Abstract
Rationale
Reactivity to stressors and environmental cues, a putative cause of relapse in addiction, may be a useful target for relapse-prevention medication. In rodents, alpha-2 adrenergic agonists such as clonidine block stress-induced reinstatement of drug seeking, but not drug cue-induced reinstatement.
Objective
The objective of this study is to test the effect of clonidine on stress- and cue-induced craving in human cocaine users.
Methods
Healthy, non-treatment-seeking cocaine users (n = 59) were randomly assigned to three groups receiving clonidine 0, 0.1, or 0.2 mg orally under double-blind conditions. In a single test session, each participant received clonidine or placebo followed 3 h later by exposure to two pairs of standardized auditory-imagery scripts (neutral/stress and neutral/drug). Subjective measures of craving were collected.
Results
Subjective responsivity (“crave cocaine” Visual Analog Scale) to stress scripts was significantly attenuated in the 0.1- and 0.2-mg clonidine groups; for drug-cue scripts, this attenuation occurred only in the 0.2-mg group. Other subjective measures of craving showed similar patterns of effects but Dose × Script interactions were not significant.
Conclusions
Clonidine was effective in reducing stress-induced (and, at a higher dose, cue-induced) craving in a pattern consistent with preclinical findings, although this was significant on only one of several measures. Our results, though modest and preliminary, converge with other evidence to suggest that alpha-2 adrenergic agonists may help prevent relapse in drug abusers experiencing stress or situations that remind them of drug use.
Keywords: Clonidine, Cocaine, Stress-induced craving, Cue-induced craving, Imagery scripts, Reinstatement
Relapse to drug use after sustained abstinence is common among individuals with substance-use disorders (McLellan et al. 1993). Identifying and targeting the precipitants of relapse may improve the long-term prognosis for addiction (O’Brien 2005; Sinha 2009). Two of the most frequently cited precipitants are stress and environmental cues (Carter and Tiffany 1999; Epstein et al. 2009; Heinz et al. 2006; Sinha 2001, 2008).
In the reinstatement model of relapse to drug use, stress has been modeled in rodents with electric foot shocks or yohimbine, each of which can reinstate seeking of heroin and cocaine (Erb et al. 1996; Shaham et al. 1996; Shaham and Stewart 1994, 1995). In this model, “relapse” is blocked by clonidine, a potent agonist at alpha-2 adrenergic autoreceptors that decreases noradrenergic cell firing (Erb et al. 2000; Shaham et al. 2000; Wang et al. 2001). These findings suggest that stress-induced relapse in humans should be responsive to clonidine.
Like stress, environmental cues (“people, places, and things,” in 12-step parlance) are frequently thought to precipitate relapse (Berger et al. 1996; Childress et al. 1993; Ehrman et al. 1992; Margolin et al. 1994). In human laboratory studies, cocaine users reliably report increased cocaine craving after hearing scripts incorporating imagery of drug-taking contexts and behaviors (Hillebrand 2000; Kilts et al. 2001; Taylor et al. 2000; Weinstein et al. 1997). In dependent individuals, susceptibility to cue-induced craving is higher during periods of drug use (Robbins and Ehrman 1998) and persistence of cue-induced craving predicts relapse (Margolin et al. 1994).
The mechanisms of cue-induced craving may differ from those of stress-induced craving. In rodents, cue-induced reinstatement of drug seeking is prevented by several manipulations (e.g. antagonism of CB1 cannabinoid receptors or D1 dopamine receptors) that do not prevent stress-induced reinstatement (Ciccocioppo et al. 2001; De Vries et al. 2001, 2003). Conversely, stress-induced reinstatement is prevented by several manipulations (e.g., administration of lofexidine, another alpha-2 adrenergic agonist (Highfield et al. 2001)) that do not prevent cue-induced reinstatement. Consequently, one might expect a dissociation between the effect of alpha-2 adrenergic agonists on stress- and cue-induced drug craving in humans. However, cocaine cues in humans have been shown to increase negative affect and induce physiological changes similar to those produced by experimental stressors (Sinha et al. 2000); thus, the degree of dissociation may be less pronounced in humans than in rats.
In this translational study, we tested the hypotheses that clonidine would block stress-induced craving responses in humans and that it might also reduce cue-induced drug craving. Either of these effects would be a novel and important indication for clonidine, unrelated to its current use as an adjunct medication during opiate detoxification.
