Abstract
Background
Poor healing after mandibular fracture (Mfx) repair can be a devastating morbidity with significant clinical consequences. Elucidating the vascular response after Mfx may help determine potential areas for therapeutic interventions for non-unions. We performed Micro-Computed Tomography (μCT) imaging after vessel perfusion to ascertain objective measures of vascular networks. We hypothesize that despite the haversion based, highly cortical structure of the mandible, the vascular response after fracture healing will return to near normal levels soon after bony union, mirroring the results of endochondral, highly trabecular, long bones.
Methods
Sprague-Dawley rats (n=12) underwent mandibular osteotomy, and a 2.1 mm fixed gap was set. Animals were sacrificed at 40 days post surgery, and perfused with Microfil. Specimens underwent μCT analysis for stereological vascular metrics. Data was compared to non-fractured controls (n=5). Confidence Intervals (95%) and the independent samples t-test (p ≤ 0.05) were used to determine statistical differences.
Results
Quantitative measures for Mfx vs. Control revealed similarities in the following vascular metrics: Vessel Volume Fraction (0.028 vs. 0.032, CI = -0.027, 0.0169); Vessel Number (0.497 mm-1 vs. 0.472 mm-1, CI = -0.884, 0.975); Vessel Thickness (0.052 mm vs. 0.067 mm, CI = -0.037, 0.008) and Vessel Separation (2.344 mm vs. 2.081 mm, CI = -0.752, 1.278).
Conclusions
To our knowledge this is the first study utilizing μCT after perfusion to analyze vascular response following Mfx. Our findings establish quantitative similarities and qualitative differences in vascular response to fracture repair of the highly cortical mandible, when compared to the highly trabecular long bone.
Introduction
Despite the debilitating consequences and cost of managing delayed healing and non-union after mandibular fracture (Mfx) repair, the vascular response in these scenarios is poorly understood. Though the importance of angiogenesis in fracture healing is well known, efforts to quantify the vascular response to fracture in the mandible have remained inadequate. Notwithstanding that inadequacy, an intense effort to elucidate the mechanisms of angiogenesis after Mfx has ensued. Further, as the molecular mechanisms are revealed, methods for observing and quantifying how these mediators relate to the actual vascular anatomy in the mandible remain rudimentary. What is certain is that the vascular system plays an integral role from the moment of fracture with the formation of a hematoma to the delivery of inflammatory mediators, extracellular matrix proteins, and growth factors to the site of the fracture and to the eventual involvement in remodeling of the bony architecture.1
Better insight is needed to understand the specific mechanisms surrounding vascularity in the mandible after fracture. Two confounding variables exist in the literature. First is the reliance of the craniofacial literature on findings in long bones, second, is the paucity of objective outcome measures to quantify mandibular vascularity at a given point in time.
The endochondral literature has shed considerable light on the temporal aspects of the vascular response after fracture with experiments utilizing quantitative measures of vascular flow such as microsphere injection and quantitative Scintigraphy. What has been observed is a peak in blood flow occurring between 7-14 days after fracture, followed by a prolonged remodeling period before a return to near pre-fracture flow rates.2,3,4,5These findings have correlated with innovatively designed studies investigating molecular angiogenic mediators and their relationship to endochondral fracture healing. The peak levels of these angiogenic mediators correlated with the peak rates of blood flow and were noted to coincide with the time of cartilage resorption, thus plausibly linking the two events. 6,7 Further, this peak angiogenic effect has also been quantified via μCT after vessel perfusion at the 14-day mark, though not after complete fracture healing.8
Despite the extensive work that has been completed regarding the endochondral bone vascular response after fracture, simple extrapolation of this data to the mandible without direct investigation is problematic. We believe the unique structural and functional complexity of the mandible dictates an effort to more clearly study its biological phenomenon as a separate entity.
The mandible is a flat membranous bone that is predominantly cortical and highly dependent on a haversion based vascular network. These characteristics are in contradistinction to the tubular, predominantly trabecular structure of long bones which principally gets its blood supply from a medullary system. Despite these differences it is our hypothesis that the underlying reparative processes of both long bones and the mandible are quite similar and therefore will ultimately result in an analogous vascular response to fracture healing.
