Abstract
Objectives
The in vivo inflammatory effects of the Bacillus anthracis cell wall are unknown. We therefore investigated these effects in rats and, for comparison, those of known inflammatory stimulants, Staphylococcus aureus cell wall or lipopolysaccharide (LPS).
Method and Results
Sprague–Dawley rats (n = 103) were challenged with increasing B. anthracis cell wall doses (10, 20, 40, 80, or 160 mg/kg) or diluent (control) as a bolus or 24-h infusion. The three highest bolus doses were lethal (20–64% lethality rates) as were the two highest infused doses (13% with each). Comparisons among lethal or nonlethal doses on other measured parameters were not significantly different, and these were combined for analysis. Over the 24 h after challenge initiation with lethal bolus or infusion, compared to controls, ten inflammatory cytokines and NO levels were increased and circulating neutrophils and platelets decreased (P ≤ 0.05). Changes with lethal doses were greater than changes with nonlethal doses (P ≤ 0.01). Lethal bolus or infusion doses produced hypotension or hypoxemia, respectively (P ≤ 0.05). The effects with B. anthracis cell wall were similar to those of S. aureus cell wall or LPS. However, paradoxically administration of B. anthracis cell wall or LPS decreased the lethality of concurrently administered B. anthracis lethal toxin (P < 0.0001 and 0.04, respectively).
Conclusion
B. anthracis cell wall has the potential to produce inflammatory injury during anthrax infection clinically. However, understanding why cell wall or LPS paradoxically reduced lethality with lethal toxin may help understand this toxin’s pathogenic effects.
Keywords: Bacillus anthracis, Cell wall, Cardiopulmonary dysfunction, Cytokine release, Inflammation
Introduction
The influence of Bacillus anthracis cell wall on inflammation and tissue injury has not been tested in vivo. The structure and inflammatory effects of cell wall components differ among gram-positive bacteria. While Staphylococcus aureus cell wall components have consistently been shown to stimulate an inflammatory response, those of other gram-positives have been more variable [1–3]. In this regard, B. anthracis cell wall has been shown to stimulate production of tumor necrosis factor (TNFα), interleukin 1 (IL-1β) and IL-6 in peripheral blood mononuclear cells [4]. In cultured epithelial cells B. anthracis cell wall activates NF-kB via TLR2 and TLR6 [5]. Finally, infection with B. anthracis in vitro results in NOD2-associated IL-1β production [6].
Based on such in vitro results, we hypothesized that B. anthracis cell wall in vivo has the potential to produce systemic inflammation and organ dysfunction. To test this, increasing doses of B. anthracis cell wall administered either as an intravenous bolus or, to simulate its gradual release during infection, as a 24-h infusion were investigated in a rat model [7, 8]. For comparison, infusions of similar weight doses of cell wall from a S. aureus strain pathogenic for humans and of similarly lethal doses of lipopolysaccharide (LPS) were also studied. Finally, to investigate the effects of cell wall on the lethal effects of anthrax lethal toxin (LeTx), animals were challenged with these components either alone or in combination, and survival was assessed. We hypothesized that the potentially injurious effects of the cell wall would likely add to the lethal effect of LeTx.
Materials and methods
Animal care
The protocol used in this study was approved by the Animal Care and Use Committee of the Clinical Center of the National Institutes of Health.
Study design
Sprague–Dawley rats (n = 103) with carotid arterial and jugular venous catheters were randomized to receive B. anthracis cell wall in a total dose of 10, 20, 40, 80, or 160 mg/kg or diluent (control), as either a bolus injection or 24-h continuous infusion [7, 8]. All animals received equivalent volumes of cell wall or diluent. Immediately before challenge and at 15-min intervals in the first hour, 2-h intervals from 2 to 8 h and 4-h intervals from 12 to 24 h after the initiation of cell wall challenge, mean arterial blood pressures (MBP) and heart rates (HR) were measured. At 4, 8 and 24 h, arterial blood was collected in all animals for blood gas (ABG), complete blood count (CBC), plasma cytokine and nitrite/nitrate (NO) measures. Animals had similar volumes (0.5 ml) of blood drawn and normal saline replaced at each time point, and were observed for 168 h. For comparison to B. anthracis cell wall, additional animals (n = 84) were challenged with 24-h infusions of either similar doses of S. aureus cell wall, doses of LPS (0.25, 0.5 and 1 mg/kg per 24 h) producing a similar range of lethality rates [7, 8] or diluent (control), all in equivalent volumes.
In order to investigate the effects of B. anthracis cell wall on the lethal effects of LeTx, animals (n = 55) were challenged with 24-h infusions of either cell wall (40 mg/kg per 24 h) or LeTx (100 µg/kg protective antigen (PA) + 50 µg/kg lethal factor (LF) over 24 h) alone or together or diluent. For comparison, additional animals (n = 82) were challenged with LPS (0.1 mg/kg per 24 h) or the same dose of LeTx alone or together. Survival was assessed at 168 h.