Methods and materials
Participants and setting
We recruited non-treatment-seeking outpatient volunteers 18–55 years old who reported using cocaine for at least 1 year and at least once in the previous 30 days (confirmed by urine screen). Participants were excluded for hypersensitivity to clonidine and for other medical and psychiatric contraindications. Participants gave written informed consent as approved by the Institutional Review Board of the NIDA Intramural Research Program.
General procedures
The study used a between-subjects design in which each participant underwent a single experimental lab session. Participants were randomized to one of three dose groups receiving 0 mg (placebo), 0.1 or 0.2 mg clonidine, double blind. Randomization was stratified by sex and by frequency and route of cocaine use. Participants were instructed not to take unprescribed drugs, including alcohol, for 72 h before the day of the study and not to eat breakfast on the morning of the session. For consistency in collection of measures sensitive to diurnal variation, participants were instructed to arrive by 9:00 a.m.
On the morning of the session, cocaine and alcohol abstinence were confirmed by interview and by a negative breath alcohol (BAL < .001; Alco-Sensor IV, St. Louis, MO) and urine cocaine screen (OnTrak TesTstik, Palo Alto, CA). Urine pregnancy tests were also conducted on the day of session to confirm that none of the female participants were pregnant. Participants were then given a standardized breakfast. Following breakfast, participants listened to two practice scripts and were familiarized with the study measures. The session was then conducted as described below.
After the last script and data collection, participants were instructed to relax and to focus on deep breathing for 10 min. This was repeated until participants’ feelings of stress or craving dissipated, after which they were discharged from the session.
Experimental session
After pre-session activities, baseline measures were collected. Self-report measures included the 14-question Cocaine Craving Questionnaire (CCQ) (Heinz et al. 2006), the 20-item Positive Affect and Negative Affect Schedule (PANAS) (Watson et al. 1988), and three Visual Analog Scale (VAS) questions worded, respectively, “Right now, how much do you [crave/want/need] cocaine?” The items on the CCQ reflect five conceptual domains: Desire to Use, Intention to Use, Anticipation of Positive Outcome, Anticipation of Relief from Dysphoria, and Lack of Control over use; each domain is represented by two to four questions. The three VAS and CCQ were designated as co-primary outcome measures reflecting urge to use cocaine, whereas the PANAS was included to check for more nonspecific changes in affect. Heart rate, blood pressure, and skin temperature were measured with a Coulbourn Instruments LabLinc V, Human Measurement System (Coulbourn Instruments, White Hall, PA); saliva was collected for measurement of alpha-amylase and cortisol levels. Results for these measures (not statistically significant) are in the supplementary online material.
A single capsule containing clonidine or placebo was administered orally 30 min after baseline measurement and 3 h before the imagery session because peak plasma concentrations of clonidine are reached approximately 3 h after administration (Gilman et al. 1991). Placebo and clonidine (commercial generic preparations) were administered in identical size 0 capsules containing dextrose (placebo) or dextrose plus one or two 0.1-mg clonidine tablets. During the 3-h break, participants rested quietly and could watch TV or read magazines.
The imagery session consisted of presentation of four standardized audiotaped scripts, with measurement collection after each. There were two active scripts (one stress-inducing script, one drug-cue script) and two neutral-affect scripts. The stress-inducing script described sitting in a dentist’s waiting room anticipating a painful procedure. The drug-cue script described the urge to use cocaine, customized to the participant’s preferred route of administration. The neutral scripts described everyday tasks (changing a light bulb and then visiting a laundromat) and a day at the beach. Each script lasted 60 s and was followed by a 30-s quiet period of active imagery. The scripts were presented in pairs of neutral and active, with neutral always presented first. There were four possible script-pair combinations, which were assigned randomly, with stratification by clonidine dose group.
Participants were asked to close their eyes, listen carefully to the script, imagine themselves in the scene, and continue to do so (i.e., engage in active imagery) until told to stop. There were 2- to 3-min breaks between the neutral and active scripts and a 20-min break between the script pairs. The CCQ, VAS, and PANAS were repeated after each of the four scripts.
Data analysis
The study had 80% power to detect at least small-to-medium interactions between dose and script type (as small as Cohen’s f = 0.19 at a two-tailed alpha of 0.05) and to detect specific differences across doses.