Previous work in our laboratory has allowed us to generate quantitative data utilizing μ-CT after vessel perfusion.9 This is a highly accurate, non-destructive, rapid, assessment of vascular network microarchitecture dependant on automated computer algorithms capable of accurately assessing differences in vessel stereology, volume and projection.
Although microsphere injection and Scintigraphy have been the mainstay techniques for the studies addressing vascular response, they are primarily measurements of flow and are not specifically designed to focus on quantitative stereological analysis of vascular networks. In addition, those former techniques are typically suited for larger animal models and make the examination of murine mandibular vascular networks impractical.
Here, we perform an in-depth vascular analysis of Mfx in a murine model utilizing μCT after vessel perfusion. Our aim is to objectively quantify the vascular response to Mfx in order to allow for future experimentation regarding the optimization of fracture repair in the cases of delayed healing as well as non-union.
Materials and Methods
Preoperative Animal Care
Adult male Sprague-Dawley rats weighing approximately 400 g were paired in cages and maintained in a pathogen-free environment on a 12-hour light/dark schedule. Rats were fed standard hard chow and water ad libitum during a seven day acclimation period prior to surgery. All animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Michigan Animal Care and Use Committee.
Surgical Procedure and Device Placement
Male Sprague-Dawley rats (n=14) underwent placement of external mandibular fixator devices followed by surgical osteotomy as previously described.10 Briefly, animals received preoperative subcutaneous injections of Gentamycin (5 mg/kg), Buprenorphine (0.03 mg/kg) and Lactated Ringer’s solution (25cc/kg) prior to surgery. Anesthesia was achieved with the inhalation of isoflurane. After induction of anesthesia, the animals were placed in the supine position, and prepped and draped in the normal sterile fashion. A 2-cm midline incision was placed ventrally from the anterior submentum to the neck crease. After pre-drilling, a stainless steel threaded rod was inserted horizontally across the anterior mandible, with the ends brought externally through the skin, creating the anterior portion of our modified external fixator. A 1-cm incision was made through the masseter, down to and in-line with the inferior border of the mandible, just 3mm anterior to the angle. After pre-drilling, bilateral threaded stainless steel pins were inserted buccal-to-lingual, and then secured with our custom titanium washer and nut. The pin ends were brought externally through the skin for the posterior fixator placement with titanium caps. The right side was fixed rigidly, whereas the left side received a fixator screw for postoperative manipulation. A vertical osteotomy on the left hemimandible was created using a 10 mm micro-reciprocating blade attached to a power saw (Stryker, Portage, Mich.). The osteotomy extended from the inferior mandibular border superiorly to the sigmoid notch along the anterior aspect of the coronoid process. The osteotomy edges were reduced with the fixator. The wound was irrigated, hemostasis verified, tissues approximated and closed in layers. Four hours after surgery a fixed 2 mm fracture gap was set using the fixator device (Figure 1).
Figure 1.
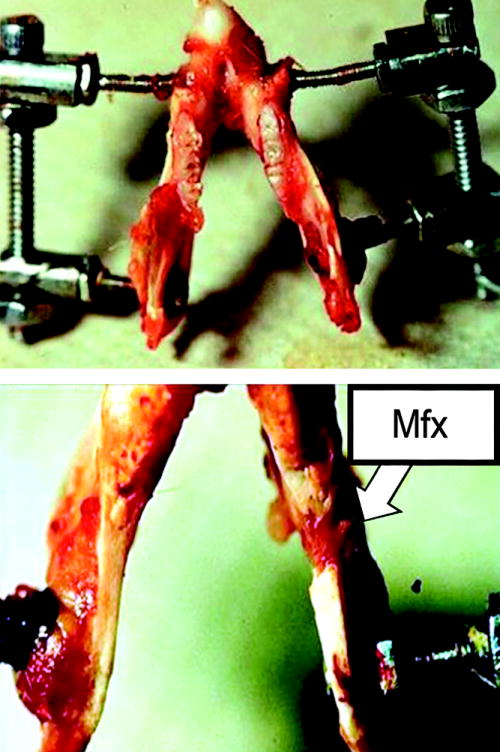
a.) Custom external fixator device on a dissected rat mandible- superior view. b.) Close-up inferior view showing healed fracture of the left hemi-mandible.
