Abstract
Background
The purpose of this study was to establish biomechanical outcomes measures to evaluate how mandibular distraction osteogenesis affects the overall quality of bone healing in the mandible. Strength and functional integrity of the regenerate was quantitatively determined after unilateral mandibular distraction osteogenesis in comparison to the contralateral mandible, and a partially reduced fracture. We hypothesized that the breaking load, yield and stiffness of mandibular distraction osteogenesis would be significantly reduced in comparison to both the contralateral mandible and a partially reduced fracture.
Methods
Sprague-Dawley rats underwent mandibular distraction osteogenesis (n=8) or a partially reduced fracture (n=6). External fixators were secured, unilateral osteotomies created behind the 3rd molar, followed by either mandibular distraction osteogenesis: 4days latency then 0.3mm Q12hrs × 8days (5.1mm) or a partially reduced mandibular fracture: fixed gap post-operatively (2.1mm). Both groups underwent 4 weeks consolidation. The contralateral mandibles were used as controls (n = 14). Mandibles were tension tested at 0.5mm/s to failure and then breaking load, yield and stiffness were determined.
Results
mandibular distraction osteogenesis had significantly lower breaking load, yield and stiffness when compared to the contralateral mandible, by 40%, 30% and 60%, respectively. Breaking load was reduced in partially reduced mandibular fractures by 40% when compared to mandibular distraction osteogenesis.
Conclusion
Utilizing a standard Ilizarov protocol, the strength and durability of the regenerate, as measured by breaking load, yield and stiffness in mandibular distraction osteogenesis was significantly lower than contralateral mandibles. Surprisingly, the breaking load of mandibular distraction osteogenesis was significantly greater than partially reduced mandibular fracture. These verifiable metrics of regenerate integrity can be utilized to discern optimal outcomes of mandibular distraction osteogenesis, and to extrapolate the data from the bench and bring it to the bedside, enhancing the clinical applications of this powerful technique.
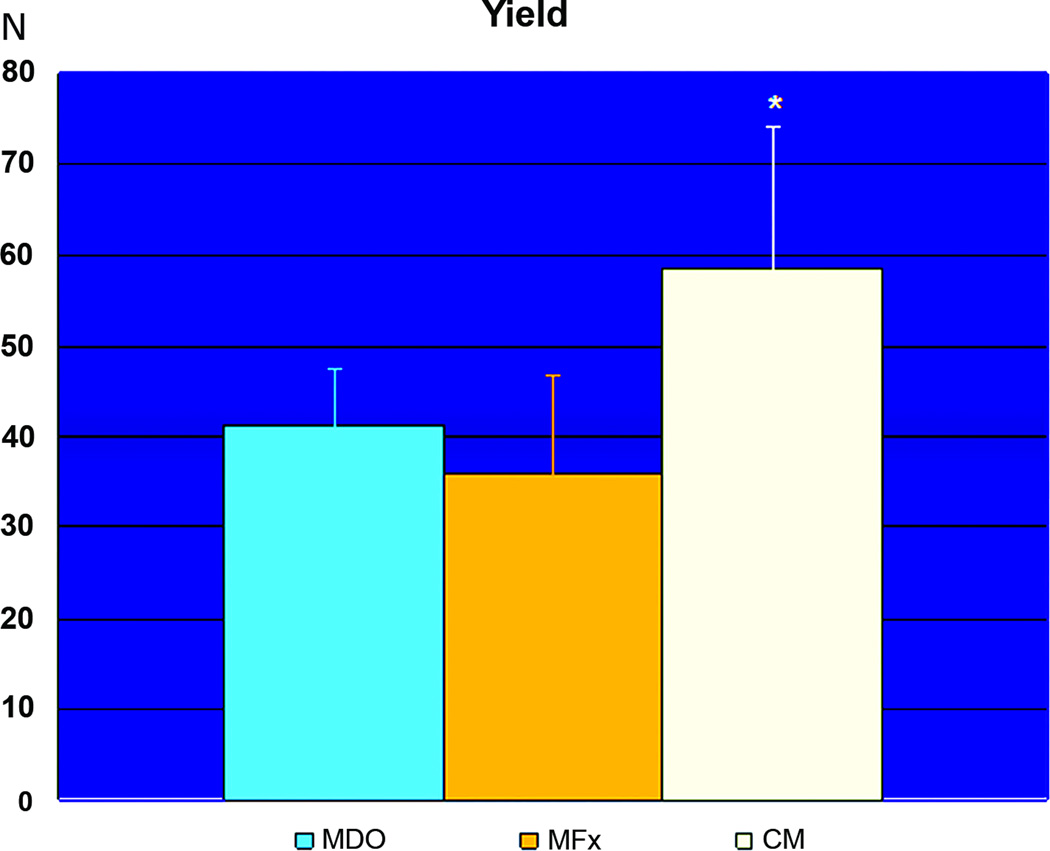
INTRODUCTION
While the clinical utilization of Distraction Osteogenesis (DO) has been steadily increasing (1–5) since its discovery over a century ago (6), surprisingly, we don’t have quantitative metrics to evaluate either the degree of success or failure of the technique. Unfortunately, the utility of DO as a reconstructive option currently relies on clinical protocols which are predicated on empiric evidence as well as trial and error. Anecdotal data gained from large animal models have been used to drive treatment design and development of new devices but have not been rigorously tested (7–10). Despite the increasing use of this powerful tool for regeneration of bony deficiencies in the craniofacial skeleton (11–16) since its initial application in the mandible (17), the outcome measures that demonstrate optimal mandibular DO (MDO) are unknown, with consolidation periods varying widely among surgeons (14, 18).
Establishing precise and reproducible parameters to assess regenerate healing during MDO should provide a better understanding of the overall process and lead to advances in the application of the technique as well as improved distraction protocols. More to the point, appropriate verifiable defined metrics of functional integrity need to be properly correlated to overall efficacy of MDO in order to take the data from the bench and bring it to the bedside for potential clinical use.
We proposed to establish biomechanical outcomes evaluating how DO affects the overall quality of bone healing in the mandible. The purpose of this study was to objectively quantify bone healing in MDO compared to a both a partially reduced fracture (MFx) and the contralateral mandible (CM), using a single, consolidation period based upon Ilizarov’s original design (19, 20). Our specific aim was to quantitatively determine the strength and functional integrity of the bony regenerate (RG) after unilateral MDO in comparison to the contralateral mandible (CM). A further specific aim of this study was to quantitatively determine whether a mandible which has undergone distraction to a length greater than its critical size defect is stronger and structurally more durable than a partially reduced fracture (MFx).
Our laboratory has developed a rat model of MDO that differentiates new bone formation attributable to distraction, versus secondary bone formation from unaided fracture healing (21). This model has permitted isolation of the variables necessary for identification of the significant differences between DO and osteotomy alone and to more accurately attribute the changes to the specific stimuli produced by the process of distraction. Utilizing tension testing of the affected hemi-mandible in order to measure RG integrity, we hypothesized that the yield (Y), breaking load (BL) and stiffness (S) of MDO would be significantly reduced in comparison to CM. We further posited that MDO would similarly show a decrease in those same biomechanical parameters in comparison to MFx and that the degree of strength and functional durability of these partially reduced fractures would be intermediate between the CM and the MDO group.