For data analysis, each measurement was expressed as a change score by subtracting the baseline value (taken just before drug administration in the morning). These change scores were the dependent variables in repeated-measures regressions (Proc Mixed in SAS 9.2) with one between-subjects factor (Dose Group), one within-subjects factor (Script, with the two neutral scripts for each participant averaged together), and their interaction. These analyses tolerate missing data points but produce output like that of a repeated-measures ANOVA, including least-squares means (accompanied by post hoc t tests comparing each change score to 0). Each model used an unstructured covariance matrix, which provided the best fit to the data by Akaike’s Information Criterion. The criterion for statistical significance was set at p ≤ .05, two-tailed; p values ≤ .10 are reported as trends.
Results
Fifty-nine participants completed the study: 0 mg, n = 19; 0.1 mg, n = 21; 0.2 mg, n = 19. Overall, 84.5% were male; average age was 41 years (range 20–55). Demographics of the participants by clonidine dose group are shown in Table 1. There were no significant differences between groups on any of these demographic characteristics.
Table 1.
Participant demographics
| Demographics | Clonidine dose | ||
|---|---|---|---|
| 0 mg | 0.1 mg | 0.2 mg | |
| Sex n (%) | |||
| Male | 16 (27.1%) | 18 (30.5%) | 16 (27.1%) |
| Female | 3 (5.1%) | 3 (5.1%) | 3 (5.1%) |
| Age | |||
| Average (years) | 39.5 | 41.7 | 42.3 |
| Range (years) | 20–49 | 21–55 | 26–54 |
| Race n (%) | |||
| African American | 11 (18.6%) | 17 (28.8%) | 15 (25.4%) |
| Caucasian | 7 (11.9%) | 4 (6.8%) | 3 (5.1%) |
| More than one race | 1 (1.7%) | 0 (0%) | 1 (1.7%) |
| Cocaine-use history | |||
| Frequency n (%) | |||
| <2 times per week | 8 (13.6%) | 8 (13.6%) | 5 (8.5%) |
| ≥2 times per week | 11 (18.6%) | 13 (22.0%) | 14 (23.7%) |
| Route of administration n (%) | |||
| Intranasal | 4 (6.8%) | 4 (6.8%) | 1 (1.7%) |
| Smoke/Inhalation | 15 (25.4%) | 17 (28.8%) | 18 (30.5%) |
Experimental session
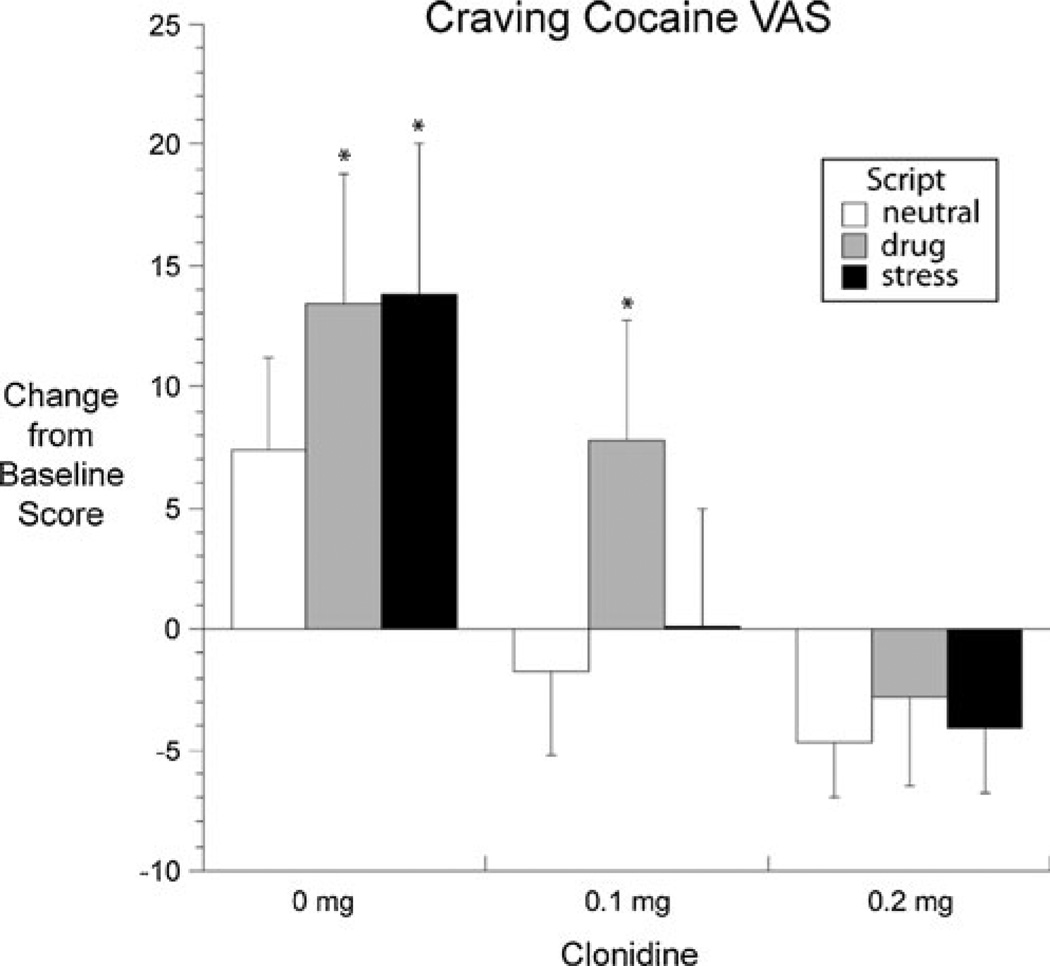
The Crave Cocaine VAS showed a significant main effect of Script [F(2,56) = 9.77, p < .001] and, as hypothesized, a significant interaction between Dose and Script type [F(4,56) = 3.63, p = .011]. There was a trend for a main effect of Dose [F(2,56) = 2.72, p = 0.074]. The Dose × Script interaction reflected the pattern of results shown in Fig. 1: in the placebo group, Crave Cocaine was significantly increased from baseline after both the stress script and the drug-cue script, but not after the neutral script; in the 0.1-mg group, Crave Cocaine increased only after the drug-cue script, not the stress script; in the 0.2-mg group, Crave Cocaine did not increase after either active script.
Fig. 1.
Least-squares mean (±SEM) change scores for the VAS item “Crave cocaine.” Raw scores could range from 0 to 100; change scores were calculated by subtracting the participant’s pre-drug baseline score from each midsession score; scores for the two neutral scripts in each session were averaged together. The interaction of Dose and Condition was significant (see “Results”). The values here are adjusted values from the output of a repeated-measures regression (SAS Proc Mixed); unadjusted values are shown in the first rows of Table 2. The asterisk indicates change score significantly different from 0 in a post hoc t test