Postoperative Animal Care
Animals were housed one per cage and fed moist chow with Hill’s high-calorie diet (Columbus Serum, Columbus, Ohio) and water at libitum. Two postoperative doses of Gentamycin (5 mg/kg subcutaneously every 12 hours) were given as well as continuation of Buprenorphine (0.03 mg/kg) and Lactated Ringer’s solution (10cc) subcutaneously every 12 hours through postoperative day 4 and as needed thereafter. Weights were monitored daily and diets adjusted as needed. Pin care was performed with Silvadene (Monarch Pharmaceuticals, Inc., Bristol, Tenn.) every other day. Animals were allowed to complete a 40-day recovery period prior to perfusion and sacrifice.
Perfusion Protocol
Rats were anesthetized prior to thoracotomy and underwent left ventricular catheterization. Perfusion with heparinized normal saline followed by pressure fixation with normal buffered formalin solution ensued and ensured euthanasia. After fixation, the vasculature was injected with Microfil (MV-122, Flow Tech, Carver, Mass.). Mandibles were harvested and demineralized using Cal-Ex II solution (Fisher Scientifics; Fairlawn, NJ). Leeching of mineral was confirmed with serial radiographs to ensure adequate demineralization prior to scanning.
Micro-Computed Tomography
Specimens were scanned at 18 μm voxel size with μCT. The region of interest was defined as a distance measuring 2 mm after the third molar corresponding to the surgical site of the osteotomy and set gap distance. Analysis of the left hemimandible region of interest was then performed with MicroView 2.2 software (GE Healthcare, Milwaukee, Wis.). Contours were defined highlighting the region of interest using the spline tool in MicroView. Analysis of vascularity in this region is accomplished by setting a global grayscale threshold of 1000 to differentiate vessels from surrounding tissue as previously described.11 Using this information, MicroView allocates only the number of voxels above this threshold and uses stereologic algorithms to assign relative densities to the voxels based on the contrast content within the vessels. The region of interest was analyzed for vessel volume fraction, number, thickness and separation. The data was then compared to an established non-fractured control (n=5) that underwent identical housing and animal care without being subjected to osteotomy surgery or device placement.9 In addition, for qualitative comparison, visualization of vascular anatomy was accomplished utilizing maximal intensity projection (MIP) imaging. This enables instant volume rendering of a volumetric data set yielding a three dimensional representation of the scanned specimen.
Statistical Analysis
The independent samples t test was used to analyze differences between group means (SPSS version 16.0; SPSS, Inc., Chicago, Ill.). Vessel volume fraction, number, thickness, and separation were compared between groups and reported as their respective means with confidence intervals for differences between the means reported at the 95th percentile.
Results
All animals in the Mfx group tolerated the osteotomy procedure without incident. Gross union of the left hemi-mandibles was verified upon harvest prior to μCT scanning. Two animals were excluded from the study due to technical difficulties resulting in poor perfusion confirmed via μCT in the Mfx group.
Qualitative Assessments
MIP’s are shown demonstrating 3-dimensional representations of the vascular networks. For orientation purposes, a schematic is shown outlining the figure of the normal left hemi-mandible with the region of interest inverted for visual comparison (Figure 2). Appraisal of mandibular vascular networks revealed the following: Control group vascularity characteristically demonstrated a clearly visualized inferior alveolar artery coursing through the region of interest with major branches supplying the mandibular body, alveolar regions as well as the incisor root. Notably, the adjacent periosteal circulations, largely stemming from the coronoid, condylar and angular processes, did not appear to contribute largely to the region of interest prior to fracture (Figure 3). In contrast, after Mfx, our findings indicate a large periosteal vascular component emanating from these adjacent surrounding vascular networks towards the fracture site (Figure 4). Interestingly, the reestablishment of the nutrient inferior alveolar artery demonstrated great variability between Mfx specimens (Figure 5). Despite this finding, union still ensued in all Mfx cases.
Figure 2.
