Abstract
A functional ubiquitin proteasome pathway (UPP) is vital for all eukaryotic cellular systems and therefore any alteration in this critical component of proteostasis machinery has rpotential pathological consequences. A proteostasis imbalance can be induced by environmental pollutants, age or genetic factors. Though the exact underlying mechanisms are unclear, a decrease in proteasome activity weakens the homeostatic cellular capacity to remove proteins that are either misfolded or need to be replenished, which favors the development of neurodegenerative, cardiac and other conformational diseases. In contrast, induction of proteasome activity is an attribute of many diseases including muscle wasting, sepsis, cachexia and uraemia. In the case of misfolded protein disorders, higher degradation of a single protein leads to the pathophysiological consequences due to the absence of functional protein. Therefore, selective proteostasis inhibition is a potential treatment strategy for misfolded protein disorders, while broad-spectrum proteasome inhibitor drugs are designed to target tumor metastasis. In contrast, for muscle wasting and neurodegeneration, the use of proteostasis-activating or modulating compounds could be more effective.
Keywords: degradation, proteasome, proteostasis, therapeutics, ubiquitin
1. Introduction
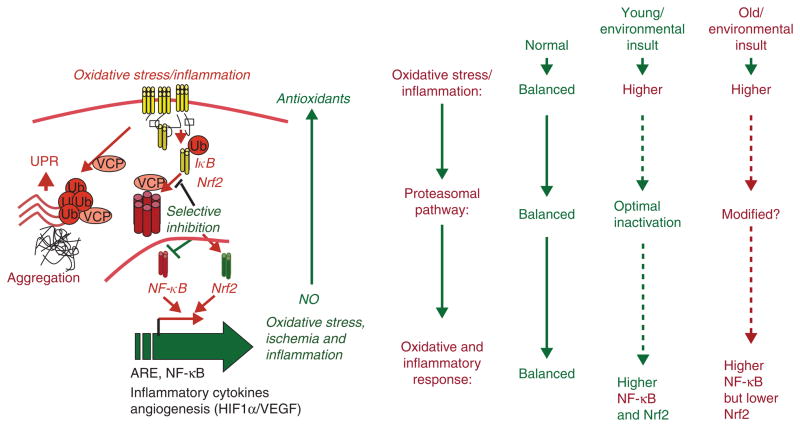
The proteostasis imbalance and its possible involvement in the development of diseases has been studied extensively but there is a clear gap in our understanding of the precise molecular mechanisms involved in this process. Environmental pollutants, age and/or genetic factors deter proteasomal activity and autophagy response by diverse mechanisms resulting in accumulation of ubiquitinated protein that induces inflammatory signaling, oxidative stress and apoptosis (Figure 1). Though the exact underlying mechanisms are unclear, decrease in proteasome activity or defective autophagy induced by variety of pathogenetic and environmental factors weakens the cellular capacity to remove misfolded or normal proteins and favors the development of neurodegenerative, cardiac and conformational diseases. In contrast, induction of proteasome activity is an attribute of diseases such as muscle wasting, sepsis, cachexia and uraemia. In addition, different individuals may have varied constitutive ability to combat proteostatic challenge. This is supported by the fact that proteasomes in various cells and tissues are not a multitude of identical 20S proteasomes but a mixture of proteasome subpopulations (standard and immuno-proteasomes) and intermediates of each subpopulation [1,2] that have different substrate specificities. In the case of misfolded protein disorders, higher degradation of single protein leads to the pathophysiological consequences due to the absence of functional protein. Therefore, selective proteostasis inhibition is a potential treatment strategy for misfolded protein disorders, while broad-spectrum proteasome inhibitor drugs like PS-341 (Bortezomib/Velcade) are designed to target tumor metastasis. In contrast, for muscle wasting and neurodegeneration, the use of proteostasis-activating or modulating compounds could be more effective.
Figure 1. Schematic on the role of ubiquitin proteasome pathway (UPP) imbalance in chronic diseases.
Under normal conditions, optimal activation of UPP balances Nrf2 and IκB (endogenous inhibitor of NF-κB) levels so that there is no induction of antioxidant and inflammatory pathways. While in a normal healthy young person, exposure to infection, cigarette smoke or injury induces oxidative stress that results in optimal activation of proteasomal pathway via Nrf2 resulting in increased NF-κB-mediated stress response that is balanced by induction of protective Nrf2 response. Notably, the UPP modification (inherent and/or environmental insult or age-related change) induces degradation of both IκB and Nrf2. The lack of compensatory antioxidant gene activation and chronic NF-κB activation, thus resulting in persistent oxidative stress and chronic inflammation. The inability to regulate protein degradation machinery and aberrant-autophagy results in fatal disease.
