To the Editor
The serum neutral mast cell protease tryptase is widely used as a marker of mast cell activation and clonal expansion. An increase in serum tryptase values above normal baseline measurements can be used to document an episode of systemic anaphylaxis. An increase in tryptase values that persistently exceeds 20 ng/mL in the absence of systemic allergic reactions constitutes a minor diagnostic criteria for mastocytosis. Normal reference values for serum mast cell tryptase have been established in the healthy adult population and range from 1 to 15 ng/mL, with an average of approximately 5 ng/mL,1 and serum tryptase values between atopic and nonatopic adults are not different.2 However, no comparable reference standards for children were found by using an all-inclusive PubMed search for articles published in English on serum tryptase values.
The aims of this study were thus to establish normal serum tryptase values in the pediatric population, to determine whether there are any differences in tryptase values in atopic versus nonatopic children, and to analyze tryptase values based on sex, race, ethnicity, total IgE level, weight, weight percentile, and dermatitis status.
In pursuit of these goals, we determined the tryptase values in 197 consecutive children (age range, 6 months to 18 years) presenting to the Pediatric Allergy Clinic at the National Institutes of Health, Bethesda, Maryland, for evaluation of allergic symptoms between July 2005 and August 2008. The patients were categorized as either nonatopic (n = 44) or atopic (n = 153), according to the presence of allergic symptoms and a tendency to produce IgE antibodies, as indicated by increased total IgE levels, specific IgE testing (ImmunoCAP; Pharmacia, Uppsala, Sweden), or cutaneous prick testing to commonly encountered environmental allergens.3 None of the patients had a documented history of Hymenoptera venom allergy or a concurrent illness that would cause an increase in tryptase values nor could we identify a confounding effect of therapies used on tryptase values.
Serum tryptase values were obtained at the initial visit and determined by using a commercial fluoroenzyme immunoassay (Pharmacia ImmunoCAP 100) with a detection range of 1 to 200 ng/mL (undiluted) as performed by Mayo Medical Labs, Rochester, Minnesota.
Statistical analysis used the Wilcoxon rank sum test, the Kruskal-Wallis test, and the Spearman correlation coefficient. For the atopic and nonatopic groups, 95% prediction intervals of tryptase values were estimated, assuming a log-normal distribution.
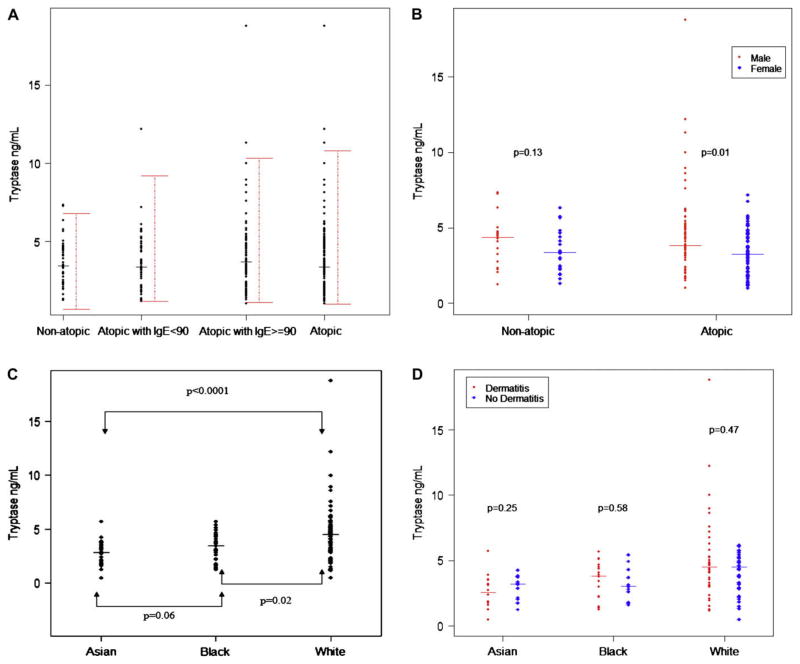
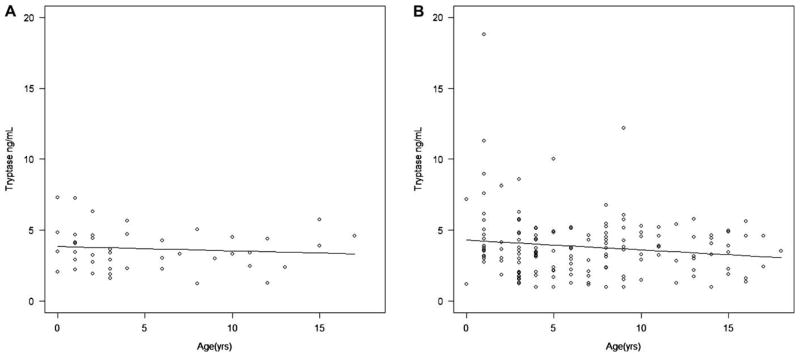