METHODS
Experimental Groups And Model
A total of 20 male SD rats were randomly assigned pre-operatively to the following 3 experimental groups: 1. MDO (n=8), 2. MFx (n=6) and 3. CM (n=14). Both surgical groups underwent 28 days of consolidation post-operatively, excluding 4 days latency and 8 days distraction to a 5.1mm total gap width.
Animals
Male Sprague-Dawley Rats, approximately 375 gm were housed 3/cage in a pathogen-free, restricted-access facility upon arrival to our laboratory during a 4 day acclimation prior to surgery. The diet was changed to moist chow 48 hrs pre-operatively along with Hill’s® high calorie diet (Columbus Serum, Columbus, OH), and then continued post-operatively in individual cages. All animal procedures were performed according to the NIH Guide for Animal Care and approved by the University of Michigan Animal Care and Use Committee (22).
Surgical Procedure
Pre-operatively, animals were given Chloramphenicol (30mg/kg SQ) prophylactically; Buprenorphine (0.15mg/kg SQ) along with 25cc/kg SQ LR and then the surgical procedure was performed under sterile conditions and general anesthesia using an isoflurane/oxygen mixture. A 2 cm midline incision was placed ventrally from the anterior submentum to the neck crease. Skin flaps were elevated exposing the anterolateral mandible. After percutaneous pre-drilling (3/32 drill bit) 0.5mm posterior to the symphysis, a 1.5” #0–80 stainless steel threaded rod was horizontally inserted across the anterior mandible, with the ends brought externally through the skin, creating the anterior portion of our modified external fixator. A 1cm incision was made through the masseter, down to, and in-line with the inferior border of the mandible, just 3mm anterior to the angle. Bilateral #0–80 threaded stainless steel pins were inserted buccal-to-lingual, after pre-drilling approximately 2mm superior to the inferior border, and 6mm anterior to the angle, and then secured with our custom titanium washer and nut. The pin ends were brought externally through the skin for the posterior fixator placement with titanium caps. The right side was rigidly fixed, while the left side received a distraction screw for post-operative manipulation.
A vertical osteotomy was created ~2mm anterior to the titanium washer on the left hemi-mandible using a 10mm micro-reciprocating blade (Stryker, Portage, MI) attached to a power saw (Aesculap, Center Valley, PA). The osteotomy extended from the inferior mandible border superiorly to the sigmoid notch along the anterior aspect of the coronoid process. The osteotomy edges were reduced and then re-secured with the fixator. The wound was irrigated, hemostasis verified, tissues approximated using 4-0 Vicryl™ suture, and the midline incision closed with staples.
Post-op Animal Care
Buprenorphine was continued (0.15 – 0.5 mg/kg) with 10cc LR SQ Q12 hrs through POD#4 and as needed thereafter. Animals were fed moist chow/Hill’s and water ad libitum. Pin sites were cleaned daily; maxillary incisors were clipped weekly due to overgrowth from crossbite and staples removed by POD#14. Weights were monitored daily and diets adjusted as needed.
Distraction Protocol
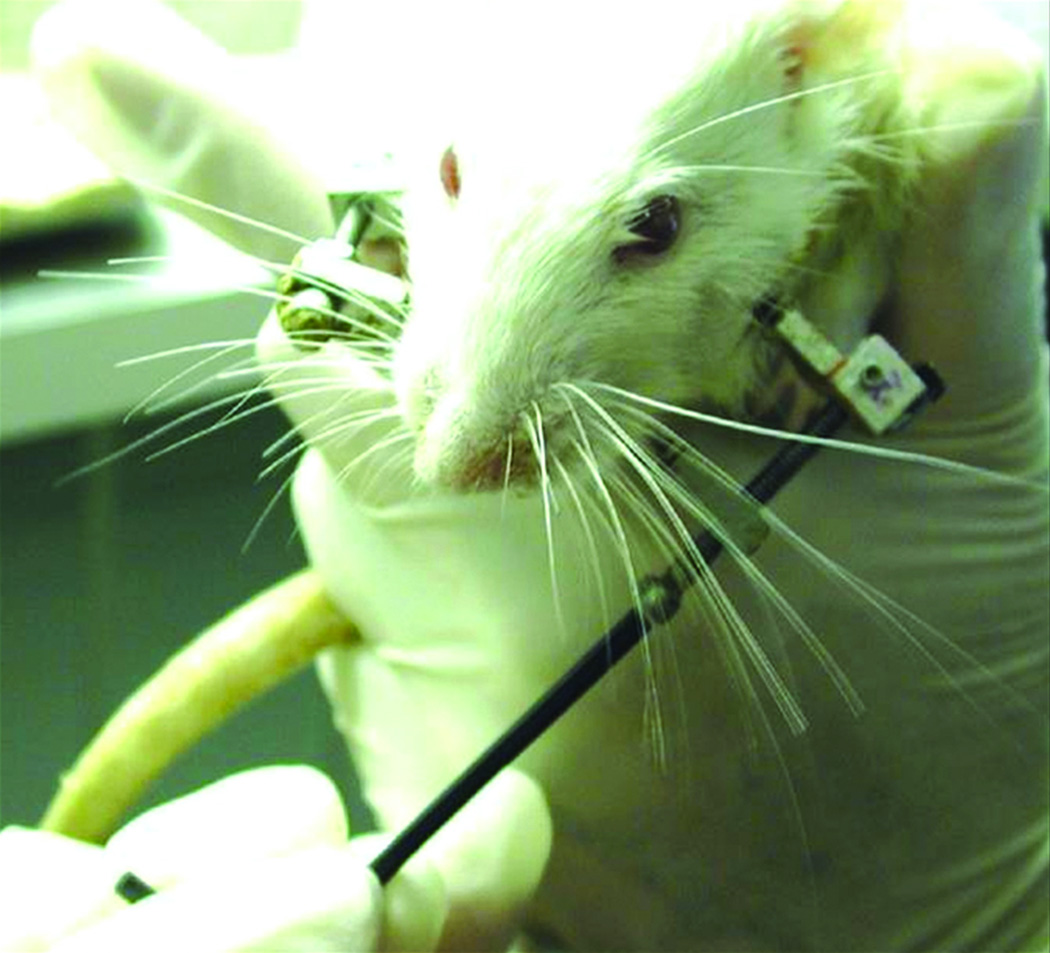
MDO were distracted after 4 days latency. One 180-degree clockwise turn of the distraction screw corresponded to a 0.3mm separation of the osteotomy fronts. A total of 17 half-turns were performed on a Q12 hr interval, resulting in a maximum 5.1mm distraction gap over 8 days. No analgesic or sedation was required during the distraction (figure 1).
Figure 1.
Performing manual distraction of left hemi-mandible on animal with external fixator/distractor in place.
The MFx group underwent 7 half turns in immediate succession at ~4–8 hrs post operatively for a total gap distance of 2.1mm, then immediately re-secured and remained fixed throughout the 4 weeks of consolidation. The MFx separation is performed immediately after the animals received their initial post-operative analgesic injection.
