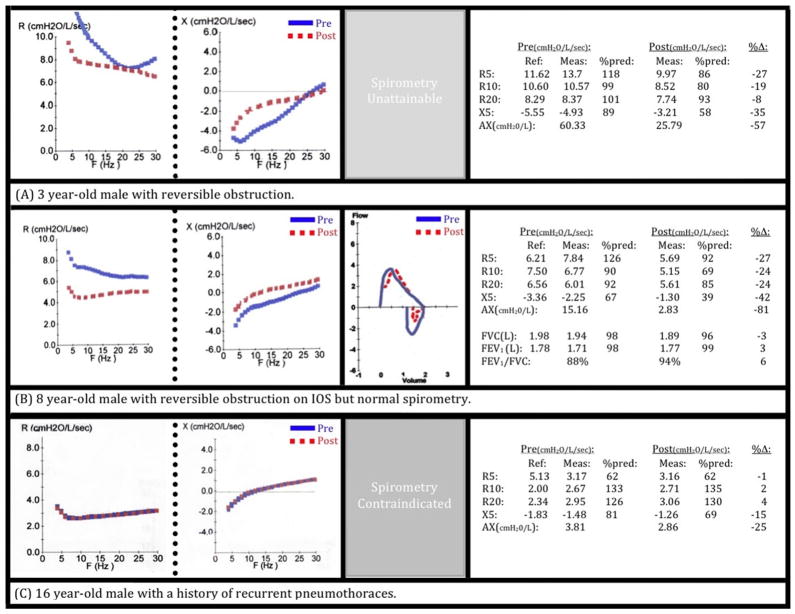
Impulse oscillometry may provide objective information that is beneficial to patient care in cases in which spirometry was either normal or could not be performed. Figure 4 outlines several instructive cases of children that were evaluated in the National Institutes of Health Pediatric Allergy Clinic. Panel A shows the impedance curves from a 3-year-old white boy with a history of food-induced systemic reactions and skin test positivity to wheat, egg, peanuts, and tree nuts; and who suffered from chronic atopic dermatitis. He presented for evaluation with a clinical diagnosis of asthma based on recurrent cough and wheezing and was well maintained with daily inhaled corticosteroids. The patient was too young to correctly perform the maneuvers for spirometry, but IOS was easily executed and reproducibly showed reversible obstruction (ΔR5: −27%, ΔAX: −57%) that could thereafter be objectively followed. Panel B shows the pulmonary function testing in an 8-year-old boy with a history of nocturnal cough, rhinorrhea, nasal congestion, conjunctivitis, intermittent “difficulty breathing,” and wheezing. He was on oral antihistamines, a leukotriene inhibitor, and daily short-acting β2 agonists. Lung studies revealed normal spirometry based on American Thoracic Society guidelines (ΔFEV1: 3%). Impulse oscillometry, however, showed significant reversible obstruction (ΔR5: −27%, ΔAX: −81%). Based on the IOS results, he was placed on daily-inhaled corticosteroids; and upon subsequent evaluation, he had full resolution of symptoms and normalization of IOS parameters. The impedance curve in Panel C was generated from a 16-year-old adolescent boy with a history of recurrent pneumothoraces and chronic respiratory symptoms; several occurred spontaneously and the others with physical exertion. There was serious concern that the increase in intrathoracic pressure required for spirometry testing could trigger a pneumothorax, and thus this type of testing was excluded from his evaluation. He was able to perform IOS on repeated clinic visits without incident. The representative impedance curve was normal, without significant reversibility.
Figure 4.
Clinical scenarios highlight utility of IOS. A, Three-year-old boy with food allergy, eczema, and chest symptoms; unable to perform spirometry. IOS indicates significant reversible obstruction. B, Eight-year-old boy with allergic rhinoconjunctivitis, dyspnea, and wheezing on examination. Normal spirometry but abnormal findings consistent with clinical presentation shown from IOS. C, Sixteen-year-old male adolescent with recurrent pneumothoraces unable to perform spirometry. Impulse oscillometry performed without event, showing normal lung function.
CONCLUSIONS
Impulse oscillometry is a noninvasive, rapid, safe and validated technique that measures respiratory impedance that is used as an indicator of lung function. It requires minimal cooperation from the subject and is therefore of great utility in preschool children, as well as in older children and adults. IOS may be used to diagnose, evaluate, and determine treatment response in those with asthma or other pulmonary disease states, with an accuracy and reproducibility comparable to spirometry and other lung function tests. IOS is similarly used in research endeavors, in part because it is objective and reproducible, although standardization for challenge testing has not been clearly established. IOS provides objective measurements of patient performance, whereas spirometry requires subjective judgments of patient effort and cooperation. Thus, impulse oscillometry is an excellent choice for the evaluation of lung disease in children.