Cell wall, LeTx and LPS preparations
B. anthracis cell wall (Sterne strain) was prepared based on previously reported methods and obtained from List Biological Laboratories (Campbell, CA) [4, 9]. S. aureus cell wall was prepared in the laboratory of one of the authors (JS) using similar methods. For both B. anthracis and S. aureus cell wall, agarose gel electrophoresis with ethidium eromide staining and SDS-PAGE with comassie blue staining did not show any detectable DNA/RNA or protein contamination, respectively.
Based on kinetic chromogenic limulus amoebocyte lysate assays performed [Lonza (Basel, Switzerland) or Clogen Laboratories (Germantown, MD)], endotoxin was undetectable in either B. anthracis or S. aureus cell wall preparations. The lowest level of endotoxin detection by these assays is <0.1 EU/mg cell wall (or 0.01 ng/mg cell wall). Challenge (24 h infusion) in rats (n = 6) with a total dose of LPS comparable to what would be received if the highest cell wall dose tested (160 mg/kg) was contaminated with LPS at a level of 0.01 ng/mg cell wall did not alter any parameter measured in this study compared to diluent control (n = 6).
Cell wall preparations were diluted with 1 × phosphate buffered saline (PBS) to deliver doses of 10, 20, 40, 80 and 160 mg/kg bw when administered either as an infusion over 24 h in 12 ml at a rate of 0.5 ml/h or as a bolus over 2 min in a volume of 0.7 ml. LPS from Escherichia coli 0111:B4 (Sigma–Aldrich, St. Louis, MO) and lethal toxin were prepared and administered as previously described [10].
Laboratory measures
Arterial blood pressure, heart rate, ABG, lactate, and CBC measures and samples for cytokine and NO levels were obtained as previously described [7]. Cytokines [IL-1β, IL-6, IL-10 TNFα, migratory inhibitory protein 1α (MIP-1α), regulated on activation, normal T cell expressed and secreted (RANTES), granulocyte macrophage-colony stimulating factor (GM-CSF) and interferon γ (INFγ)] were measured using standard kits [Searchlight® Proteome Array Multiplex system (Pierce Biotechnology, Rockford, IL, or Cytokine Multiplex Immunoasssy Kit, Millipore, Danvers, MA)]. Plasma nitrite/nitrate (NO) was measured with a fluorometric assay kit (Cayman Chemical, Ann Arbor, MI).
Statistics
Kaplan–Meier survival curves were used to show the survival effect of individual doses of cell wall or LPS. All other parameters were analyzed with repeated measures ANOVA using PROC MIXED in Statistical Analysis System Version 9.1 software (SAS Institute, Inc., Cary, NC), and least squares means and associated standard errors were reported. For each of the three challenges (B. anthracis or S. aureus cell wall or LPS), comparison among nonlethal doses or among lethal doses on measured parameters did not show significant differences throughout. Therefore, to increase the power to detect the effects of cell wall or LPS, nonlethal doses were combined into one group and lethal doses into another for comparison to respective control (diluent) groups. All results are expressed as least square means ± SEM, and two-sided P values ≤ 0.05 were considered significant. For clarity of presentation in some figures in which both the challenge effect and challenge effect versus time interaction were significant, a single P value is shown and corresponds to the less significant comparison. However, in these cases it is also denoted that each effect reached significance.