Mechanical Testing
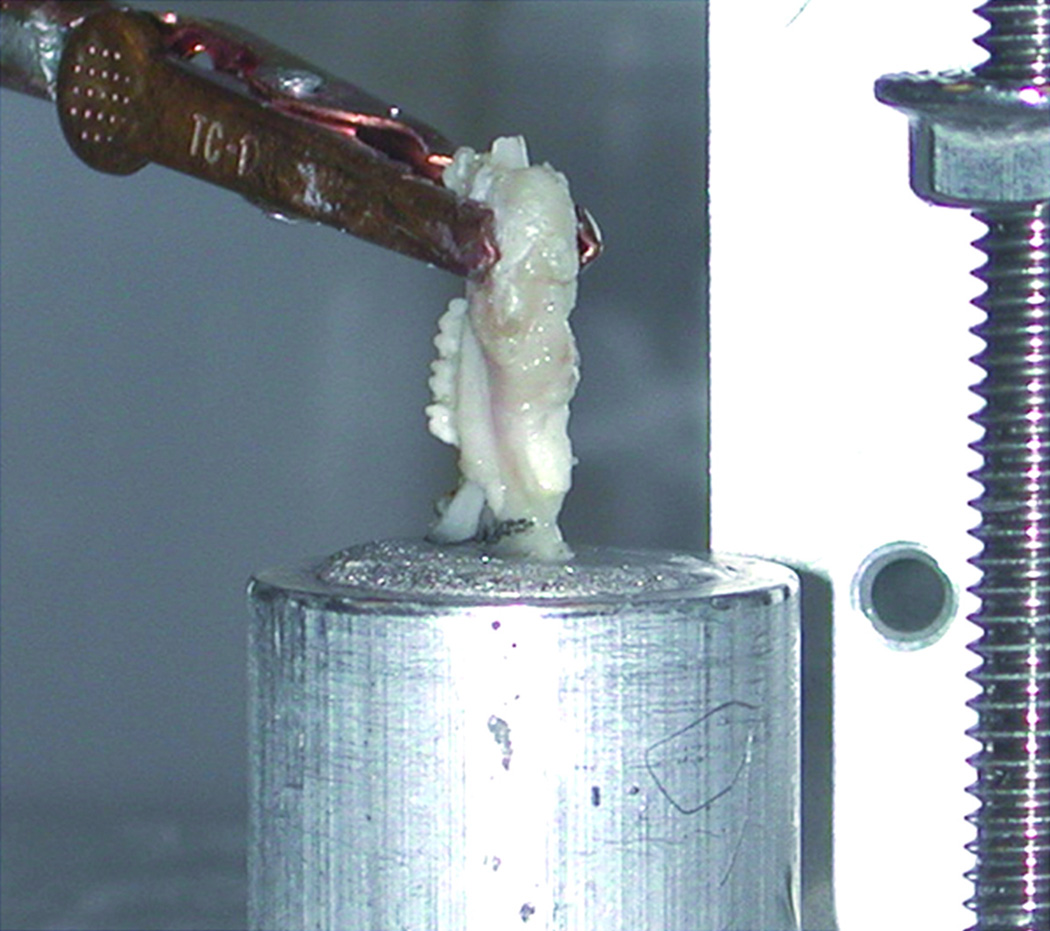
MDO and MFx animals, along with CM were sacrificed after 4 weeks of consolidation. The mandibles were harvested en-bloc, split between the incisors, and frozen at −20°C until testing. The posterior aspect of the mandible was secured first into an open cylindrical shaped “pot” using a bismuth alloy (Cerrobend, Cerro Products, Bellefont, PA) which when melted became an easy-to-use, conforming liquid, and when cooled secured the bone in place (figure 2). Potting secures the specimen, thus enabling it to be attached within the mechanical testing apparatus. These were placed as close to the healed regenerate as possible, without crossing into the gap to ensure that mechanical forces were limited to the regenerate (figure 3), and not other regions of the mandible. CM specimens were potted analogously. The potential for mal-alignment during potting, which is irreversible, may preclude the use of some specimens from mechanical testing. In our experiment, 3 animals were excluded for this reason.
Figure 2.
Left hemi-mandible with the posterior aspect potted in bismuth alloy covering the condyle and coronoid process, without adhering to the regenerate. The un-potted anterior region reveals molars and clipped incisor exposed.
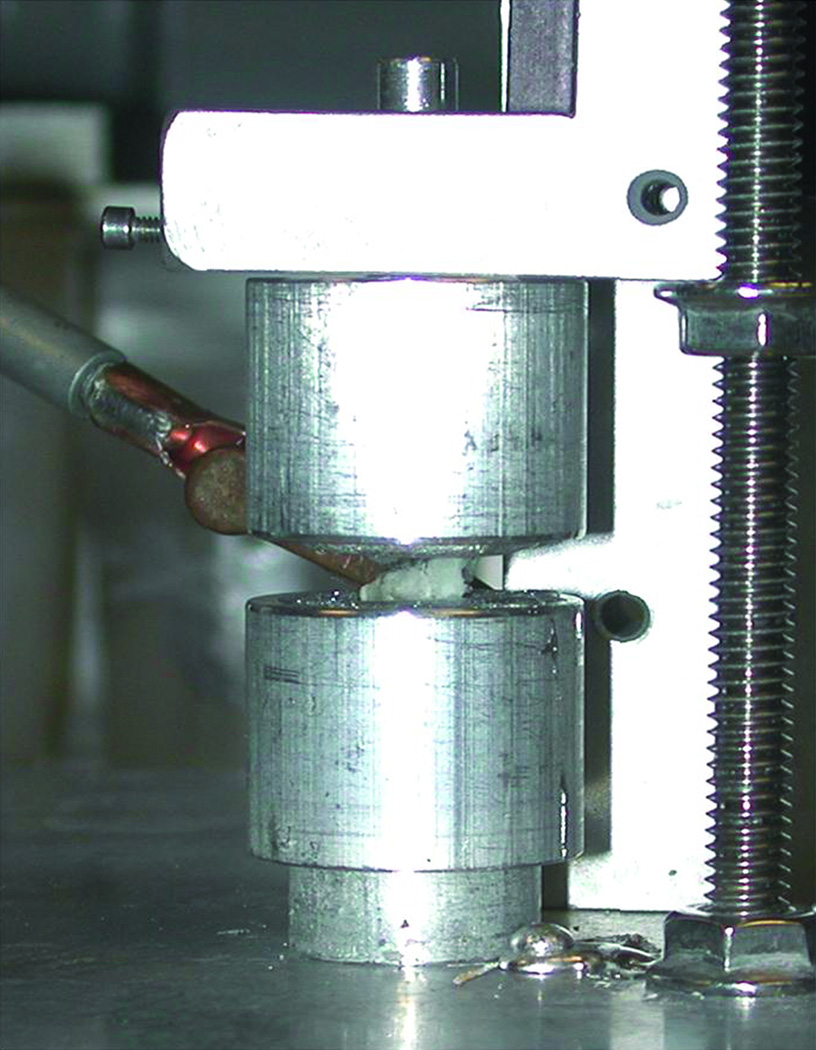
Figure 3.
Left hemi-mandible is completely potted exposing only the fracture/regenerate region, loaded (posterior aspect at the base) on the mechanical testing apparatus, and ready for biomechanical tension testing.