Acknowledgments
Funding Source: This work was supported by the DIR Intramural Research Program of the NIAID.
Footnotes
Disclosures: Authors have nothing to disclose.
References
- 1.Foliaki S, Annesi-Maesano I, Daniel R, et al. Prevalence of symptoms of childhood asthma, allergic rhinoconjunctivitis and eczema in the Pacific: the International Study of Asthma and Allergies in Childhood (ISAAC) Allergy. 2007;62:259–264. doi: 10.1111/j.1398-9995.2007.01343.x. [DOI] [PubMed] [Google Scholar]
- 2.Gerald LB, Sockrider MM, Grad R, et al. An official ATS workshop report: issues in screening for asthma in children. Proc Am Thorac Soc. 2007;4:133–141. doi: 10.1513/pats.200604-103ST. [DOI] [PubMed] [Google Scholar]
- 3.Marotta A, Klinnert MD, Price MR, Larsen GL, Liu AH. Impulse oscillometry provides an effective measure of lung dysfunction in 4-year-old children at risk for persistent asthma. J Allergy Clin Immunol. 2003;112:317–322. doi: 10.1067/mai.2003.1627. [DOI] [PubMed] [Google Scholar]
- 4.Neve V, Edme JL, Devos P, et al. Spirometry in 3–5-year-old children with asthma. Pediatr Pulmonol. 2006;41:735–743. doi: 10.1002/ppul.20389. [DOI] [PubMed] [Google Scholar]
- 5.Dubois AB, Brody AW, Lewis DH, Burgess BF., Jr Oscillation mechanics of lungs and chest in man. J Appl Physiol. 1956;8:587–594. doi: 10.1152/jappl.1956.8.6.587. [DOI] [PubMed] [Google Scholar]
- 6.Smith HJ, Reinhold P, Goldman MD. Forced oscillation technique and impulse oscillometry. In: Gosselink R, Stam H, editors. Lung Function Testing: European Respiratory Society Monograph. Vol. 31. Shef-field, UK: European Respiratory Society; 2005. Chapter 5. [Google Scholar]
- 7.Klug B. The impulse oscillation technique applied for measurements of respiratory function in young children. Pediatr Pulmonol. 1997;16(suppl):240–241. doi: 10.1002/ppul.19502308125. [DOI] [PubMed] [Google Scholar]
- 8.Beydon N, Davis SD, Lombardi E, et al. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med. 2007;175:1304–1345. doi: 10.1164/rccm.200605-642ST. [DOI] [PubMed] [Google Scholar]
- 9.Clement J, Dumoulin B, Gubbelmans R, Hendriks S, van de Woestijne KP. Reference values of total respiratory resistance and reactance between 4 and 26 Hz in children and adolescents aged 4 –20 years. Bull Eur Physiopathol Respir. 1987;23:441– 48. [PubMed] [Google Scholar]
- 10.Goldman MD. Clinical application of forced oscillation. Pulm Pharmacol Ther. 2001;14:341–350. doi: 10.1006/pupt.2001.0310. [DOI] [PubMed] [Google Scholar]
- 11.Michaelson ED, Grassman ED, Peters WR. Pulmonary mechanics by spectral analysis of forced random noise. J Clin Invest. 1975;56:1210–1230. doi: 10.1172/JCI108198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frei J, Jutla J, Kramer G, Hatzakis GE, Ducharme FM, Davis GM. Impulse oscillometry: reference values in children 100 to 150 cm in height and 3 to 10 years of age. Chest. 2005;128:1266–1273. doi: 10.1378/chest.128.3.1266. [DOI] [PubMed] [Google Scholar]
- 13.Oostveen E, MacLeod D, Lorino H, et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003;22:1026–1041. doi: 10.1183/09031936.03.00089403. [DOI] [PubMed] [Google Scholar]
- 14.Dencker M, Malmberg LP, Valind S, et al. Reference values for respiratory system impedance by using impulse oscillometry in children aged 2–11 years. Clin Physiol Funct Imaging. 2006;26:247–250. doi: 10.1111/j.1475-097X.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- 15.Morgan W, Mansfield L, Wolf J, Souhrada JF. The measurement of total respiratory resistance in small children. J Asthma. 1982;19:233–240. doi: 10.3109/02770908209104766. [DOI] [PubMed] [Google Scholar]
- 16.Ducharme FM, Davis GM. Measurement of respiratory resistance in the emergency department: feasibility in young children with acute asthma. Chest. 1997;111:1519–1525. doi: 10.1378/chest.111.6.1519. [DOI] [PubMed] [Google Scholar]
- 17.Michels A, Decoster K, Derde L, Vleurinck C, Van de Woestijne KP. Influence of posture on lung volumes and impedance of respiratory system in healthy smokers and nonsmokers. J Appl Physiol. 1991;71:294–299. doi: 10.1152/jappl.1991.71.1.294. [DOI] [PubMed] [Google Scholar]