Fig 1, A, shows the 95% prediction intervals and median of tryptase values. There was no statistically significant difference between nonatopic subjects and atopic subjects (median, 3.44 vs 3.56 ng/mL; P = .93; 95% prediction intervals, 0.64–6.77 and 0.98–10.80, respectively). Because these data were not normally distributed, nonparametric statistical analysis was performed based on the median (Wilcoxon rank sum test). The mean and SD, however are shown in Table I in comparison with the adult data from Schwartz et al,2 and indicate that although the Richmond adult data with a mean of 4.9 ng/mL diverges somewhat from the National Institutes of Health pediatric data, there is clear overlap with the German adult data (mean of 3.5 ng/mL for atopic subjects and 3.8 ng/mL for nonatopic subjects). When the atopic subjects were subdivided into those with normal (<90 IU/mL; median, 3.33 ng/mL) and increased (>90 IU/mL; median, 3.68 ng/mL) total IgE levels, similar prediction intervals were obtained. When evaluating sex differences in tryptase values, a statistically significantly higher value of tryptase was observed in male than in female subjects within the atopic group (P = .01; Fig 1, B), and a similar pattern was observed in the non-atopic group; lack of statistical significance might be due to smaller sample size. In addition, among the atopic subjects, there was a statistically significant difference in tryptase values among the racial subgroups of white, black, and Asian (P < .001, Kruskal-Wallis rank sum test; Fig 1, C). The white subgroup had significantly higher tryptase values than both the Asian and black subgroups (P < .001 and P = .02, respectively). Among the atopic subjects, no statistically significant associations with tryptase were observed for dermatitis status (Fig 1, D), Hispanic/Latino versus not Hispanic/Latino ethnicity, age, IgE levels, weight, and weight percentiles. There was a slight but statistically insignificant trend toward lower tryptase values with increasing age in the atopic group but not in the nonatopic group (Fig 2).
FIG 1.
Analysis of tryptase values. A, Serum tryptase values in pediatric patients showing 95% prediction intervals and median in nonatopic subjects, atopic subjects, and atopic subjects with normal (<90 IU/mL) and increased (>90 IU/mL) total IgE levels. B–D, Comparison of tryptase values in male subjects (solid circles) and female subjects (solid diamonds; Fig 1, B); the atopic group according to race (Fig 1, C), and in those with (solid circles) and without (solid diamonds) active dermatitis (Fig 1, D).
TABLE I.
Comparison of serum tryptase values in children and adults
| Study | Atopic status | Sample size | Mean | SD | Median |
|---|---|---|---|---|---|
| NIH pediatric | Nonatopic | 44 | 3.7 | 1.5 | 3.44 |
| Atopic | 153 | 3.8 | 2.3 | 3.56 | |
| Germany adults2 | Nonatopic | 19 | 3.8 | 2.8 | — |
| Atopic | 62 | 3.5 | 1.6 | — | |
| Richmond adults2 | Nonatopic | 56 | 4.9 | 2.3 | — |
Values are presented in nanograms per milliliter.
NIH, National Institutes of Health.
FIG 2.
Tryptase values versus age with trend line in nonatopic (A) and atopic (B) subjects.