Potted mandibles were tested in uniaxial tension until failure at 0.5 mm/s in the anterior-posterior direction using a servohydraulic testing machine (858 Mini Bionix II; MTS Systems, Eden Prairie, MN). Load head displacement was monitored using an external linear variable differential transducer (LVDT; Lucas Schavitts, Hampton, VA), and load data were collected with a 10 and 50-lb load cell (Sensotec, Columbus, OH). Load and displacement data were acquired using the TestStar IIs system (version 2.4; MTS) at a sampling frequency of 2000 Hz. Results were expressed in a load-displacement (LD) curve, and numeric data were transferred to Microsoft Excel for further analysis to determine BL, Y and S.
BL was defined as the highest load, in Newtons (N) resulting in partial or complete failure of the regenerate callus with at least 20% reduction from the maximum load; Y was determined as the first major right-shift deviation from the initial linear portion of the LD curve, greater than 2% displacement; and S extrapolated from the initial 20% to 80% of the Y on the LD curve as N/mm displacement. Each mechanical metric was determined from the individual LD curves by at least three independent reviewers, results compiled, averaged, and analyzed statistically.
Statistics
Two CM specimens had stiffness (one had both Y and S) values > 3 standard deviations from the mean and were determined as outliers. CM final number was n = 12 for S and n = 5 for Y. Modified Levene’s test for equal variance determined One-way ANOVA with appropriate post-hoc Tukey-Kramer test was utilized for analyzing S between MDO, MFx and CM groups (SPSS 16.0, Chicago). One-way ANOVA on ranks, with z-value and separate Kruskal-Wallis non-parametric test was used to analyze BL and Y between MDO, MFx and CM groups. Results were accepted as statistically significant at a p value less than 0.05.
RESULTS
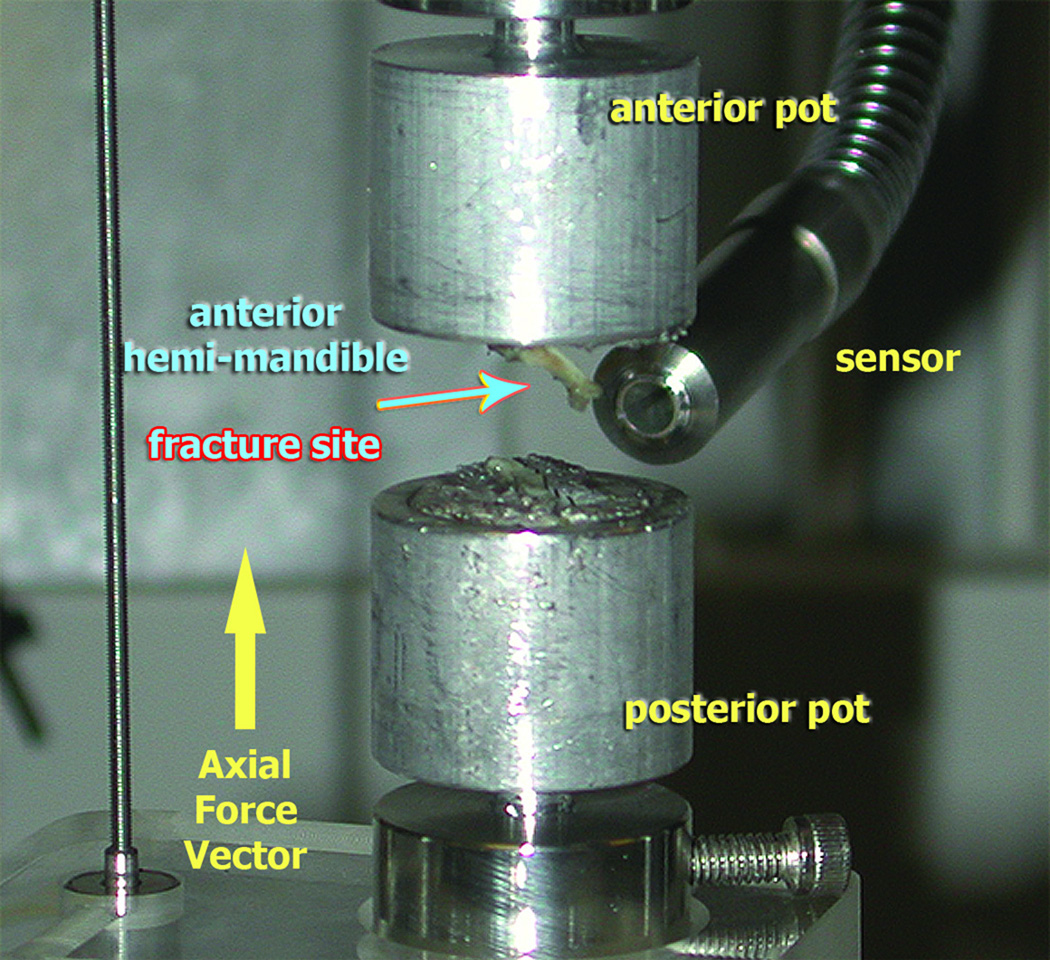
Under tension testing, all the MDO specimens failed (broke) posterior to the third molar, extending from the inferior border of the mandible cephalad into the coronoid process, within the area of the regenerate (figure 4). The MFx specimens uniformly failed along the fracture site, while the CM specimens broke in various regions. The most common failure location in CM specimens occurred between the first and second molars, while two broke between the second and third molars, and one specimen broke in the condylar region. Two of the CM specimens exceeded the load cell limits, resulting in an incomplete failure, as they remained attached via the incisor root.
Figure 4.
Broken MDO hemi-mandible after uniaxial tension testing to failure shows break occurring through the exposed regenerate region.