- 18.Kuhnle GE, Brandt T, Roth U, Goetz AE, Smith HJ, Peter K. Measurement of respiratory impedance by impulse oscillometry: effects of endotracheal tubes. Res Exp Med (Berl) 2000;200:17–26. [PubMed] [Google Scholar]
- 19.Desager KN, Cauberghs M, Naudts J, van de Woestijne KP. Influence of upper airway shunt on total respiratory impedance in infants. J Appl Physiol. 1999;87:902–909. doi: 10.1152/jappl.1999.87.3.902. [DOI] [PubMed] [Google Scholar]
- 20.Ortiz G, Menendez R. The effects of inhaled albuterol and salmeterol in 2- to 5-year-old asthmatic children as measured by impulse oscillometry. J Asthma. 2002;39:531–536. doi: 10.1081/jas-120004923. [DOI] [PubMed] [Google Scholar]
- 21.Nieto A, Pamies R, Oliver F, Medina A, Caballero L, Mazon A. Montelukast improves pulmonary function measured by impulse oscillometry in children with asthma (Mio study) Respir Med. 2006;100:1180–1185. doi: 10.1016/j.rmed.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 22.Jartti T, Lehtinen P, Vanto T, et al. Efficacy of prednisolone in children hospitalized for recurrent wheezing. Pediatr Allergy Immunol. 2007;18:326–334. doi: 10.1111/j.1399-3038.2007.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duiverman EJ, Den Boer JA, Roorda RJ, Rooyackers CM, Valstar M, Kerrebijn KF. Lung function and bronchial responsiveness measured by forced oscillometry after bronchopulmonary dysplasia. Arch Dis Child. 1988;63:727–732. doi: 10.1136/adc.63.7_spec_no.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gangell CL, Horak F, Jr, Patterson HJ, Sly PD, Stick SM, Hall GL. Respiratory impedance in children with cystic fibrosis using forced oscillations in clinic. Eur Respir J. 2007;30:892– 897. doi: 10.1183/09031936.00003407. [DOI] [PubMed] [Google Scholar]
- 25.Van Noord JA, Clement J, Van de Woestijne KP, Demedts M. Total respiratory resistance and reactance in patients with asthma, chronic bronchitis, and emphysema. Am Rev Respir Dis. 1991;143:922–927. doi: 10.1164/ajrccm/143.5_Pt_1.922. [DOI] [PubMed] [Google Scholar]
- 26.Mansur AH, Manney S, Ayres JG. Methacholine-induced asthma symptoms correlate with impulse oscillometry but not spirometry. Respir Med. 2008;102:42– 49. doi: 10.1016/j.rmed.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Pairon JC, Iwatsubo Y, Hubert C, Lorino H, Nouaigui H, Gharbi R, et al. Measurement of bronchial responsiveness by forced oscillation technique in occupational epidemiology. Eur Respir J. 1994;7:484– 489. doi: 10.1183/09031936.94.07030484. [DOI] [PubMed] [Google Scholar]
- 28.Song TW, Kim KW, Kim ES, Kim KE, Sohn MH. Correlation between spirometry and impulse oscillometry in children with asthma. Acta Paediatr. 2008;97:51–54. doi: 10.1111/j.1651-2227.2007.00526.x. [DOI] [PubMed] [Google Scholar]
- 29.Boccaccino A, Peroni DG, Pietrobelli A, et al. Forced oscillometry is applicable to epidemiological settings to detect asthmatic children. Allergy Asthma Proc. 2007;28:170–173. doi: 10.2500/aap.2007.28.2965. [DOI] [PubMed] [Google Scholar]
- 30.Farre R, Mancini M, Rotger M, Ferrer M, Roca J, Navajas D. Oscillatory resistance measured during noninvasive proportional assist ventilation. Am J Respir Crit Care Med. 2001;164:790–794. doi: 10.1164/ajrccm.164.5.2102049. [DOI] [PubMed] [Google Scholar]
- 31.Liu H, Ni W, Zhao J, Xiong S, Xu Y, Zhang Z. The diagnosis value and its implication of impulse oscillometry in obstructive sleep apnea syndrome patients. J Tongji Med Univ. 2000;20:280–282. doi: 10.1007/BF02888179. [DOI] [PubMed] [Google Scholar]
- 32.Badia JR, Farre RO, John Kimoff R, et al. Clinical application of the forced oscillation technique for CPAP titration in the sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;160:1550–1554. doi: 10.1164/ajrccm.160.5.9902085. [DOI] [PubMed] [Google Scholar]
- 33.Lin CC, Lee KS, Chang KC, Wu KM, Chou CS. Effect of laser-assisted uvulopalatoplasty on oral airway resistance during wakefulness in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2006;263:241–247. doi: 10.1007/s00405-005-0994-2. [DOI] [PubMed] [Google Scholar]