Results
B. anthracis cell wall bolus
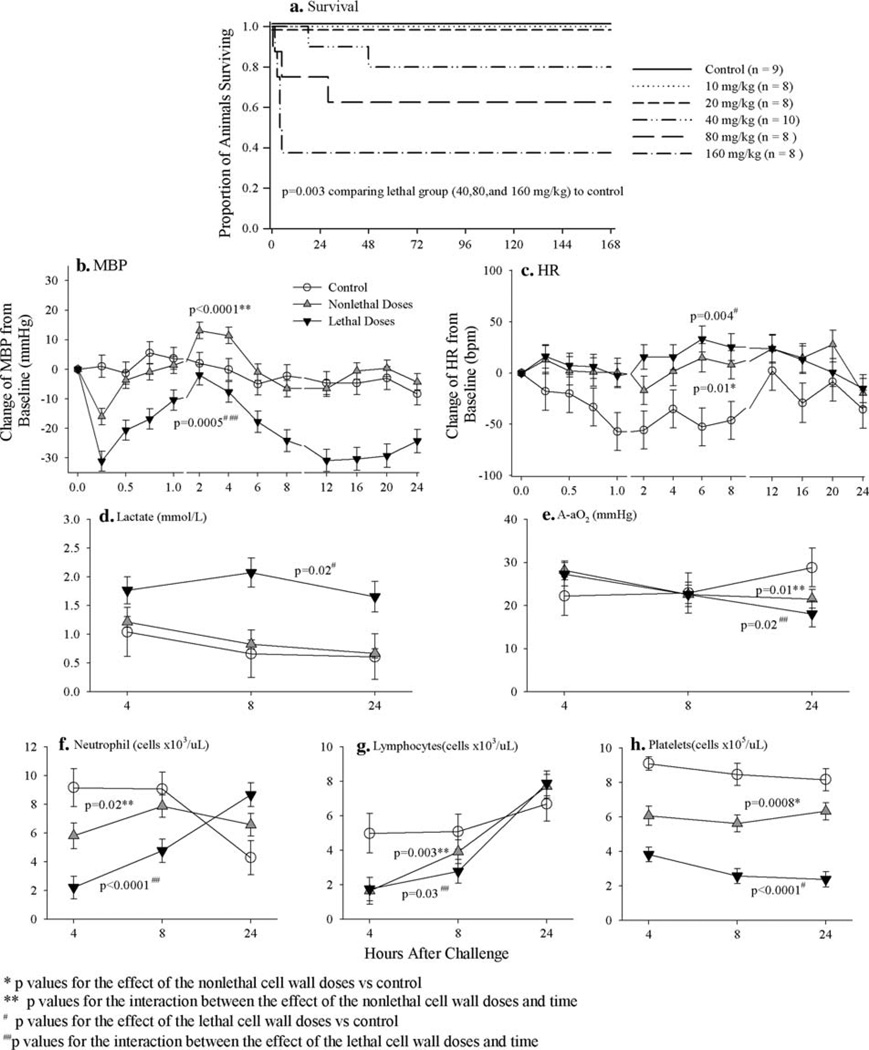
While the two lower B. anthracis bolus cell wall doses (10 and 20 mg/kg) did not worsen survival, the three higher ones (40, 80 and 160 mg/kg) did (Fig. 1a) (P = 0.003 comparing lethal doses to control, Wilcoxon test). Compared to controls, over the 24 h following challenge lethal cell wall doses decreased MBP and increased lactate (P < 0.0001 and P = 0.02, respectively), although the effects on MBP varied with time (P < 0.0001 for the interaction between cell wall effect and time) (Fig. 1). While both nonlethal and lethal doses increased heart rate (P ≤ 0.01 for each compared to controls), they had variable effects on A-aO2 (P ≤ 0.02 for the interaction between cell wall effect and time for each) (Fig. 1). Compared to controls, both nonlethal and lethal cell wall doses first decreased (4 and 8 h) and then increased (24 h) circulating neutrophils and lymphocytes (P ≤ 0.03 for the interaction between cell wall effect and time for all) but decreased platelets throughout (P = 0.001 and P < 0.0001 compared to controls, respectively) (Fig. 1). Changes in neutrophils (decreases and then increases) and platelets were greater with lethal compared to nonlethal cell wall doses (P ≤ 0.001 for each).
Fig. 1.
a Compares the effects of B. anthracis cell wall administered as a bolus in doses of 10, 20, 40, 80 or 160 mg/kg to a diluent (control) on the proportion of animals surviving. Based on the results shown in a, b–h show the effects of either nonlethal doses (10 and 20 mg/kg) or lethal doses (40, 80 and 160 mg/kg) of B. anthracis cell wall bolus combined compared to control on: the serial mean (±SEM) changes from baseline in mean arterial blood pressure (MBP, mmHg, b) and heart rate (HR, BPM, c) measured q15 min from 0 to 1 h, q2 h from 2 to 8 h and q4 h from 12 to 24 h and serial mean (±SEM) arterial lactates (d), arterial–alveolar oxygen gradients (A-aO2, e) and circulating neutrophils (f), lymphocytes (g) and platelets (h) measured at 4, 8 and 24 h
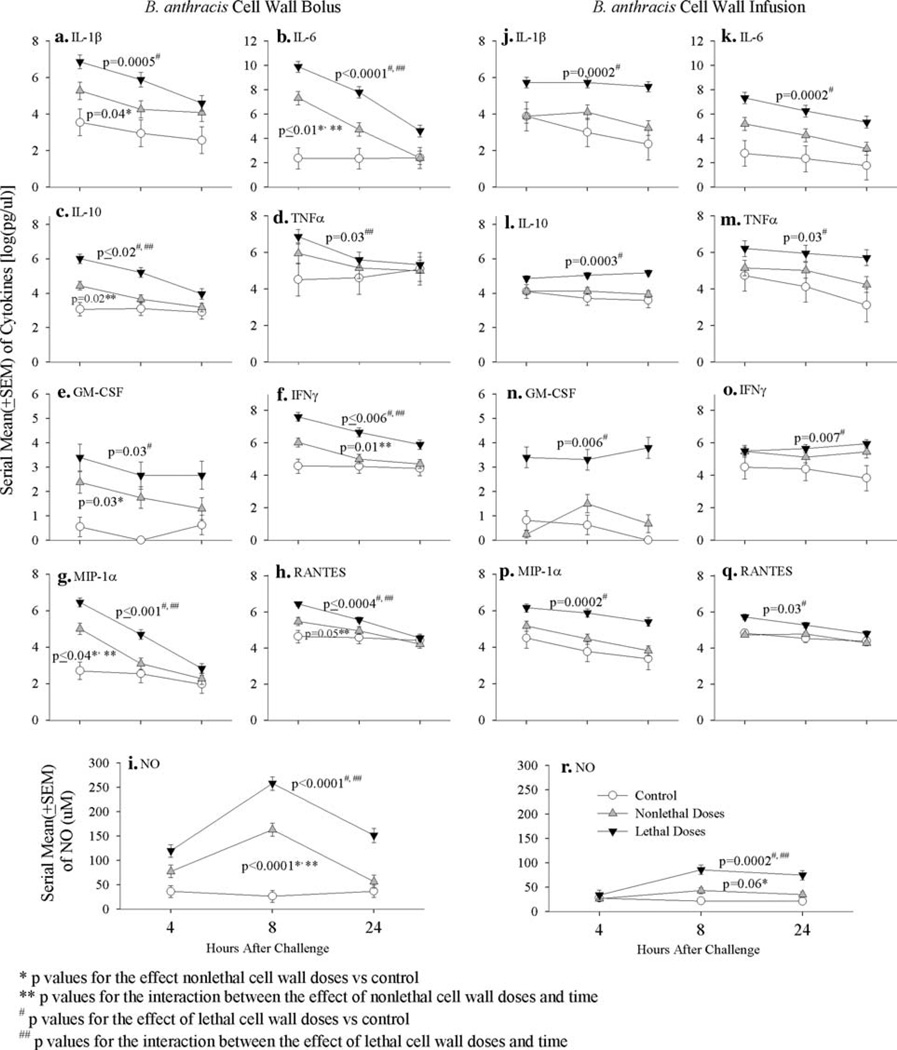
Compared to controls, while both nonlethal and lethal cell wall bolus doses significantly increased IL-1β, IL-6, MIP-1α, and GM-CSF levels (P ≤ 0.04 for all) over the 24 h after initiation of challenge, these changes except for GM-CSF were significantly greater with lethal compared to nonlethal doses (P ≤ 0.01 for all) (Fig. 2). Furthermore, only lethal cell wall significantly increased IL-10, IFNγ and RANTES across all time points (P ≤ 0.02 for all compared to controls) or TNFα at early time points (P = 0.03 for the interaction between cell wall effect and time) (Fig. 2). Finally, both nonlethal and lethal cell wall doses increased NO levels significantly (P < 0.0001 compared to controls for all) (Fig. 2). However, these increases were greatest at 8 h (P < 0.0001 for the interaction between the effects of both nonlethal and lethal cell wall doses and time), and they were always greater with lethal cell wall doses (P = 0.001).