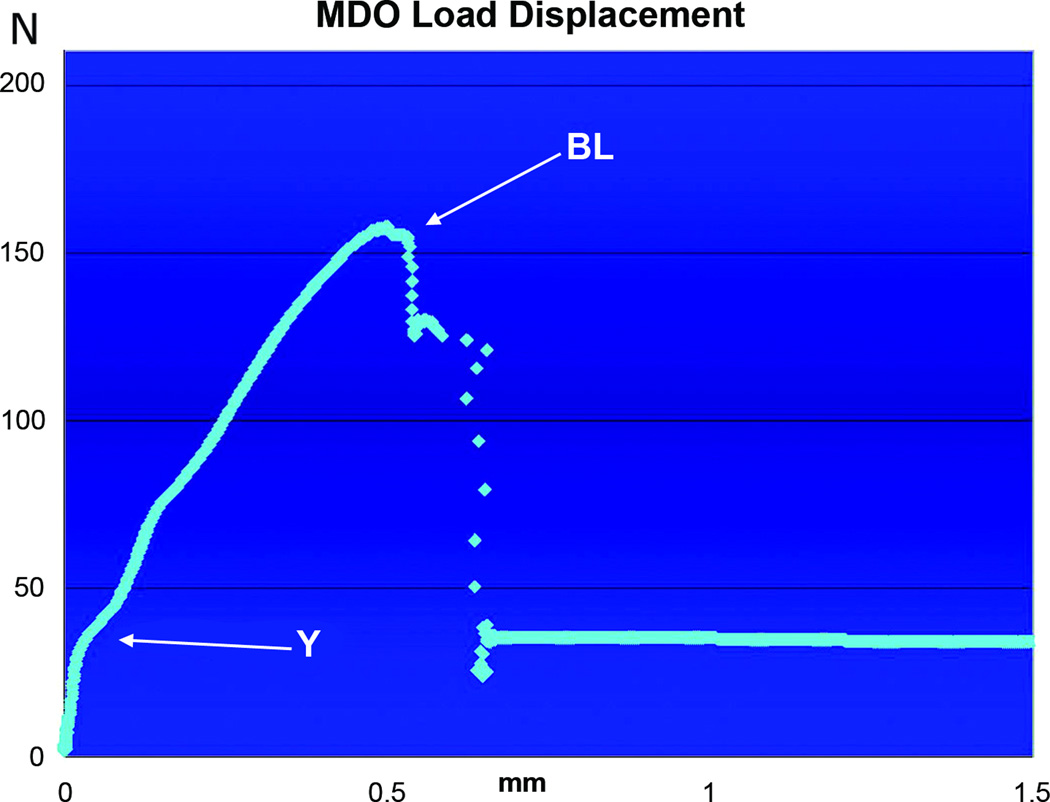
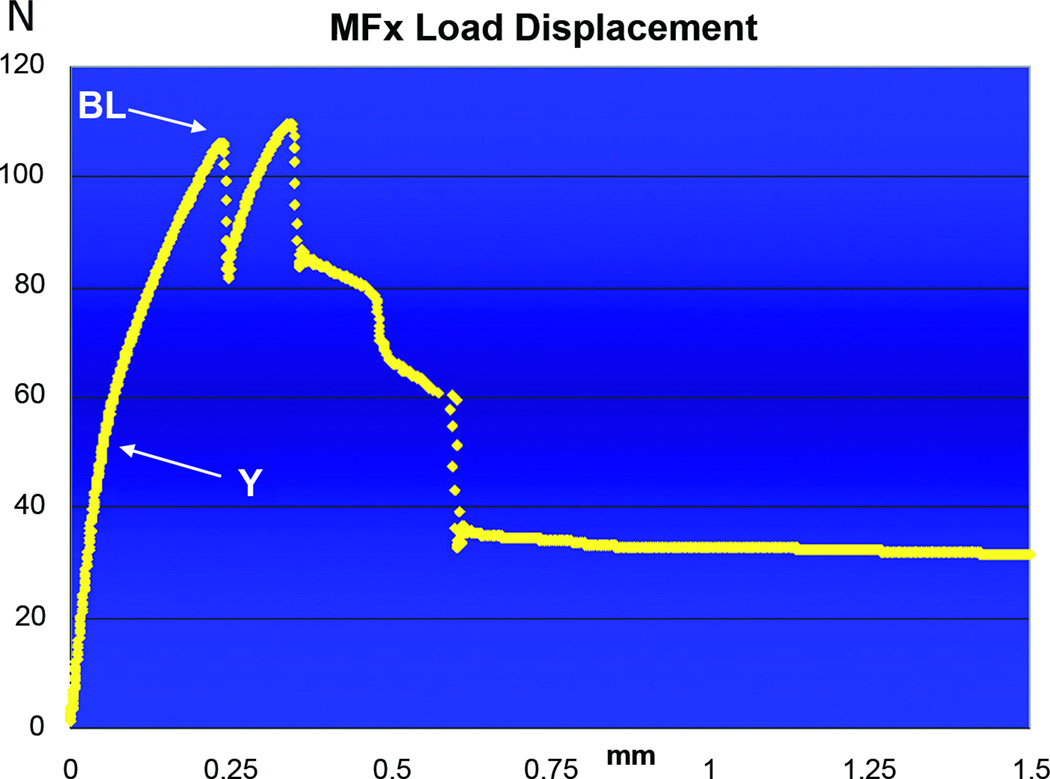
The LD curves from MDO revealed a steep upward slope until their average Y at 41.34 Newtons ± 6SD, then continued through an average BL of 166.88 Newtons ± 21SD, which occurred at a displacement ranging from 0.4 to 0.6 mm (figure5). In a similar fashion, the LD curves for MFx revealed a steep initial slope through their average Y at 35.78 Newtons ± 11SD (figure 6). However, the average BL of MFx (92.58 Newtons ± 11SD) was significantly lower than the average BL of the MDO specimens, and it occurred at a lower displacement range of 0.2 to 0.4mm.
Figure 5.
LD curve of MDO tension tested to failure with the X-axis representing mm of displacement, with the Y-axis representing Newtons (N) of force. For this specimen, the Yield (~36Newtons) occurs at a displacement of 0.035mm, while the BL (~157Newtons) occurs here along the X-axis at 0.5mm (MDO displacement ranges from 0.4 – 0.6mm).
Figure 6.
LD curve of MFx tension tested to failure. For this specimen, the Y (~45Newtons) occurs at a displacement of 0.045mm, while the BL (~105Newtons) is significantly lower than that for MDO, and occurs at 0.25mm displacement, also significantly lower than for MDO.
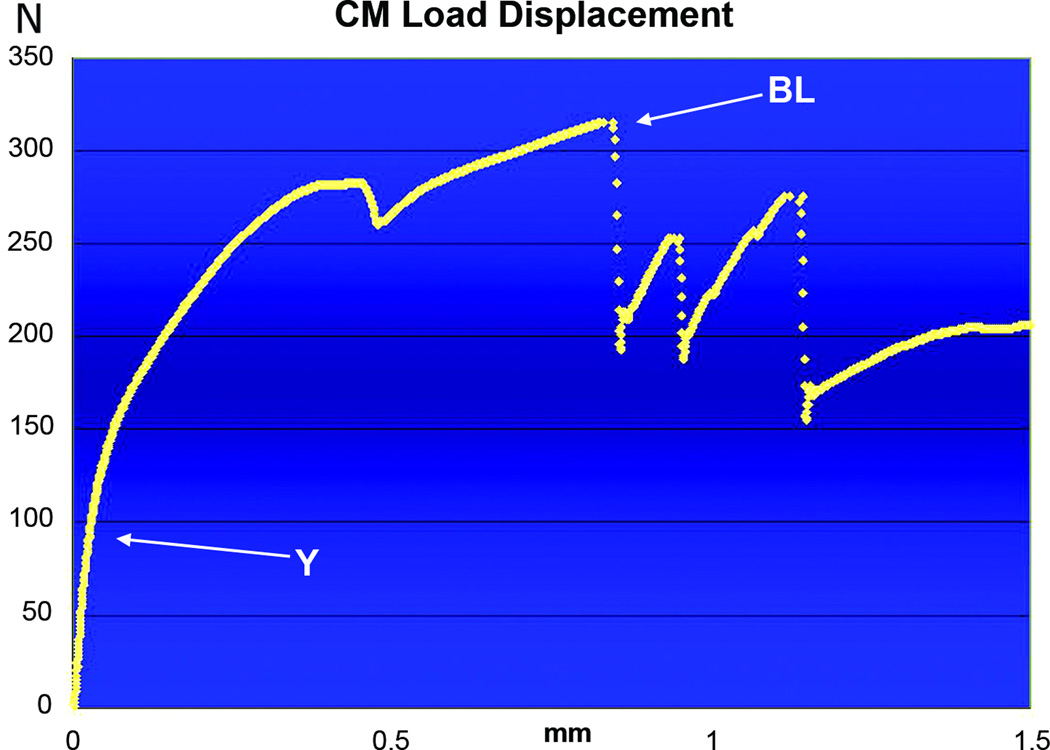
Similar to both surgical groups, the LD curves from CM specimens also showed a steep initial curve, although the Y occurred at a significantly higher level of 58.42 Newtons ± 16SD. In stark contrast to both surgical groups, the LD curve from CM specimens had a much more gradual and prolonged slope after the Y and continued through a statistically greater BL of 275.82 Newtons ± 59 SD, which occurred at a much higher displacement range of 0.75 to 1.0mm (figure 7).