We observed a statistically significant increase in tryptase values in the atopic group among male subjects, yet in an adult nonatopic population there is a report of increased tryptase values among 109 healthy female subjects.4 This difference might be related to the age of the patients (pediatric vs adults), atopic versus nonatopic status, or other factors.
We have shown statistically significant variability among racial groups. Ethnic variability has been shown in patients with genetic α-tryptase deficiency,5 which highlights a possible genetic predisposition that might relate to our findings. However, it is unclear whether the difference in baseline serum tryptase values among the racial subgroups reflects the difference in mast cell burden or is due to other unknown factors.
Although patients with atopic dermatitis tend to have increased IgE levels,6 by using Spearman correlation coefficients, we did not find a statistically significant correlation between IgE and tryptase values in the nonatopic or atopic groups. We did not find a statistically significant correlation between age and tryptase values, although higher tryptase values (>5 ng/mL) tended to occur among younger children (<5 years old).
Of the 197 patients tested, 7 were considered to be high outliers (tryptase, >8 ng/mL), as defined by greater than 1.5 times the interquartile range above the third quartile. All of these patients were atopic, had active eczema, and were male. Six had documented food allergy, and 5 were white. None of these patients had a history of Hymenoptera allergy, recent anaphylaxis, or a rheumatologic disorder, and IgA levels measured in all of these patients were within normal limits. The probability of interference by heterophilic antibodies, which has been reported to result in a false increase in tryptase values,7 is low given the history of atopic disease, relatively low increase in tryptase values (range, 8.13–18.8 ng/mL [repeated value, 13.8 ng/mL]) and lack of IgA deficiency in these patients, which has been associated with heterophilic antibodies.8 The significance of these findings is unknown.
In summary, this study presents data that support the conclusion that tryptase values in the pediatric population are comparable with those reported in the adult population. There was no statistically significant difference in baseline serum tryptase levels between atopic and nonatopic children, which is in common with the adult study of 62 atopic and 19 nonatopic subjects from Germany.2 Among the atopic children, there was a statistically significant association of tryptase values with sex and race but not with ethnicity, age, total IgE level, weight, weight percentile, or dermatitis status.
Acknowledgments
Supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
References
- 1.Schwartz LB. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006;26:451–63. doi: 10.1016/j.iac.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz LB, Bradford TR, Rouse C, Irani AM, Rasp G, Van der Zwan JK, et al. Development of a new, more sensitive immunoassay for human tryptase: use in systemic anaphylaxis. J Clin Immunol. 1994;14:190–204. doi: 10.1007/BF01533368. [DOI] [PubMed] [Google Scholar]
- 3.Kay AB. Allergy and allergic diseases. First of two parts. N Engl J Med. 2001;344:30–7. doi: 10.1056/NEJM200101043440106. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz LB, Min HK, Ren S, Xia HZ, Hu J, Zhao W, et al. Tryptase precursors are preferentially and spontaneously released, whereas mature tryptase is retained by HMC-1 cells, Mono-Mac-6 cells, and human skin-derived mast cells. J Immunol. 2003;170:5667–73. doi: 10.4049/jimmunol.170.11.5667. [DOI] [PubMed] [Google Scholar]
- 5.Soto D, Malmsten C, Blount JL, Muilenburg DJ, Caughey GH. Genetic deficiency of human mast cell alpha-tryptase. Clin Exp Allergy. 2002;32:1000–6. doi: 10.1046/j.1365-2222.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 6.Laske N, Niggemann B. Does the severity of atopic dermatitis correlate with serum IgE levels? Pediatr Allergy Immunol. 2004;15:86–8. doi: 10.1046/j.0905-6157.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 7.van Toorenenbergen AW, van Daele PL, Boonstra JG. False-elevated serum tryptase assay result caused by heterophilic antibodies. J Allergy Clin Immunol. 2005;116:1159–60. doi: 10.1016/j.jaci.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Knight AK, Bingemann T, Cole L, Cunningham-Rundles C. Frequent false positive beta human chorionic gonadotropin tests in immunoglobulin A deficiency. Clin Exp Immunol. 2005;141:333–7. doi: 10.1111/j.1365-2249.2005.02837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]