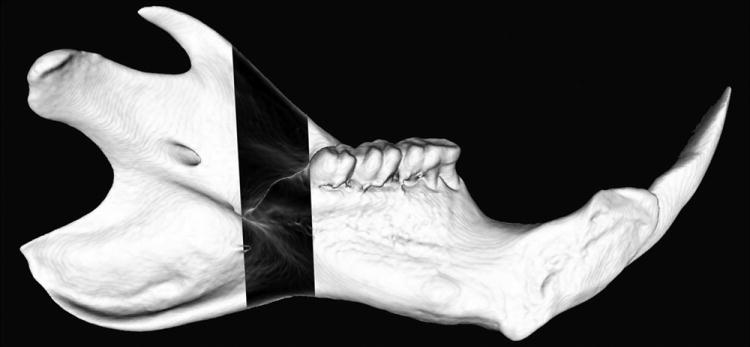
Schematic outlining the figure of the normal left hemi-mandible with the region of interest inverted for visual comparison.
Figure 3.

Control- Maximal Intensity Projection image after vessel perfusion.
Figure 4.

Mfx- Maximal Intensity Projection image after vessel perfusion.
Figure 5.
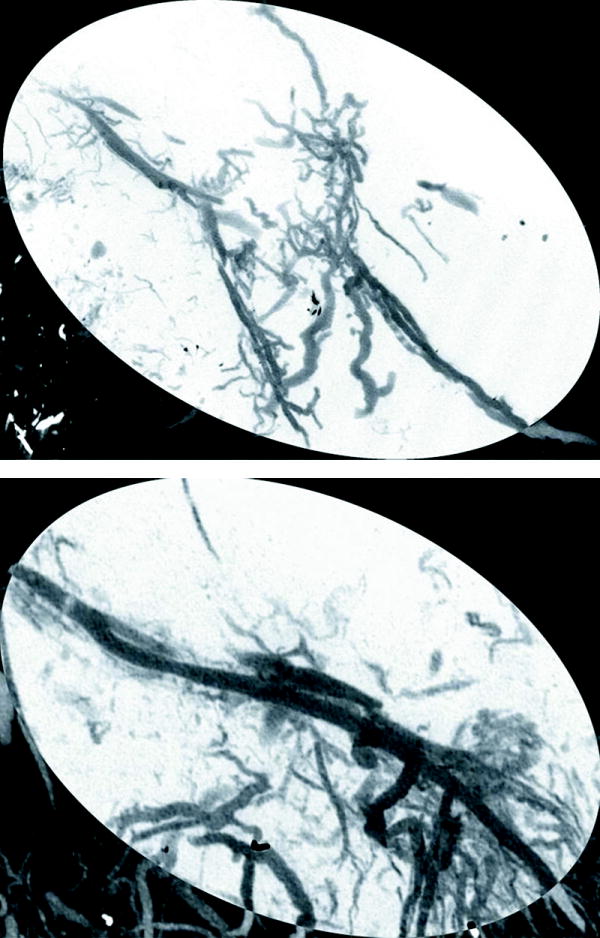
Maximal Intensity Projections demonstrating variable reconstitution of the nutrient inferior alveolar artery in two Mfx specimens. a.) Non-reconstituted vs. b.) Reconstituted.
Quantitative Assessments
Stereological analysis demonstrated no statistically significant differences in the Mfx vasculature when compared to control animals. Our quantitative measures for Mfx vs. Control were as follows: (Table 1)
Table 1.
Quantitative Stereological Analysis of Vascularity in Mfx (n=12) vs. Control (n=5).
| Mfx vs. Control μCT Vascularity Metrics | |||||
|---|---|---|---|---|---|
| Measurement | Means (Mfx vs. Control) | SD (Mfx, Control) | Δ in Means | 95% CI | p |
| VVF | 2.8% vs. 3.2% | 2.2%, 0.8% | 0.4% | -2.7, 1.7 | 0.644 |
| VN | 0.497 mm-1 vs. 0.472 mm-1 | 0.221 mm-1, 0.064 mm-1 | 0.025 mm-1 | -0.884, 0.975 | 0.808 |
| VT | 0.052 mm vs. 0.067 mm | 0.021 mm, 0.067 mm | 0.029 mm | -0.037, 0.008 | 0.191 |
| VS | 2.344 mm vs. 2.081 mm | 1.03 mm, .281 mm | 0.26mm | -0.752, 1.278 | 0.589 |
VVF=Vessel Volume Fraction, VN=Vessel Number, VT=Vessel Thickness, and VS= Vessel Separation.