Responses on the Want Cocaine VAS showed a pattern very similar to that of the Crave Cocaine VAS (Table 2). There was trend for a main effect of Dose [F(2,56) = 2.53, p = 0.088] and a significant effect of Script [F(2,56) = 7.87, p = 0.001]; however, the Dose × Script interaction was not significant. On the Need Cocaine VAS, there was a trend for a main effect of Script [F(2,56) = 2.90, p = 0.063], but no effect of Dose alone or Dose × Script interaction.
Table 2.
Subjective measures
| Clonidine dose | Statisticsc | |||||
|---|---|---|---|---|---|---|
| 0 mg mean (SEM) |
0.1 mg mean (SEM) |
0.2 mg mean (SEM) |
Dose effect | Script effect | Dose × script effect | |
| Crave Cocaine VASa | p=0.074 | p=0.0002 | p=0.011 | |||
| Neutral scriptsb | 7.58 (3.95) | 0.14 (3.04) | −1.95 (1.47) | |||
| Drug script | 12.84 (5.92)* | 12.70 (5.00)* | −0.42 (2.68) | |||
| Stress script | 14.53 (6.14)* | 2.67 (3.88) | −3.37 (1.75) | |||
| Want Cocaine VASa | p=0.088 | p=0.001 | n.s. | |||
| Neutral scriptsb | 7.42 (3.78) | −1.71 (3.56) | −4.63 (2.33) | |||
| Drug script | 13.47 (5.28)* | 7.81 (4.92) | −2.84 (3.63) | |||
| Stress script | 13.79 (6.26)* | 0.10 (4.91) | −4.11 (2.72) | |||
| Need Cocaine VASa | n.s. | p=0.063 | n.s. | |||
| Neutral scriptsb | 3.16 (3.19) | −3.81 (1.84) | 0.47 (0.77) | |||
| Drug script | 5.37 (5.46) | 0.48 (2.56) | 0.53 (2.05) | |||
| Stress script | 7.58 (5.22) | 3.24 (4.66) | −1.05 (1.37) | |||
| Cocaine Craving Questionnaire (CCQ)a | p=0.010 | p=0.007 | n.s. | |||
| Neutral scriptsb | 7.26 (1.97) | −0.38 (1.56) | −1.79 (1.73) | |||
| Drug script | 11.74 (3.26) | 4.85 (3.02) | −2.58 (2.53) | |||
| Stress script | 8.47 (3.23) | 1.48 (2.27) | 0.0 (2.17) | |||
| PANASa | ||||||
| Negative Affect Scale | p=0.060 | n.s. | p=0.060 | |||
| Neutral scriptsb | 0.16 (0.29) | −1.52 (0.50)* | −0.50 (0.19) | |||
| Drug script | 0.0 (0.36) | −1.05 (0.74)* | −0.16 (0.30) | |||
| Stress script | 0.63 (0.51) | −1.76 (0.71)* | −0.58 (0.37) | |||
| Positive Affect Scale | n.s. | p=0.061 | n.s. | |||
| Neutral scriptsb | −3.00 (0.94)* | −5.19 (0.95)* | −4.26 (0.82)* | |||
| Drug script | −3.16 (1.12)* | −5.00 (1.44)* | −4.74 (1.16)* | |||
| Stress script | −3.68 (1.53)* | −6.48 (1.51)* | −4.11 (1.09)* | |||
Expressed as change from baseline
Change from 30 min pre-medication administration baseline questionnaires
Scores for the neutral scripts are the average of the two scripts presented in each session
p Values from repeated-measures regressions (SAS Proc Mixed); values are shown for p ≤ 0.10 to indicate trends; statistical significance accepted at p ≤ 0.05. Means and SEMs in this table are unadjusted values
p ≤ 0.05 in post hoc t test comparing change score to 0 from output of repeated-measures regression (SAS Proc Mixed)