Figure 7.
Load-Displacement curve of a contralateral control (CM) hemi-mandible tension tested to failure. The Y (~85Newtons) is almost twice that of MDO and MFx and occurs at a displacement of 0.02mm. The BL, 315Newtons, is also significantly higher than MDO and MFx, and occurs at approximately 0.9mm displacement.
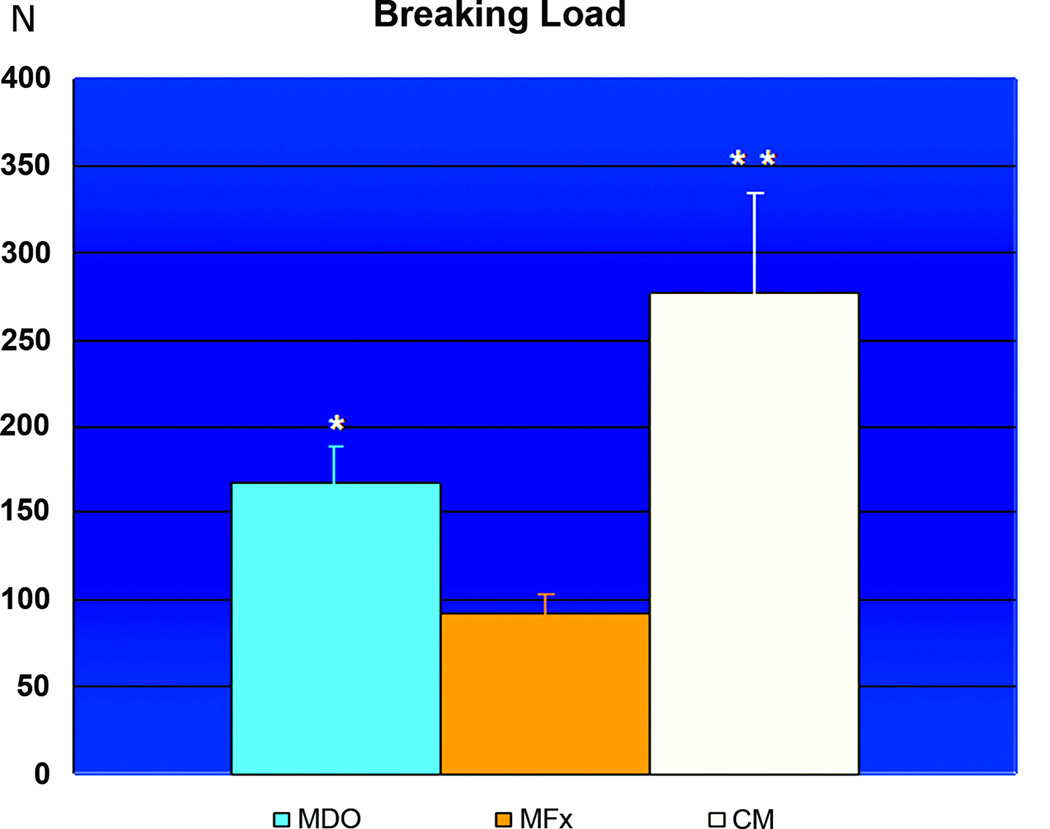
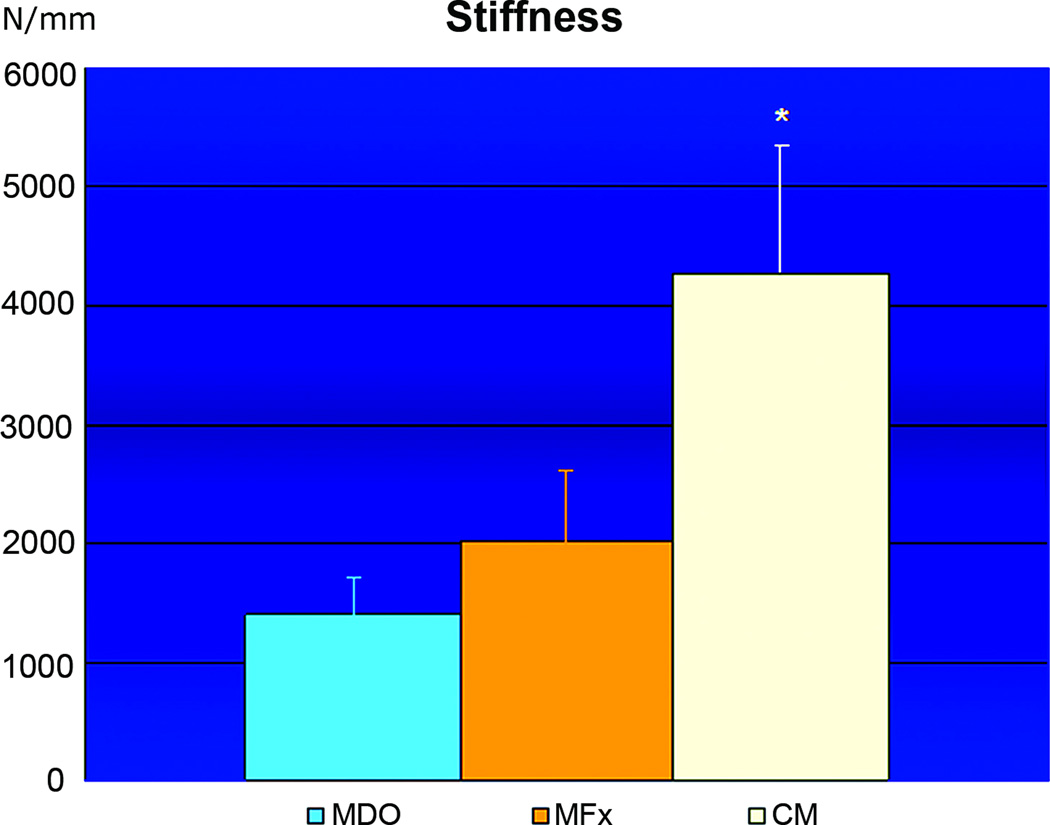
When comparing the biomechanical measures from all groups, we found that the BL of the MDO group (166.88 Newtons ± 21SD) was statistically different than the BL of the MFx group (92.58 Newtons ± 11SD), while the BL from CM group (275.82 Newtons ± 59SD) was statistically different than both surgical groups (figure 8). We did not however, find any statistically significant difference in Y between the MDO (41.34 Newtons ± 6SD) and the MFx groups (35.78 Newtons ± 11SD). The Y from the CM group (58.42 Newtons ± 16SD) again was demonstrated to be statistically different than either surgical group (figure 9). Analogous to Y results, we did not find any statistical difference in S between the MDO (1400 Newtons/mm ± 295SD) and the MFx (2007 Newtons/mm ± 600SD) groups, while the CM group showed statistically significant S (4276 Newtons/mm ± 1074SD) than both MDO and MFx surgical groups (figure 10).