Fig. 2.
This figure compares the effects of either nonlethal or lethal doses combined of B. anthracis cell wall administered as a bolus (a–i) or as a 24-h infusion (j–r) to their respective diluent controls on serial mean (±SEM) interleukin-1β (IL-1β, a, j), IL-6 (b, k), IL-10 (c, l), tumor necrosis factor α (TNFα, d, m), granulocyte macrophage-colony stimulating factor (GM-CSF, e, n), interferon γ (INFγ, f, o), migratory inhibitor protein-1α (MIP-1α, g, p) and RANTES (h, q) [all log (pg/ml)] and nitrite/nitrate (NO, µM) (i, r) at 4, 8 and 24 h. As shown in Figs. 1 and 3, nonlethal and lethal bolus doses were 10 and 20 and 40, 80 and 160 mg/kg, respectively, while nonlethal and lethal infused cell wall doses were 10, 20 and 40 and 80 and 160 mg/kg, respectively
B. anthracis cell wall infusion
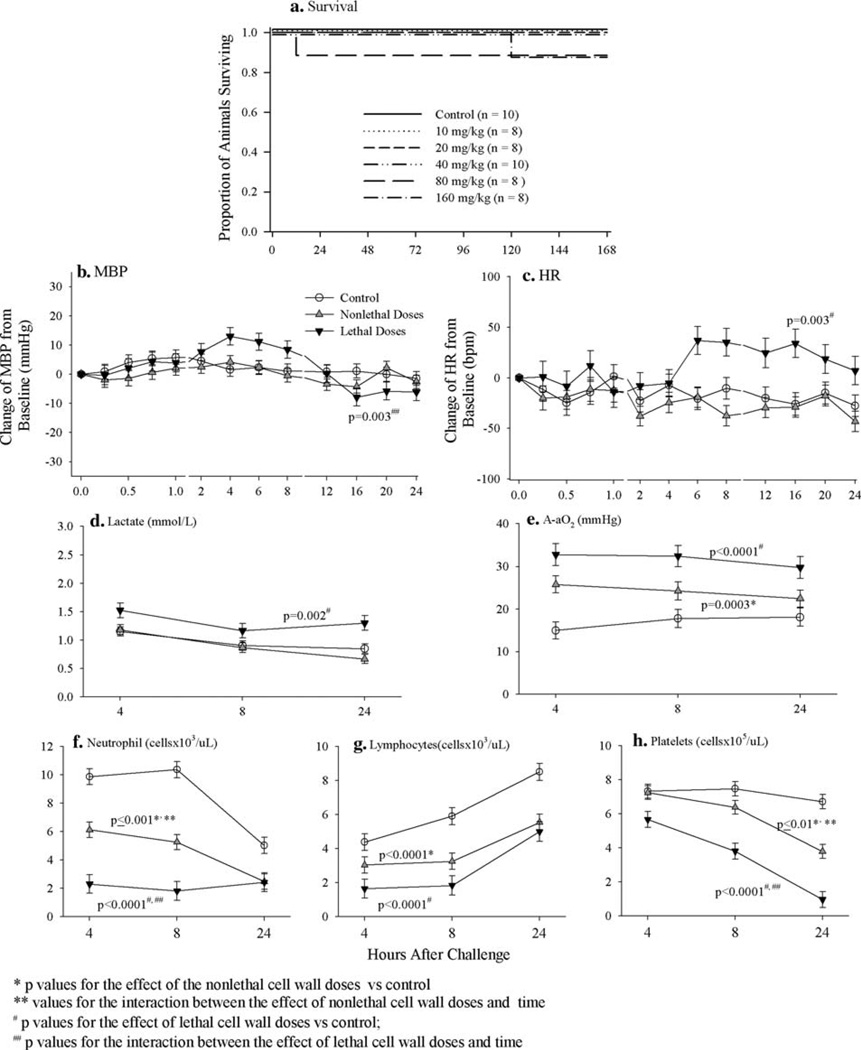
When administered as 24-h infusions, none of the three lower B. anthracis cell wall doses (10, 20 and 40 mg/kg) worsened survival, but the two higher doses did (Fig. 3), although these changes were not statistically significant (P = ns comparing lethal cell wall doses to control). Compared to controls, while neither nonlethal or lethal cell wall decreased MBP consistently during the 24-h challenge infusion, lethal dose increased heart rate and lactate levels (P ≤ 0.003 for each) (Fig. 3). However, both nonlethal and lethal cell wall doses worsened gas exchange and increased the A-aO2 (P ≤ 0.0003 for each compared to controls) (Fig. 3). Furthermore, both nonlethal and lethal doses decreased circulating neutrophil, lymphocyte and platelet counts (P ≤ 0.01 for all compared to controls) during the 24-h infusion (Fig. 3). These changes in A-aO2 and circulating cells were significantly greater with lethal compared to nonlethal cell wall doses (P ≤ 0.01).
Fig. 3.
a Compares the effects of B. anthracis cell wall administered as a 24-h infusion in total doses of 10, 20, 40, 80 or 160 mg/kg to a diluent (control) on the proportion of animals surviving. Based on the results shown in a, b–h show the effects of either nonlethal doses (10 and 20 mg/kg) or lethal doses (40, 80 and 160 mg/kg) of B. anthracis cell wall infusion combined compared to control on: the serial mean (±SEM) changes from baseline in mean arterial blood pressure (MBP, mmHg, b) and heart rate (HR, BPM, c) measured q15 min from 0 to 1 h, q2 h from 2 to 8 h and q4 h from 12 to 24 h and serial mean (±SEM) arterial lactates (d), arterial–alveolar oxygen gradients (A-aO2, e) and circulating neutrophils (f), lymphocytes (g) and platelets (b) measured at 4, 8 and 24 h