Our results indicate that these two groups are quantitatively similar with regards to vascularity at 40 days post surgery. Though it is not possible to prove equality, we can reasonably quantify the boundaries of similarity with some level of accuracy utilizing confidence intervals for differences between the means giving us a valuable metric for future comparison.12,13
Discussion
Mfx and its subsequent surgical repair impose a metabolic toll on the reparative process. This quality can be most apparent when the metabolic demand is exceeded and costly complications ensue. The most common complications cited after Mfx repair are aberrant wound healing, infections, and malunions.14, 15 Though it is commonly known that the reparative response of the vascular system is critically important to successful Mfx repair, it has not been clearly quantified. The inquiry of avoidance of Mfx complications has centered on the aspects of: Utility of prophylactic antibiotics, timing of repair, location of fracture, and hardware selection preference.14,15,16,17 Due to the global involvement of the vascular system in all aspects of fracture pathology and repair, an effort to more clearly observe its involvement in these scenarios was warranted.
Previous efforts examining mandibular vascularity have succeeded in characterizing the complex vascular anatomy of the mandible.18, 19 Despite this, little is known about the mandibular vascular response after fracture. This paucity of information has led to discontinuance in knowledge and a reliance on the examination of the endochondral response to fracture to draw scientific conclusions about what is happening in the mandible.
Our aim was to begin to fill this gap in knowledge utilizing the most accurate methods available today, thus establishing a valuable body of evidence for the continuation of experimentation based on quantifiable data in the mandible.
We have succeeded in demonstrating quantitative as well as qualitative evaluations of the vascular response after Mfx utilizing μCT with vessel perfusion. This powerful analytic technique was able to directly highlight changes in vessel number, vessel volume, and vascular density resulting from Mfx and its subsequent repair. In addition, this valuable investigative tool provided 3-dimensional angiographic images clearly delineating the vascular supply of the murine mandible both before and after fracture healing.
As hypothesized we have established a quantitative similarity in vascular response to fracture repair in the murine mandible in comparison to those reported in long bones. Furthermore, our findings corroborate our suspicion that the structural differences of long bones and flat bones as well as their strikingly dissimilar anatomic vascular networks were of significantly less consequence than the overwhelming similarities inherent in the reparative processes of fracture healing.
Our findings using MIP imaging indicate variable reconstitution of the nutrient inferior alveolar artery with a consistent, large periosteal contribution from surrounding vascular networks. Regardless of the variable reestablishment of the nutrient artery, however, a consistent union still ensued at our fracture site in all cases.
Rheinlander’s classic work eloquently characterized the qualitative aspects of the vascular response in the long bone diaphysis after fracture.20 He has described a reparative process highly dependent on the medullary circulation with only an initial contribution from the periosteal vascular component. Reportedly, displaced fractures with significant interruption of the medullary blood supply characteristically demonstrated delayed-union. In our findings, however, gross union was routinely evidenced regardless of reconstitution of the medullary blood supply, thus highlighting a unique difference between the healing processes of highly trabecular long bone in comparison to the predominantly cortical mandible.
Our findings of variability in the anatomic microcirculation but constancy in the quantitative metrics of vascularity may suggest that though the volume of vasculature remains the same, the source of vascular supply changes as contributions from surrounding vascular networks are recruited to assist in the repair process, and persist even after Mfx healing. Further, we have quantified the amount of vascularity characteristically observed to consistently deliver the metabolic demands required for successful Mfx union regardless of the source of vascular supply.
Conclusions
For the first time we have quantified the mandibular vascular response to fracture utilizing μCT after vessel perfusion as an accurate method for generating valuable objective stereologic outcomes. Observed similarities in the quantity of vascularity, but qualitative differences regarding the sources of vascular networks supplying a fracture site are reported. Our findings suggest that vascularity after successful fracture repair returns to a baseline pre-fracture level. Despite this, however, the source of vascular networks supplying the fracture site may change considerably. These results highlight notable similarities and distinctions existing between the well-characterized long bone, and the mandible.