Figure 8.
Graph of Breaking Load comparison between MDO, MFx and CM groups with mean values in Newtons of applied load on the Y-axis and standard deviation as Y-error bars. The BL is significantly greater in CM (** p<0.05) than in both surgical groups, and the BL for the MDO group (* p<0.05) is significantly higher than the MFx group.
Figure 9.
Graph of Yield for MDO, MFx and CM with mean values listed as Newtons of applied load and standard deviation as Y-error bars. While CM had significantly higher Yield than either surgical group (* p<0.05), there was no statistically significant difference between MDO and MFx.
Figure 10.
Graph of Stiffness for MDO, MFx and CM with mean values listed as Newtons of applied load and standard deviation as Y-error bars. While CM had significantly higher S than both surgical groups (* p<0.05), there was no significant difference in S between MDO and MFx.
DISCUSSION
The utilization of DO in the facial skeleton is rapidly expanding and is now being performed in many craniofacial centers throughout the world (16, 23–25). The rush to apply this new technique to an eager and willing population of patients has resulted in clinical protocols which are predicated on empiric evidence as well as trial and error. Anecdotal data gained from large animal models have been used to drive treatment design and development of new devices but have not been rigorously tested (7–10). Despite the increasing use of this powerful tool for regeneration of bony deficiencies in the craniofacial skeleton, the outcome measures that demonstrate optimal DO are unknown. Our ultimate purpose in this experiment was to quantitate biomechanical measures of bone healing that may be extrapolated for eventual clinical use to best determine functional healing.
Our experimental protocol allowed the determination of the mechanical integrity of MDO regenerate, in comparison to MFx and CM, by assessing BL, Y and S at a single consolidation period, based on the standardized Ilizarov principles still being used clinically today (19, 20, 26). Specifically, we used the commonly practiced guideline of two times the distraction period for consolidation, which assumes adequate functional bone healing and stability prior to removal of the distractor. Clinically, surgeons utilize simple radiographs as a check of bony consolidation to give them confidence that the Ilizarov protocol was sufficient for hardware removal without the fear of instability of the mandible. The specific aim of this report was to test the actual biomechanical integrity of the regenerate utilizing the Ilizarov protocol in our own rodent model.
We established tension testing as the most appropriate biomechanical assessment to evaluate functional integrity of the regenerate and fracture healing in our MDO and MFx specimens because this type of analysis allowed us to isolate mechanical testing to the specific region of interest (27, 28). In comparison, other mechanical testing methods, such as the cantilever method (7, 8, 10), which primarily assesses overall mandibular function in relationship to perpendicular occlusal forces, and the modified three point bending analyses (9, 29, 30), which has the potential to convert a significant portion of forces to shear, do not allow such a specific isolation of mechanical forces to the region of interest(28). These latter methods assess aspects of the entire hemi-mandible which would add unwanted and problematic confounding variables.
We utilized MFx as a surgical comparison group in order to distinguish the biomechanical parameters of new bone formation attributable to distraction from secondary bone formation resulting from unaided fracture healing (21). We utilized CM as a control group to represent the upper limit of biomechanical properties that could potentially be obtained after complete fracture repair and regenerate consolidation (31–35).
In keeping with our initial hypothesis, the BL of the mandibles that underwent DO demonstrated a 40% decrease in comparison to the BL of the CM. Although a reduction in strength and functional integrity of the distracted hemi-mandible in comparison to the unoperated opposite side was certainly anticipated, the degree by which that principal biomechanical parameter decreased is of great importance. It has long been felt that the Ilizarov protocol of doubling the distraction period for full consolidation would be sufficient time to achieve “adequate” healing. However, there has been a critical lack of hard data to understand the extent and quality of healing utilizing that protocol. Clinically, quantifying the degree of stability and strength of the healing regenerate could drive treatment strategies. Decisions regarding how long to keep the distractor on subsequent to a period of consolidation, and how long to protect the mandible and restrict diet once the distractor is removed, could be predicated on precise data rather than intuition. In addition, specific phases of distraction, such as latency, periodicity, and consolidation could potentially be modified and subsequently outcomes measured in order to facilitate optimizing the overall distraction process. A 40% reduction in BL did give us pause as the maximum load resulting in partial or complete failure of the regenerate callus is an important biomechanical metric emblematic of the overall sturdiness of the distracted mandible. Our results suggest a significant and persistent diminution in functional integrity even at the end of consolidation, within our rodent model of MDO, raising uncertainty in regards to the durability of overall healing and the potential for relapse, which is a potential complication noted in some short and long term clinical follow-up studies (36–41). Our results highlight the need to correlate these experimental findings with clinical outcome measures in order to evaluate the degree of success or failure of MDO and to intelligently guide advances in the application of the technique. Recent clinical studies have in fact utilized longer consolidation periods (3–4x distraction) than Ilizarov’s initial protocol in order to achieve adequate bone healing (42, 43).
In contrast to our initial hypothesis, the BL of the mandibles that underwent partially reduced fracture repair demonstrated a further diminution of force required to achieve failure when compare to MDO. Specifically the MFx group demonstrated a 60% reduction in overall BL in comparison to the CM and a 25% further reduction in comparison to the MDO group. Therefore instead of the MFx group showing strength and integrity intermediate between the CM and the MDO group, it was notably less than both of those groups. We posit that the significantly greater load required to achieve failure of the distraction regenerate when compared to partially reduced fracture may be due to the tremendous osteoinductive force of DO from the cyclic, repeated axial forces (32, 33, 44), in addition to the MFx being partially rather than fully reduced. Unpublished work in our lab has demonstrated increased highly mineralized bone formation in distraction regenerate in comparison to MFx which may indeed be responsible for the increased mechanical integrity seen in the MDO group. Differences in the microarchitectural orientation of the constituents of the DO regenerate in comparison the MFx fracture callus may also help to explain differences in the mechanical properties of the two groups (45, 46).
In keeping with our initial hypothesis the force required to damage the underlying bony architecture, or Y, in CM specimens was almost twice that of either MDO or MFx specimens. Y is a particularly important biomechanical metric as it sets the limit for irreversible damage to the regenerate. Such a measurement could guide the extent of biomechanical manipulation of the regenerate that could potentially be used to enhance healing or increase distraction length without detrimental effects to the bony architecture (i.e. set maximal biomechanical parameters for pumping the regenerate(44)).
Although we demonstrated a greater BL in MDO in comparison to MFx, surprisingly we found no similar significant difference in Y between the MFx and MDO groups. Since Yield measures the ability of a material to return to its normal shape, or its elastic properties, then more non-mineralized matrix should support greater loads before becoming irreversibly deformed (28). Indeed, unpublished data from our laboratory using quantitative histomorphometry demonstrated that there was 30% more osteoid volume in the region of interest in the MDO specimens when compared to the MFx specimens. This substantially increased osteoid formation may function to maintain elasticity within the regenerate and result in the lack of statistical significance we saw in Y between MDO and MFx. Y, therefore, is now the second biomechanical parameter to negate our hypothesis of superior mechanical integrity of fracture callus in comparison to the regenerate of DO.