Compared to controls, although nonlethal cell wall consistently increased all cytokine levels throughout the 24-h infusion period, these changes did not reach significance. In contrast lethal cell wall significantly increased all cytokines measured at almost all time points (P ≤ 0.03 compared to controls) (Fig. 2). These changes were all significantly greater with lethal compared to nonlethal doses (P ≤ 0.01) except for IFNγ. Finally, although not as great as with cell wall administered as a bolus, both nonlethal and lethal infused cell wall doses increased NO levels, but these were only significant with the latter (P = 0.0002) (Fig. 2).
S. aureus cell wall and LPS infusions
While the two lower S. aureus doses (10 and 20 mg/kg) did not worsen survival, the three higher doses (40, 80 and 160 mg/kg) did (Figure E1), although these changes were not statistically significant (P = ns comparing lethal cell wall doses to control). Compared to controls, neither nonlethal nor lethal S. aureus cell wall doses had consistent effects on MBP or HR (Figure E1). However, compared controls, both nonlethal and lethal doses increased lactate levels (P < 0.0001 for each) and lethal dose increased A-aO2 (P = 0.04), although all these changes were greater at earlier time points (P ≤ 0.04 for the interaction between cell wall effect and time) (Figure E1). Both nonlethal and lethal S. aureus doses decreased neutrophils (P ≤ 0.005 and 0.002 compared to controls for each, respectively) and platelets and lethal dose decreased lymphocytes (P = 0.0006 compared to controls) (Figure E1). Changes were greater with neutrophils earlier and with platelets later (P ≤ 0.002 for the interaction between cell wall effect and time), and changes in all three cell types were significantly greater with lethal cell wall doses (P ≤ 0.001 for all). Both nonlethal and lethal S. aureus cell wall doses significantly increased IL-1β, IL-10, TNFα, MIP-1α and NO levels (P ≤ 0.01 compared to controls for all) (Figure E2), although these changes were not consistently greater with the lethal doses.
The lowest dose of infused LPS (0.25 mg/kg) did not alter survival, but the two higher ones (0.5 and 1.0 mg/kg) did (Figure E1), although these changes were not statistically significant (P = ns comparing lethal cell wall doses to control). While only the nonlethal LPS dose reduced MBP at later time points (P = 0.0003 for the interaction between the effect of LPS and time) and neither dose had consistent effects on HR, both doses increased lactate levels (P ≤ 0.004 for each compared to controls) (Figure E1). LPS did not increase A-aO2 (Figure E1). Comparing controls, both doses decreased neutrophils early and increased them late (P < 0.0001 for the interaction between cell wall effect and time) and decreased lymphocytes (P ≤ 0.03 for each) and platelets (P ≤ 0.001 for each) at all time points (Figure E1). Both LPS doses significantly increased IL-1β, IL-6, IL-10, TNFα and MIP-1α levels (all P ≤ 0.05), and lethal doses increased IFNγ and RANTES (P ≤ 0.002), and decreased GM-CSF levels (P = 0.001) (Figure E2). Both LPS doses also increased NO levels (P ≤ 0.003), and these increases were significantly (P = 0.001) greater with lethal doses (Figure E2).