Our results can now serve as a quantitative comparison for future experimentation regarding optimization of Mfx repair. Future studies will consider therapeutic interventions to maintain or enhance this observed reparative level of vascularity after mandibular fracture in various complex clinical scenarios such as vessel injury, delayed healing, and non-union in the mandible.
Acknowledgments
Funding was provided by National Institutes of Health grant RO1 CA 12587-01 to Steven R. Buchman, M.D. The authors thank Elizabeth R. Razdolsky, Aria J. Zehtabzadeh, and Melody Merati for technical assistance during surgery, animal care, and Micro–Computed Tomographic analysis.
Funding supported by: NIH RO1 #CA12587-01 “Optimization of Bone Regeneration in the Irradiated Mandible” to PI: Steven R. Buchman, MD.
Footnotes
Accepted for presentation at the American Society of Plastic Surgeons meeting, October 2010 in Toronto, Canada.
No commercial association or financial interests exist by any author.
Contributor Information
Alexis Donneys, University of Michigan Department of Plastic Surgery, Ann Arbor, MI.
Catherine N. Tchanque-Fossuo, University of Michigan Department of Plastic Surgery, Ann Arbor, MI.
Aaron S. Farberg, University of Michigan Medical School, Ann Arbor, MI.
Xi L. Jing, Henry Ford Health System, Detroit, MI.
Sagar S. Deshpande, University of Michigan, Ann Arbor, MI
Steven A. Goldstein, University of Michigan, Department of Orthopaedic Surgery, Ann Arbor, MI.
Steven R. Buchman, University of Michigan Department of Plastic Surgery, Ann Arbor, MI.
References
- 1.Diegelmann R, Evans M. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. Available at: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.116.3683&rep=rep1&type=pdf. [DOI] [PubMed] [Google Scholar]
- 2.Aronson J. Temporal and Spatial Increases in Blood Flow During Distraction Osteogenesis. Clinical orthopaedics and related research. 1994;(301):124–31. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8156663. [PubMed]
- 3.Williams E, Rand J, An K, Chao E, Kelly P. The early healing of tibial osteotomies stabilized by one-plane or two-plane external fixation. J Bone Joint Surg Am. 1987;69(3):355–365. Available at: http://www.ejbjs.org/cgi/content/abstract/69/3/355. [PubMed] [Google Scholar]
- 4.Wu J, Shyr H, Chao E, Kelly P. Comparison of osteotomy healing under external fixation devices with different stiffness characteristics. J Bone Joint Surg Am. 1984;66(8):1258–1264. Available at: http://www.ejbjs.org/cgi/content/abstract/66/8/1258. [PubMed] [Google Scholar]
- 5.Hughes S, Khan R, Davies R, Lavender P. The uptake by the canine tibia of the bone-scanning agent 99mTc-MDP before and after an osteotomy. J Bone Joint Surg Br. 1978;60-B(4):579–582. doi: 10.1302/0301-620X.60B4.711811. Available at: http://web.jbjs.org.uk/cgi/content/abstract/60-B/4/579. [DOI] [PubMed] [Google Scholar]
- 6.Duvall CL, Taylor WR, Weiss D, Wojtowicz AM, Guldberg RE. Impaired Angiogenesis, Early Callus Formation, and Late Stage Remodeling in Fracture Healing of Osteopontin-Deficient Mice. Journal of Bone and Mineral Research. 2007;22(2):286–297. doi: 10.1359/jbmr.061103. [DOI] [PubMed] [Google Scholar]
- 7.AI-Aql Z, Alagl a, Graves D, Gerstenfeld L, Einhorn T. Molecular Mechanisms Controlling Bone Formation during Fracture Healing and Distraction Osteogenesis. Journal of Dental Research. 2008;87(2):107–118. doi: 10.1177/154405910808700215. Available at: http://jdr.sagepub.com/cgi/doi/10.1177/154405910808700215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen X, Wan C, Ramaswamy G, et al. Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2009;27(10):1298–305. doi: 10.1002/jor.20886. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19338032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jing XL, Farberg AS, Monson L, et al. Radiomorphometric Quantitative Analysis of Vasculature in Rat Mandible Utilizing Microcomputed Tomography. Plastic and Reconstructive Surgery. 2009;24(4S):26–27. [Google Scholar]