Cocaine craving as measured on the CCQ showed a significant effect of Script, with the drug scripts inducing greater increases than the stress scripts [F(2,56) = 5.03, p = 0.010] (Table 2). There was also a significant effect of clonidine Dose, with a dose-related decrease in craving scores [F(2,56) = 5.49, p = 0.007]. Clonidine did not differentially affect craving induced by the drug versus stress scripts, as indicated by a nonsignificant Dose × Script interaction. However, overall the pattern was similar to that seen in the craving VAS. In post hoc analyses of the five conceptual domains of the CCQ, there was a Dose × Script interaction [F(4,56) = 3.75, p = 0.009] on just one domain, Intention to Use (“If I were offered some cocaine, I would use it immediately” and “I am going to use cocaine as soon as possible”), with a pattern of changes similar to those for VAS Crave Cocaine (data not shown).
On the PANAS, which was included to check for nonspecific effects of clonidine on mood or arousal, there were no significant effects of Dose or Script and no significant interaction between Dose and Script, either on the positive or negative affect scale (Table 2). Positive-affect ratings on the PANAS significantly, but not differentially, decreased in all conditions (dose and script) compared with pre-drug baseline. Negative-affect ratings on the PANAS were significantly decreased in the clonidine 0.1-mg group compared with pre-drug baseline.
Discussion
Clonidine at 0.1 and 0.2 mg blocked increases in VAS ratings of Crave Cocaine after a stress script. The effect on stress-induced craving was somewhat specific; craving after a drug-cue script was blocked only by the higher dose of clonidine. These findings are consistent with rodent data that show alpha-2 adrenergic agonists can block reinstatement of cocaine seeking after a stressor and also support clinical data suggesting that in humans, the effect can generalize to drug cues (Sinha et al. 2007). Based on the preclinical literature (Highfield et al. 2001; Shaham et al. 2000), this generalization is unexpected; it may reflect a tendency for drug-associated cues to induce stress-like responses in humans, but not in rats (Sinha 2001).
One limitation of our findings is that clonidine’s reactivity-reducing effects were significant only on the VAS measure Crave Cocaine, though with a similar pattern on the VAS measure Want Cocaine and the CCQ. There were no significant effects on the VAS measure Need Cocaine. We had designated all of these (and no other measures) as our co-primary outcome measures before starting the study. Our findings would be more clear-cut if clonidine had significantly affected all four measures, but the effect on Crave Cocaine was clear and dose-dependent and seems worth attempting to replicate. The words “want” and “need” presumably had different meanings for participants than “crave”; similarly, the CCQ’s multifactorial operationalization of craving may have obscured increases in particular aspects of craving. This possibility is supported by our exploratory finding of a Dose × Script interaction in the CCQ’s Intention to Use subscale; when graphed (data not shown), the Intention to Use data resembled the VAS “crave” data shown in Fig. 1.