Influence of B. anthracis cell wall on the lethal effects of LeTx
Infusion with diluent or B. anthracis cell wall alone produced no lethality, while anthrax LeTx alone was very lethal (68% lethality rate). Unexpectedly, this same dose of LeTx in combination with cell wall produced no lethality (P < 0.0001 comparing LeTx alone to cell wall with LeTx on survival) (Fig. 4). To explore this interaction further, animals were challenged with LeTx alone or in combination with LPS (0.1 mg/kg). This LPS dose, as with the anthrax cell wall dose employed, was just below the dosage range associated with lethality in the model and had been shown previously to produce cytokine and NO release. Consistent with B. anthracis cell wall, LPS in combination with LeTx resulted in a significant reduction in lethality compared to LeTx alone (P = 0.04) (Fig. 4).
Fig. 4.
a Shows the proportion of animals surviving over time with cell wall and LeTx challenge alone or together or with diluent only (control). b Shows the proportion of animals surviving over time after challenge with LPS and LeTx alone or together or diluent only (control)
Discussion
These in vivo findings are the first showing that B. anthracis cell wall produces a systemic inflammatory response with organ dysfunction and, at higher doses, lethality. Whether administered rapidly or slowly, both lethal and nonlethal cell wall doses increased intravascular cytokines and NO levels. Lethal cell wall doses produced greater changes in these mediators than nonlethal ones. While early increases in six cytokines and chemokines later decreased following cell wall bolus, all eight increased with cell wall infusion remaining elevated throughout. Along with its effects on cytokines and chemokines, B. anthracis cell wall reduced circulating neutrophils, lymphocytes and platelets. This is consistent with the observation that intravascular inflammation caused by microbial components increases adhesion molecule expression with adherence of circulating leukocytes and platelets to the endothelium [11, 12]. Reductions in these cells were in almost all cases greater with lethal compared to nonlethal cell wall doses.
Although both bolus and infusion of B. anthracis cell wall doses activated systemic inflammation, the physiologic basis for their lethal effects appeared different. Lethal bolus doses caused persistent hypotension and increases in lactate but not hypoxemia (i.e., unchanged A-aO2). In contrast, lethal infused cell wall doses, although increasing heart rate and lactate levels, did not reduce blood pressure, but worsened oxygenation (i.e., increased A-aO2). One basis for these differences is that bolus B. anthracis cell wall caused greater increases in NO than infusion. NO is closely associated with hypotension during sepsis [13]. On the other hand, reductions in circulating leukocyte number persisted throughout cell wall infusion, whereas these reversed with a bolus. Excessive and persistent activation of circulating leukocytes is implicated in septic lung injury [14, 15]. The effects of B. anthracis cell wall infusion were very similar to those noted with S. aureus cell wall.
Although LeTx is closely associated with the pathogenesis of B. anthracis infection, its isolated effects occur independent of excessive inflammation [7, 10, 16–18]. Studies in baboons demonstrated however that 2-h infusions of increasing B. anthracis (Sterne strain) concentrations produced dose-ordered decreases in blood pressure, neutrophils and platelets and increases in respiratory rate, TNFα, IL-1β and MIP-1α [19] and lung injury. These findings are very consistent with those noted with cell wall alone in this rat model. In combination these findings suggest that patients with B. anthracis sepsis may benefit from adjunctive therapies neutralizing toxin as well as cellular components like cell wall.
It required 1 × 1010 CFU bacteria to produce 1 mg of cell wall for these experiments. Thus, lethal doses of B. anthracis cell wall (40–160 mg/kg) were equivalent to infection with 40–160 × 1010 CFU/kg bw or 6–24 × 109 CFU/ml blood (based on an estimated rat blood volume of 17 ml per 250 g animal). While these circulating B. anthracis counts are equivalent to ones lethal in guinea pigs, (5–15 × 109 CFU/ml blood [20]), they are higher than those noted in baboons [19].
Challenge with LPS produced lethality and inflammatory change at much lower doses than B. anthracis cell wall. Separation of LPS from other gram-negative cell wall components may have increased its stimulatory effects. Whether challenge with individual components of B. anthracis cell wall (i.e., peptidoglycan or lipotechoic acid) would increase their inflammatory effects requires study. Breakdown of peptidoglycan chain into its muramyl subunits by bacterial autolysins might increase the chain’s stimulatory capacity during infection [21, 22]. The large bacterial burden in patients and animals with B. anthracis could provide a high concentration of cell wall or its components [20, 23–25].