ARE: Antioxidant response element; HIF: Hypoxia-inducible factor; Nrf2: Nuclear factor erythroid 2-related factor 2; Ub: Ubiquitin; UPR: Ubiquitin proteasome response; VCP: Valosin containing protein; VEGF: Vascular endothelial growth factor.
2. Proteostasis-imbalance in chronic diseases
Recent studies indicate that the unfolded protein response (UPR) plays an important homeostatic function in combating proteostasis-imbalance by regulating inflammatory and anti-oxidant responses [3,4]. The UPR activates in response to cellular stress such as injury, infection, reactive oxygen species (ROS) and environmental pollutants that impair protein folding in the lumen of the endoplasmic reticulum (ER). Activation of the UPR compensates for abnormalities in protein folding by transcriptional activation and increased expression of proteostasis machinery that includes protein chaperones, folding, translation and degradation [3–5]. It is of interest that many basic cellular processes (e.g., cell cycle regulation, apoptosis, energy metabolism, inflammation, ion transport and acute phase reactants) that depend on an adequate supply of fully functional membrane and secreted proteins are also regulated by the UPR [3,6].
As discussed above, proteostasis-imbalance can be induced by environmental pollutants, age or genetic factors. Although the role of aberrant proteasomal, autophagic and/or protease activities in pathogenesis of various chronic inflammatory diseases is well documented, the exact function of the immunoproteasome is not clear [7]. A recent review [7] discusses in detail, how age-related increases in the concentration of immunoproteasome subunits in brain and muscle tissues may reflect a state of constant inflammation or cell stress. Thus, increased concentration of immunoproteasomes in muscle of patients suffering from myofibrillar myopathy and inclusion body myositis [8], as well as in neurons of a mouse model of Huntington’s disease [9], can be regarded as a consequence of, rather than the cause of, these diseases. Alternatively, this increased concentration can have a protective function, since induction of immunoproteasome subunits by NO via cGMP/cAMP-mediated mechanisms was recently shown to occur in endothelial cells. The NO-induced synthesis of immunoproteasomes protected the cells against transferrin iron-induced oxidative stress by regulating the level of transferrin receptor [10]. Since NO regulates processes such as vasodilatation, neurotransmittance, the immune response and apoptosis, an imbalance in this mediator has many pathological consequences. A NO-dependent change in the ratio of standard to immunoproteasomes is thought to contribute to these consequences [11–14]. Similarly, treatment of SH-SY5Y neural cells with non-toxic doses (1 – 10 μM) of H2O2 induced not only the formation of oxidized proteins but also synthesis of immunoproteasome subunits (detected by western blotting and real-time RT-PCR), indicating the sensitivity of the proteasome system to react and cope with cell stress. Such an adaptation of the proteasome system was lost in senescent human fibroblasts, which displayed a decreased concentration of standard proteasome subunits but retention of the immunoproteasome subunits β1i, β2i and β5i. In contrast to confluent young fibroblasts, the concentration of immunoproteasome subunits could not be augmented by treatment with gamma interferon [15] confirming the critical affect of aging on proteasomal activity. In addition, a recent study clearly demonstrates that immunoproteasomes increase the MHC class I antigen supply for antigen presentation to maintain protein homeostasis [1]. The study also clarifies the role of immunoproteasomes in modulating protein aggregation in mammalian cells.