Another limitation of our findings is that the stress and drug-cue scripts had very few effects on physiological measures (see supplementary online material).
The doses of clonidine used in this study are within the range of doses used clinically to treat high blood pressure and opioid withdrawal. We did not observe any clinically significant adverse effects with either dose of clonidine. Our clinic is currently conducting a double-blind clinical trial of clonidine (up to 0.3 mg/day) for relapse prevention to heroin use in buprenorphine-maintained outpatients, many of whom are also using cocaine; to date, participants appear to be tolerating the study medication well, with minimal hypotension or sedation.
Preliminary clinical-trial data now support the efficacy of another alpha-2 adrenergic agonist, lofexidine, for prevention of exogenously induced heroin craving and use. Specifically, in a paper published while our study was already in progress, Sinha and colleagues (2007) reported that lofexidine, when given double-blind as an adjuvant to naltrexone in eight of 18 recently opiate-tapered outpatients, tended to increase abstinence during a 4-week monitoring period (75% opioid-negative urines, versus 40% for the placebo group). In laboratory sessions with the same participants, lofexidine reduced opioid-craving reactivity to both stress imagery and drug-cue imagery. Our own prior data suggest that lofexidine maintenance should be used with caution, if at all, in patients who are concurrently maintained on methadone, due to the possibility of QTc prolongation (Schmittner et al. 2004, 2009; Schroeder et al. 2007). Nonetheless, data appear to be converging in favor of continued testing of alpha-2 adrenergic agonists for prevention of exogenously induced relapse to opioid misuse. The effects of alpha-2 adrenergic agonists in addicted humans are consistent with findings in the reinstatement model of relapse but may also be more promising, with possible protection against drug-cue reactivity as well as stress reactivity.
Supplementary Material
Acknowledgements
This research was supported by the Intramural Research Program (IRP) of the National Institute on Drug Abuse (NIDA), National Institutes of Health.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00213-011-2230-7) contains supplementary material, which is available to authorized users.
Financial disclosures None of the authors has any relevant financial interests to declare.
Contributor Information
Michelle L. Jobes, Email: jobesm@nida.nih.gov, Clinical Pharmacology and Therapeutics Research Branch, Intramural Research Program, National Institute on Drug Abuse, 251 Bayview Blvd., Suite 200, Baltimore, MD 21224, USA.
Udi E. Ghitza, Center for the Clinical Trials Network, National Institute on Drug Abuse, 6001 Executive Blvd., Room 3151, MSC 9557, Bethesda, MD 20892–9557, USA
David H. Epstein, Clinical Pharmacology and Therapeutics Research Branch, Intramural Research Program, National Institute on Drug Abuse, 251 Bayview Blvd., Suite 200, Baltimore, MD 21224, USA
Karran A. Phillips, Clinical Pharmacology and Therapeutics Research Branch, Intramural Research Program, National Institute on Drug Abuse, 251 Bayview Blvd., Suite 200, Baltimore, MD 21224, USA
Stephen J. Heishman, Clinical Pharmacology and Therapeutics Research Branch, Intramural Research Program, National Institute on Drug Abuse, 251 Bayview Blvd., Suite 200, Baltimore, MD 21224, USA
Kenzie L. Preston, Clinical Pharmacology and Therapeutics Research Branch, Intramural Research Program, National Institute on Drug Abuse, 251 Bayview Blvd., Suite 200, Baltimore, MD 21224, USA
References
- Berger SP, Hall S, Mickalian JD, Reid MS, Crawford CA, Delucchi K, Carr K, Hall S. Haloperidol antagonism of cue-elicited cocaine craving. Lancet. 1996;347:504–508. doi: 10.1016/s0140-6736(96)91139-3. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D (1) antagonists. Proc Natl Acad Sci USA. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, Vanderschuren LJ, Schoffelmeer AN. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med. 2001;7:1151–1154. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Homberg JR, Binnekade R, Raaso H, Schoffelmeer AN. Cannabinoid modulation of the reinforcing and motivational properties of heroin and heroin-associated cues in rats. Psychopharmacology. 2003;168:164–169. doi: 10.1007/s00213-003-1422-1. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology. 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry. 2009;66:88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology. 1996;128:408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Gilman AG, Rall TW, Nies AS, Taylor P. Goodman and Gilman’s pharmacological basis of therapeutics. New York: Pergamon; 1991. [Google Scholar]