Different from our hypothesis, the lethal effects of LeTx were reduced rather than increased when administered with anthrax cell wall. This decrease did not appear to result from nonspecific binding of toxin by cell wall, since administration of LPS in a 400-fold lower dose than cell wall (0.1 vs. 40 mg/kg) also significantly reduced the lethal effect of LeTx. Of note, intraperitoneal lipotechoic acid administration protected mice from intravenous LeTx challenge [26]. Lethal factor, the toxigenic moiety of LeTx, is a protease that inhibits mitogen-activated protein kinase pathways important in adaptive host stress responses [8, 20]. This inhibition may contribute to the pathogenic effect of LeTx. Therefore, it is possible that stimulation of these pathways by cell wall or LPS prior to inhibition by LeTx may have contributed to protective host responses.
The present findings suggest that excessive stimulation of host inflammation by cell wall may contribute to the pathogenesis of B. anthracis. If effective methods to modulate host inflammation are developed for sepsis, they may be applicable for B. anthracis. Importantly, despite unexpected beneficial effects when cell wall was combined with LeTx, understanding this interaction may provide insights both into the pathogenic effects of LeTx as well as the treatment of anthrax sepsis itself.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s00134-009-1643-9) contains supplementary material, which is available to authorized users.
Contributor Information
Xizhong Cui, Critical Care Medicine Department, Clinical Center, National Institutes of Health, Building 10, Room 2C148, Bethesda, MD 20892, USA.
Junwu Su, Critical Care Medicine Department, Clinical Center, National Institutes of Health, Building 10, Room 2C148, Bethesda, MD 20892, USA.
Yan Li, Critical Care Medicine Department, Clinical Center, National Institutes of Health, Building 10, Room 2C148, Bethesda, MD 20892, USA.
Joseph Shiloach, NIDDK, National Institutes of Health, Bethesda, MD 20892, USA.
Steven Solomon, Critical Care Medicine Department, Clinical Center, National Institutes of Health, Building 10, Room 2C148, Bethesda, MD 20892, USA.
Jeanne B. Kaufman, NIDDK, National Institutes of Health, Bethesda, MD 20892, USA
Haresh Mani, Laboratory of Pathology, National Cancer Institutes, National Institutes of Health, Bethesda, MD 20892, USA.
Yvonne Fitz, Critical Care Medicine Department, Clinical Center, National Institutes of Health, Building 10, Room 2C148, Bethesda, MD 20892, USA.
Jia Weng, Critical Care Medicine Department, Clinical Center, National Institutes of Health, Building 10, Room 2C148, Bethesda, MD 20892, USA.
Laith Altaweel, Critical Care Medicine Department, Clinical Center, National Institutes of Health, Building 10, Room 2C148, Bethesda, MD 20892, USA.
Virginia Besch, Department of Anesthesiology, Clinical Center, National Institutes of Health, Bethesda, MD 20892, USA.
Peter Q. Eichacker, Critical Care Medicine Department, Clinical Center, National Institutes of Health, Building 10, Room 2C148, Bethesda, MD 20892, USA
References
- 1.Kengatharan KM, De Kimpe S, Robson C, Foster SJ, Thiemermann C. Mechanism of gram-positive shock: identification of peptidoglycan and lipoteichoic acid moieties essential in the induction of nitric oxide synthase, shock, and multiple organ failure. J Exp Med. 1998;188:305–315. doi: 10.1084/jem.188.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myhre AE, Aasen AO, Thiemermann C, Wang JE. Peptidoglycan—an endotoxin in its own right? Shock. 2006;25:227–235. doi: 10.1097/01.shk.0000191378.55274.37. [DOI] [PubMed] [Google Scholar]
- 3.Ryu YH, Baik JE, Yang JS, Kang SS, Im J, Yun CH, Kim DW, Lee K, Chung DK, Ju HR, Han SH. Differential immunostimulatory effects of gram-positive bacteria due to their lipoteichoic acids. Int Immunopharmacol. 2009;9:127–133. doi: 10.1016/j.intimp.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Popov SG, Villasmil R, Bernardi J, Grene E, Cardwell J, Popova T, Wu A, Alibek D, Bailey C, Alibek K. Effect of Bacillus anthracis lethal toxin on human peripheral blood mononuclear cells. FEBS Lett. 2002;527:211–215. doi: 10.1016/s0014-5793(02)03228-3. [DOI] [PubMed] [Google Scholar]
- 5.Triantafilou M, Uddin A, Maher S, Charalambous N, Hamm TS, Alsumaiti A, Triantafilou K. Anthrax toxin evades toll-like receptor recognition, whereas its cell wall components trigger activation via TLR2/6 heterodimers. Cell Microbiol. 2007;9:2880–2892. doi: 10.1111/j.1462-5822.2007.01003.x. [DOI] [PubMed] [Google Scholar]