- 10.Buchman SR, Ignelzi MA, Jr, R C. Unique rodent model of distraction osteogenesis of the mandible. Ann Plast Surg. 2002;49(2):511–519. doi: 10.1097/00000637-200211000-00012. Available at: http://journals.lww.com/annalsplasticsurgery/Abstract/2002/11000/Unique_Rodent_Model_of_Distraction_Osteogenesis_of.12.aspx. [DOI] [PubMed] [Google Scholar]
- 11.Farberg A, Jing X, Monson L. Vascular Anatomic Mapping of the Rat Mandible Using Microcomputed Tomography (micro-CT) Plastic and Reconstructive Surgery. 2009;24(4S):103. Available at: http://journals.lww.com/plasreconsurg/Abstract/2009/10002/Vascular_Anatomic_Mapping_of_the_Rat_Mandible.124.aspx. [Google Scholar]
- 12.Aberson Chris. Interpreting Null Results: Improving Presentation and Conclusions with Confidence Intervals. JASNH. 2002;1(3):36–42. Available at: http://www.jasnh.com/pdf/Vol1-No3-art1.pdf. [Google Scholar]
- 13.Porcher R. Reporting results of orthopaedic research: confidence intervals and p values. Clinical orthopaedics and related research. 2009;467(10):2736–7. doi: 10.1007/s11999-009-0952-1. Available at: http://www.springerlink.com/content/u02ux543m50m8n28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moulton-Barret R, Rubinstein AJ, Salzhauer MA, et al. Complications of Mandibular Fractures : Annals of Plastic Surgery. Ann Plast Surg. 1998;41(3):258–263. doi: 10.1097/00000637-199809000-00006. Available at: http://journals.lww.com/annalsplasticsurgery/Abstract/1998/09000/Complications_of_Mandibular_Fractures.6.aspx. [DOI] [PubMed] [Google Scholar]
- 15.Furr AM, Schweinfurth JM, May WL. Factors Associated with Long-Term Complications after Repair of Mandibular Fractures. The Laryngoscope. 2006 Mar;116:427–430. doi: 10.1097/01.MLG.0000194844.87268.ED. Available at: http://www.metroatlantaotolaryngology.org/journal/apr06/LongTermComplications_and_Mand_Fx.pdf. [DOI] [PubMed] [Google Scholar]
- 16.Bormann K, Wild S, Gellrich N, et al. Five-year retrospective study of mandibular fractures in Freiburg, Germany: incidence, etiology, treatment, and complications. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2009;67(6):1251–5. doi: 10.1016/j.joms.2008.09.022. Available at: http://dx.doi.org/10.1016/j.joms.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 17.Seemann R, Schicho K, Wutzl A, et al. Complication rates in the operative treatment of mandibular angle fractures: a 10-year retrospective. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2010;68(3):647–50. doi: 10.1016/j.joms.2009.07.109. Available at: http://dx.doi.org/10.1016/j.joms.2009.07.109. [DOI] [PubMed] [Google Scholar]
- 18.Hamparian aM. Blood supply of the human fetal mandible. The American journal of anatomy. 1973;136(1):67–75. doi: 10.1002/aja.1001360106. Available at: http://www.ncbi.nlm.nih.gov/pubmed/4682141. [DOI] [PubMed] [Google Scholar]
- 19.Huelke DF, Castelli Wa. The blood supply of the rat mandible. The Anatomical Record. 1965;153(4):335–341. doi: 10.1002/ar.1091530402. Available at: http://doi.wiley.com/10.1002/ar.1091530402. [DOI] [PubMed] [Google Scholar]
- 20.Rhinelander FW. The normal microcirculation of diaphyseal cortex and its response to fracture. The Journal of bone and joint surgery American volume. 1968;50(4):784–800. doi: 10.2106/00004623-196850040-00016. Available at: http://www.ncbi.nlm.nih.gov/pubmed/5658563. [DOI] [PubMed] [Google Scholar]