- Heinz AJ, Epstein DH, Schroeder JR, Singleton EG, Heishman SJ, Preston KL. Heroin and cocaine craving and use during treatment: measurement validation and potential relationships. J Subst Abuse Treat. 2006;31:355–364. doi: 10.1016/j.jsat.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Highfield D, Yap J, Grimm JW, Shalev U, Shaham Y. Repeated lofexidine treatment attenuates stress-induced, but not drug cues-induced reinstatement of a heroin-cocaine mixture (speedball) seeking in rats. Neuropsychopharmacology. 2001;25:320–331. doi: 10.1016/S0893-133X(01)00227-5. [DOI] [PubMed] [Google Scholar]
- Hillebrand J. New perspectives on the manipulation of opiate urges and the assessment of cognitive effort associated with opiate urges. Addict Behav. 2000;25:139–143. doi: 10.1016/s0306-4603(99)00007-6. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Margolin A, Avants SK, Kosten TR. Cue-elicited cocaine craving and autogenic relaxation. Association with treatment outcome. J Subst Abuse Treat. 1994;11:549–552. doi: 10.1016/0740-5472(94)90006-x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Arndt IO, Metzger DS, Woody GE, O’Brien CP. The effects of psychosocial services in substance abuse treatment. JAMA. 1993;269:1953–1959. [PubMed] [Google Scholar]
- O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN. Cocaine use is associated with increased craving in outpatient cocaine abusers. Exp Clin Psychopharmacol. 1998;6:217–224. doi: 10.1037//1064-1297.6.2.217. [DOI] [PubMed] [Google Scholar]
- Schmittner J, Schroeder JR, Epstein DH, Preston KL. QT interval increased after single dose of lofexidine. BMJ. 2004;329:1075. doi: 10.1136/bmj.329.7474.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittner J, Schroeder JR, Epstein DH, Krantz MJ, Eid NC, Preston KL. Electrocardiographic effects of lofexidine and methadone coadministration: secondary findings from a safety study. Pharmacotherapy. 2009;29:495–502. doi: 10.1592/phco.29.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JR, Schmittner J, Bleiberg J, Epstein DH, Krantz MJ, Preston KL. Hemodynamic and cognitive effects of lofexidine and methadone coadministration: a pilot study. Pharmacotherapy. 2007;27:1111–1119. doi: 10.1592/phco.27.8.1111. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Exposure to mild stress enhances the reinforcing efficacy of intravenous heroin self-administration in rats. Psychopharmacology. 1994;114:523–527. doi: 10.1007/BF02249346. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology. 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Rajabi H, Stewart J. Relapse to heroin-seeking in rats under opioid maintenance: the effects of stress, heroin priming, and withdrawal. J Neurosci. 1996;16:1957–1963. doi: 10.1523/JNEUROSCI.16-05-01957.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Highfield D, Delfs J, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur J Neurosci. 2000;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann NY Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology. 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, Kimmerling A, Doebrick C, Kosten TR. Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rates: preliminary findings. Psychopharmacology. 2007;190:569–574. doi: 10.1007/s00213-006-0640-8. [DOI] [PubMed] [Google Scholar]
- Taylor RC, Harris NA, Singleton EG, Moolchan ET, Heishman SJ. Tobacco craving: intensity-related effects of imagery scripts in drug abusers. Exp Clin Psychopharmacol. 2000;8:75–87. doi: 10.1037//1064-1297.8.1.75. [DOI] [PubMed] [Google Scholar]
- Wang X, Cen X, Lu L. Noradrenaline in the bed nucleus of the stria terminalis is critical for stress-induced reactivation of morphine-conditioned place preference in rats. Eur J Pharmacol. 2001;432:153–161. doi: 10.1016/s0014-2999(01)01487-x. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weinstein A, Wilson S, Bailey J, Myles J, Nutt D. Imagery of craving in opiate addicts undergoing detoxification. Drug Alcohol Depend. 1997;48:25–31. doi: 10.1016/s0376-8716(97)00098-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.