- 6.Hsu LC, Ali SR, McGillivray S, Tseng PH, Mariathasan S, Humke EW, Eckmann L, Powell JJ, Nizet V, Dixit VM, Karin M. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci USA. 2008;105:7803–7808. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui X, Moayeri M, Li Y, Li X, Haley M, Fitz Y, Correa-Araujo R, Banks SM, Leppla SH, Eichacker PQ. Lethality during continuous anthrax lethal toxin infusion is associated with circulatory shock but not inflammatory cytokine or nitric oxide release in rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R699–R709. doi: 10.1152/ajpregu.00593.2003. [DOI] [PubMed] [Google Scholar]
- 8.Sherer K, Li Y, Cui X, Eichacker PQ. Lethal and edema toxins in the pathogenesis of Bacillus anthracis septic shock: implications for therapy. Am J Respir Crit Care Med. 2007;175:211–221. doi: 10.1164/rccm.200608-1239CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medvedev AE, Flo T, Ingalls RR, Golenbock DT, Teti G, Vogel SN, Espevik T. Involvement of CD14 and complement receptors CR3 and CR4 in nuclear factor-kappaB activation and TNF production induced by lipopolysaccharide and group B streptococcal cell walls. J Immunol. 1998;160:4535–4542. [PubMed] [Google Scholar]
- 10.Cui X, Li Y, Li X, Laird MW, Subramanian M, Moayeri M, Leppla SH, Fitz Y, Su J, Sherer K, Eichacker PQ. Bacillus anthracis edema and lethal toxin have different hemodynamic effects but function together to worsen shock and outcome in a rat model. J Infect Dis. 2007;195:572–580. doi: 10.1086/510856. [DOI] [PubMed] [Google Scholar]
- 11.Parent C, Eichacker PQ. Neutrophil and endothelial cell interactions in sepsis. The role of adhesion molecules. Infect Dis Clin North Am. 1999;13:427–447. doi: 10.1016/s0891-5520(05)70084-2. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am J Emerg Med. 2008;26:711–715. doi: 10.1016/j.ajem.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 13.Assreuy J. Nitric oxide and cardiovascular dysfunction in sepsis. Endocr Metab Immune Disord Drug Targets. 2006;6:165–173. doi: 10.2174/187153006777442314. [DOI] [PubMed] [Google Scholar]
- 14.Aldridge AJ. Role of the neutrophil in septic shock and the adult respiratory distress syndrome. Eur J Surg. 2002;168:204–214. doi: 10.1080/11024150260102807. [DOI] [PubMed] [Google Scholar]
- 15.Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27:337–349. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- 16.Albrecht MT, Li H, Williamson ED, LeButt CS, Flick-Smith HC, Quinn CP, Westra H, Galloway D, Mateczun A, Goldman S, Groen H, Baillie LW. Human monoclonal antibodies against anthrax lethal factor and protective antigen act independently protect against Bacillus anthracis infection and enhance endogenous immunity to anthrax. Infect Immun. 2007;75:5425–5433. doi: 10.1128/IAI.00261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui X, Li Y, Moayeri M, Choi GH, Subramanian GM, Li X, Haley M, Fitz Y, Feng J, Banks SM, Leppla SH, Eichacker PQ. Late treatment with a protective antigen-directed monoclonal antibody improves hemodynamic function and survival in a lethal toxin-infused rat model of anthrax sepsis. J Infect Dis. 2005;191:422–434. doi: 10.1086/427189. [DOI] [PubMed] [Google Scholar]
- 18.Moayeri M, Haines D, Young HA, Leppla SH. Bacillus anthracis lethal toxin induces TNF-alpha-independent hypoxia-mediated toxicity in mice. J Clin Invest. 2003;112:670–682. doi: 10.1172/JCI17991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stearns-Kurosawa DJ, Lupu F, Taylor FB, Jr, Kinasewitz G, Kurosawa S. Sepsis and pathophysiology of anthrax in a nonhuman primate model. Am J Pathol. 2006;169:433–444. doi: 10.2353/ajpath.2006.051330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith H, Keppie J, Stanley JL. Observations on the cause of death in experimental anthrax. Lancet. 1954;267:474–476. doi: 10.1016/s0140-6736(54)91881-4. [DOI] [PubMed] [Google Scholar]
- 21.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 22.Mesnage S, Fouet A. Plasmid-encoded autolysin in Bacillus anthracis: modular structure and catalytic properties. J Bacteriol. 2002;184:331–334. doi: 10.1128/JB.184.1.331-334.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guarner J, Jernigan JA, Shieh WJ, Tatti K, Flannagan LM, Stephens DS, Popovic T, Ashford DA, Perkins BA, Zaki SR. Pathology and pathogenesis of bioterrorism-related inhalational anthrax. Am J Pathol. 2003;163:701–709. doi: 10.1016/S0002-9440(10)63697-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holty JE, Bravata DM, Liu H, Olshen RA, McDonald KM, Owens DK. Systematic review: a century of inhalational anthrax cases from 1900 to 2005. Ann Intern Med. 2006;144:270–280. doi: 10.7326/0003-4819-144-4-200602210-00009. [DOI] [PubMed] [Google Scholar]
- 25.Quintiliani R, Jr, Quintiliani R. Inhalational anthrax and bioterrorism. Curr Opin Pulm Med. 2003;9:221–226. doi: 10.1097/00063198-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Martin J Edward N, Nathan Barnett R, Scheld William M. Lipoteichoic acid (LTA) decreases anthrax lethal toxin (LeTx) induced mortality in C57bl/6 mice; Programs and abstracts of the annual meeting of IDSA; 2003. p. 150. [Google Scholar]