3. Targeting proteostasis
It is clear that primary aim while modulating the ubiquitinproteasome activity is to selectively target the proteolytic pathway. Interestingly, a recent study shows that inhibition of proteasomal activity induces the de novo synthesis of proteasomes [16] that warrants further investigation. This has potential application in disease states that are the outcome of reduced or aberrant proteasomal activity, as in aging. In support of this, treatment of endothelial cells with low, non-toxic doses of the proteasome inhibitor MG132 was found to activate an antioxidant defense program that included upregulation of eNOS, glutathione peroxidase-3, glutathione S-transferase and others, resulting in an improvement in endothelial functions [17,18]. This is not only promising in the search for treatments for patients suffering from atherosclerosis and coronary heart diseases, but also provides a strategy to prevent the pathogenesis of these diseases and other neurodegenerative conditions [19]. This is substantiated by recent studies where vascular endothelial dysfunction and neuronal death after embolic stroke were found to be restricted and terminable in animal models treated with proteasome inhibitors (PS-341 [20,21] or MLN519 [22]). PS-341 (Bortezomib/Velcade, pyrazylcarbonyl-Phe-Leu-boronate) is a FDA-approved drug for multiple myeloma [23–25] while MLN519 ((1R-[1S,4R,5S])-1-(1hydroxy-2-methylpropyl-6-oxa-2-azabi-cyclo [3.2.1] heptane-3,7-dione)) is a synthetic analog of lactacystin developed by Millennium Pharmaceuticals that is under evaluation in a Phase I clinical trial [26]. An exact knowledge of the susceptibility of the different forms of proteasomes to these and other therapeutic inhibitors will help development of optimal treatments and therapies for a wide range of diseases. Finally, aside from proteasome inhibitors or activators, proteasomes themselves could be useful as diagnostic and even prognostic markers, since the level of circulating proteasomes reflects the state of health of patients suffering from cancer and autoimmune diseases [27].
4. Expert opinion
Environmental exposure, injury, infection and age-related changes in protein processing, proteasomal activity and autophagy responses, result in accumulation of ubiquitinated protein that induces chronic inflammatory signaling, oxidative stress and apoptosis (Figure 1). Identification of critical components of protein processing that are involved in protein ubiquitination, aggregation and degradation or trafficking will lead to development of novel therapeutics for diseases caused by proteostasis-imbalance.
Footnotes
Declaration of interest
This author is supported by Cystic Fibrosis Foundation (R025-CR07 and VIJ07IO), FAMRI Young Clinical Scientist Award (YCSA), and NIH (CTSA UL RR 025005 and RHL096931) grants. The author declares no conflict of interest.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1••.Seifert U, Bialy LP, Ebstein F, et al. Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell. 2010;142:613–24. doi: 10.1016/j.cell.2010.07.036. A study demonstrating the critical role of immunoproteasomes in MHC I homeostasis, antigen presentation and protein aggregation. [DOI] [PubMed] [Google Scholar]
- 2.Zoeger A, Blau M, Egerer K, et al. Circulating proteasomes are functional and have a subtype pattern distinct from 20S proteasomes in major blood cells. Clin Chem. 2006;52:2079–86. doi: 10.1373/clinchem.2006.072496. [DOI] [PubMed] [Google Scholar]
- 3.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 4.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133–49. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 5.Zhang K, Wong HN, Song B, et al. The unfolded protein response sensor IRE1alpha is required at 2 distinct steps in B cell lymphopoiesis. J Clin Invest. 2005;115:268–81. doi: 10.1172/JCI21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–89. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 7•.Dahlmann B. Role of proteasomes in disease. BMC Biochem. 2007;8(Suppl 1):S3. doi: 10.1186/1471-2091-8-S1-S3. This paper reviews the critical role of proteasomes in pathogenesis of variety of diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrer I, Martin B, Castano JG, et al. Proteasomal expression, induction of immunoproteasome subunits, and local MHC class I presentation in myofibrillar myopathy and inclusion body myositis. J Neuropathol Exp Neurol. 2004;63:484–98. doi: 10.1093/jnen/63.5.484. [DOI] [PubMed] [Google Scholar]
- 9.Diaz-Hernandez M, Martin-Aparicio E, Avila J, et al. Enhanced induction of the immunoproteasome by interferon gamma in neurons expressing mutant Huntingtin. Neurotox Res. 2004;6:463–8. doi: 10.1007/BF03033282. [DOI] [PubMed] [Google Scholar]