- 34.Eigen H, Bieler H, Grant D, Christoph K, Terrill D, Heilman DK, et al. Spirometric pulmonary function in healthy preschool children. Am J Respir Crit Care Med. 2001;163:619– 623. doi: 10.1164/ajrccm.163.3.2002054. [DOI] [PubMed] [Google Scholar]
- 35.Bisgaard H, Nielsen KG. Plethysmographic measurements of specific airway resistance in young children. Chest. 2005;128:355–362. doi: 10.1378/chest.128.1.355. [DOI] [PubMed] [Google Scholar]
- 36.Oswald-Mammosser M, Llerena C, Speich JP, Donata L, Lonsdorfer Measurements of respiratory system resistance by the interrupter technique in healthy and asthmatic children. Pediatr Pulmonol. 1997;24:78– 85. doi: 10.1002/(sici)1099-0496(199708)24:2<78::aid-ppul2>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 37.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 38.Kanengiser S, Dozor AJ. Forced expiratory maneuvers in children aged 3 to 5 years. Pediatr Pulmonol. 1994;18:144–149. doi: 10.1002/ppul.1950180305. [DOI] [PubMed] [Google Scholar]
- 39.Crenesse D, Berlioz M, Bourrier T, Albertini M. Spirometry in children aged 3 to 5 years: reliability of forced expiratory maneuvers. Pediatr Pulmonol. 2001;32:56– 61. doi: 10.1002/ppul.1089. [DOI] [PubMed] [Google Scholar]
- 40.Piccioni P, Borraccino A, Forneris MP, et al. Reference values of forced expiratory volumes and pulmonary flows in 3– 6 year children: a cross-sectional study. Respir Res. 2007;8:14. doi: 10.1186/1465-9921-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vilozni D, Bentur L, Efrati O, et al. Exercise challenge test in 3- to 6-year-old asthmatic children. Chest. 2007;132:497–503. doi: 10.1378/chest.07-0052. [DOI] [PubMed] [Google Scholar]
- 42.Larsen GL, Morgan W, Heldt GP, et al. Impulse oscillometry versus spirometry in a long-term study of controller therapy for pediatric asthma. J Allergy Clin Immunol. 2009;123:861–867e1. doi: 10.1016/j.jaci.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nielsen KG, Bisgaard H. Bronchodilation and bronchoprotection in asthmatic preschool children from formoterol administered by mechanically actuated dry-powder inhaler and spacer. Am J Respir Crit Care Med. 2001;164:256–259. doi: 10.1164/ajrccm.164.2.2011121. [DOI] [PubMed] [Google Scholar]
- 44.Bisgaard H, Nielsen KG. Bronchoprotection with a leukotriene receptor antagonist in asthmatic preschool children. Am J Respir Crit Care Med. 2000;162:187–190. doi: 10.1164/ajrccm.162.1.9910039. [DOI] [PubMed] [Google Scholar]
- 45.Morice AH, Waterhouse JC, Peers EM, Parry-Billings M. Use of whole-body plethysmography to compare bronchodilator inhaler efficacy. Respiration. 1998;65:120–124. doi: 10.1159/000029242. [DOI] [PubMed] [Google Scholar]
- 46.Nielsen KG, Bisgaard H. Cold air challenge and specific airway resistance in preschool children. Paediatr Respir Rev. 2005;6:255–266. doi: 10.1016/j.prrv.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 47.Aurora P, Bush A, Gustafsson P, et al. Multiple-breath washout as a marker of lung disease in preschool children with cystic fibrosis. Am J Respir Crit Care Med. 2005;171:249–256. doi: 10.1164/rccm.200407-895OC. [DOI] [PubMed] [Google Scholar]
- 48.Beydon N, Pin I, Matran R, et al. Pulmonary function tests in preschool children with asthma. Am J Respir Crit Care Med. 2003;168:640– 644. doi: 10.1164/rccm.200303-449OC. [DOI] [PubMed] [Google Scholar]
- 49.Delacourt C, Lorino H, Fuhrman C, et al. Comparison of the forced oscillation technique and the interrupter technique for assessing airway obstruction and its reversibility in children. Am J Respir Crit Care Med. 2001;164:965–972. doi: 10.1164/ajrccm.164.6.2010153. [DOI] [PubMed] [Google Scholar]
- 50.Klug B, Bisgaard H. Measurement of lung function in awake 2–4-year-old asthmatic children during methacholine challenge and acute asthma: a comparison of the impulse oscillation technique, the interrupter technique, and transcutaneous measurement of oxygen versus whole-body plethysmography. Pediatr Pulmonol. 1996;21:290–300. doi: 10.1002/(SICI)1099-0496(199605)21:5<290::AID-PPUL4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]