- 10.Kotamraju S, Matalon S, Matsunaga T, et al. Upregulation of immunoproteasomes by nitric oxide: potential antioxidative mechanism in endothelial cells. Free Radic Biol Med. 2006;40:1034–44. doi: 10.1016/j.freeradbiomed.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 11.Klahr S. The role of nitric oxide in hypertension and renal disease progression. Nephrol Dial Transplant. 2001;16(Suppl 1):60–2. doi: 10.1093/ndt/16.suppl_1.60. [DOI] [PubMed] [Google Scholar]
- 12.Moreno-Lopez B, Gonzalez-Forero D. Nitric oxide and synaptic dynamics in the adult brain: physiopathological aspects. Rev Neurosci. 2006;17:309–57. doi: 10.1515/revneuro.2006.17.3.309. [DOI] [PubMed] [Google Scholar]
- 13.Niedbala W, Cai B, Liew FY. Role of nitric oxide in the regulation of T cell functions. Ann Rheum Dis. 2006;65(Suppl 3):iii37–40. doi: 10.1136/ard.2006.058446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas DD, Espey MG, Ridnour LA, et al. Hypoxic inducible factor 1alpha, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide. Proc Natl Acad Sci USA. 2004;101:8894–9. doi: 10.1073/pnas.0400453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stratford FL, Chondrogianni N, Trougakos IP, et al. Proteasome response to interferon-gamma is altered in senescent human fibroblasts. FEBS Lett. 2006;580:3989–94. doi: 10.1016/j.febslet.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 16••.Meiners S, Heyken D, Weller A, et al. Inhibition of proteasome activity induces concerted expression of proteasome genes and de novo formation of mammalian proteasomes. J Biol Chem. 2003;278:21517–25. doi: 10.1074/jbc.M301032200. This study highlights that inhibition of proteasome activity induces expression of proteasome subunits and formation of proteasomes, suggesting a novel application in age-related disorders. [DOI] [PubMed] [Google Scholar]
- 17•.Stangl V, Lorenz M, Meiners S, et al. Long-term up-regulation of eNOS and improvement of endothelial function by inhibition of the ubiquitin-proteasome pathway. FASEB J. 2004;18:272–9. doi: 10.1096/fj.03-0054com. This research demonstrates the application of ubiquitin–proteasome inhibition in correcting oxidative stress. [DOI] [PubMed] [Google Scholar]
- 18.Meiners S, Ludwig A, Lorenz M, et al. Nontoxic proteasome inhibition activates a protective antioxidant defense response in endothelial cells. Free Radic Biol Med. 2006;40:2232–41. doi: 10.1016/j.freeradbiomed.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Qian SB, Zhang X, Sun J, et al. mTORC1 links protein quality and quantity control by sensing chaperone availability. J Biol Chem. 2010;285:27385–95. doi: 10.1074/jbc.M110.120295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henninger N, Sicard KM, Bouley J, et al. The proteasome inhibitor VELCADE reduces infarction in rat models of focal cerebral ischemia. Neurosci Lett. 2006;398:300–5. doi: 10.1016/j.neulet.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Zhang ZG, Liu X, et al. Treatment of embolic stroke in rats with bortezomib and recombinant human tissue plasminogen activator. Thromb Haemost. 2006;95:166–73. [PubMed] [Google Scholar]
- 22.Williamson MJ, Blank JL, Bruzzese FJ, et al. Comparison of biochemical and biological effects of ML858 (salinosporamide A) and bortezomib. Mol Cancer Ther. 2006;5:3052–61. doi: 10.1158/1535-7163.MCT-06-0185. [DOI] [PubMed] [Google Scholar]
- 23••.Mitchell BS. The proteasome – an emerging therapeutic target in cancer. N Engl J Med. 2003;348:2597–8 . doi: 10.1056/NEJMp030092. This paper discusses the strategies for targeting proteasomes to control tumor metastasis. [DOI] [PubMed] [Google Scholar]
- 24.Bross PF, Kane R, Farrell AT, et al. Approval summary for bortezomib for injection in the treatment of multiple myeloma. Clin Cancer Res. 2004;10:3954–64. doi: 10.1158/1078-0432.CCR-03-0781. [DOI] [PubMed] [Google Scholar]
- 25.Kane RC, Bross PF, Farrell AT, Pazdur R. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist. 2003;8:508–13. doi: 10.1634/theoncologist.8-6-508. [DOI] [PubMed] [Google Scholar]
- 26.Shah IM, Lees KR, Pien CP, Elliott PJ. Early clinical experience with the novel proteasome inhibitor PS-519. Br J Clin Pharmacol. 2002;54:269–76. doi: 10.1046/j.1365-2125.2002.01638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Jakob C, Egerer K, Liebisch P, et al. Circulating proteasome levels are an independent prognostic factor for survival in multiple myeloma. Blood. 2007;109:2100–5. doi: 10.1182/blood-2006-04-016360. This research demonstrates the proteasome as an independent prognostic factor for survival in multiple myeloma. [DOI] [PubMed] [Google Scholar]