A few MDO experimental models have had limited success in measuring stiffness, accounting for the inconsistent and wide geometric variations in the mandible (8, 10). We were able to successfully institute extrinsic stiffness as another outcome metric, in establishing the significantly reduced biomechanical integrity of MDO compared to the CM. Similar to Y, we did not find a statistical difference between the MFx and MDO groups. However, we recognize the complexity that geometry and callus vs. regenerate thickness play in assessing this parameter (28, 47, 48), and thus our primary focus on BL as the key metric to ascertain functional integrity of RG. Regardless, the goal of these experiments was to ascertain statistically verifiable, defined biomechanical outcome metrics in MDO using Ilizarov’s protocol, which to our knowledge previously have not been established.
Conclusion
This study reports the use of accurate and reproducible biomechanical analysis of MDO in comparison to MFx and CM at a single consolidation point in order to establish outcomes measures of the overall quality of bone healing. Our findings demonstrate statistical data indicating a 40% decrease in BL of MDO, a Y that was 30% decreased, and S that was almost 60% less in comparison to CM, utilizing a standard Ilizarov protocol in a rodent model. Furthermore, we found a 25% reduction in BL of MFx when compared to MDO specimens. These verifiable defined metrics of strength and functional integrity need to be properly correlated with current treatment strategies so that they can be utilized to precisely discern optimal outcomes of MDO in order to take the data from the bench and bring it to the bedside to improve clinical applications of this powerful technique.
Acknowledgements
We thank Steven Goldstein, PhD and his Orthopaedic Research Laboratories’ fellows and staff for their technical assistance in mechanical testing and interpretation of results. We thank our students, Jordan Stewart, Mohammed Hussain Dar, Daniel Zeldes, Michael Jurkewicz and Dionne Okefor for their assistance with animal care and data collection.
Funding supported by the following grants:
Carls Foundation, PI: Steven R. Buchman, MD
“Optimization of Bone Regeneration in the Irradiated Mandible”, NIH RO1 #CA12587-01; PI: Steven R. Buchman, MD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No commercial association or financial interest exists by any author.
Contributor Information
Daniel A. Schwarz, University of Michigan Medical School, Ann Arbor, MI.
Krikor G. Arman, University of Michigan Medical School, Ann Arbor, MI.
Mehreen S. Kakwan, University of Michigan Medical School, Ann Arbor, MI.
Ameen M. Jamali, University of Michigan Medical School, Ann Arbor, MI
Steven R. Buchman, University of Michigan Medical School, Ann Arbor, MI.
References
- 1.Aronson J, Johnson E, Harp JH. Local bone transportation for treatment of intercalary defects by the Ilizarov technique. Biomechanical and clinical considerations. Clin Orthop Relat Res. 1989:71–79. [PubMed] [Google Scholar]
- 2.Paley D, Catagni MA, Argnani F, et al. Ilizarov treatment of tibial nonunions with bone loss. Clin Orthop Relat Res. 1989:146–165. [PubMed] [Google Scholar]
- 3.White SH, Kenwright J. The timing of distraction of an osteotomy. J Bone Joint Surg Br. 1990;72:356–361. doi: 10.1302/0301-620X.72B3.2341426. [DOI] [PubMed] [Google Scholar]
- 4.White SH, Kenwright J. The importance of delay in distraction of osteotomies. Orthop Clin North Am. 1991;22:569–579. [PubMed] [Google Scholar]
- 5.Paley D, Catagni M, Argnani F, et al. Treatment of congenital pseudoarthrosis of the tibia using the Ilizarov technique. Clin Orthop Relat Res. 1992:81–93. [PubMed] [Google Scholar]
- 6.Codivilla A. On the means of lengthening, in the lower limbs, the muscles and tissues which are shortened through deformity. 1904. Clin Orthop Relat Res. 1994:4–9. [PubMed] [Google Scholar]
- 7.Kontaxis A, Abu-Serriah M, Ayoub AF, et al. Mechanical testing of recombinant human bone morphogenetic protein-7 regenerated bone in sheep mandibles. Proc Inst Mech Eng [H] 2004;218:381–388. doi: 10.1243/0954411042632135. [DOI] [PubMed] [Google Scholar]
- 8.Perrott DH, Rahn B, Wahl D, et al. Development of a mechanical testing system for a mandibular distraction wound. Int J Oral Maxillofac Surg. 2003;32:523–527. [PubMed] [Google Scholar]
- 9.Farhadieh RD, Gianoutsos MP, Dickinson R, et al. Effect of distraction rate on biomechanical, mineralization, and histologic properties of an ovine mandible model. Plast Reconstr Surg. 2000;105:889–895. doi: 10.1097/00006534-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Kunz C, Adolphs N, Buscher P, et al. Mineralization and mechanical properties of the canine mandible distraction wound following acute molding. Int J Oral Maxillofac Surg. 2006;35:822–827. doi: 10.1016/j.ijom.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Yu JC, Fearon J, Havlik RJ, et al. Distraction Osteogenesis of the Craniofacial Skeleton. Plast Reconstr Surg. 2004;114:1E–20E. doi: 10.1097/01.prs.0000128965.52013.95. [DOI] [PubMed] [Google Scholar]
- 12.Diner PA, Tomat C, Soupre V, et al. Intraoral mandibular distraction: indications, technique and long-term results. Ann Acad Med Singapore. 1999;28:634–641. [PubMed] [Google Scholar]
- 13.Hollier LH, Kim JH, Grayson B, et al. Mandibular growth after distraction in patients under 48 months of age. Plast Reconstr Surg. 1999;103:1361–1370. doi: 10.1097/00006534-199904050-00004. [DOI] [PubMed] [Google Scholar]
- 14.Gosain AK. Distraction osteogenesis of the craniofacial skeleton. Plast Reconstr Surg. 2001;107:278–280. doi: 10.1097/00006534-200101000-00050. [DOI] [PubMed] [Google Scholar]
- 15.Monasterio FO, Drucker M, Molina F, et al. Distraction osteogenesis in Pierre Robin sequence and related respiratory problems in children. J Craniofac Surg. 2002;13:79–83. doi: 10.1097/00001665-200201000-00018. discussion 84, [DOI] [PubMed] [Google Scholar]
- 16.Carls FR, Sailer HF. Seven years clinical experience with mandibular distraction in children. J Craniomaxillofac Surg. 1998;26:197–208. doi: 10.1016/s1010-5182(98)80015-2. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy JG, Schreiber J, Karp N, et al. Lengthening the human mandible by gradual distraction. Plast Reconstr Surg. 1992;89:1–8. discussion 9–10, [PubMed] [Google Scholar]
- 18.Mofid MM, Manson PN, Robertson BC, et al. Craniofacial distraction osteogenesis: a review of 3278 cases. Plast Reconstr Surg. 2001;108:1103–1114. doi: 10.1097/00006534-200110000-00001. discussion 1115-1107, [DOI] [PubMed] [Google Scholar]
- 19.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clinical orthopaedics and related research. 1989:263–285. [PubMed] [Google Scholar]
- 20.Ilizarov GA. The tension-stress effect on the genesis and growth of tissues. Part. I. The influence of stability of fixation and soft-tissue preservation. Clin Orthop Relat Res. 1989:249–281. [PubMed] [Google Scholar]
- 21.Buchman SR, Ignelzi MA, Jr, Radu C, et al. Unique rodent model of distraction osteogenesis of the mandible. Ann Plast Surg. 2002;49:511–519. doi: 10.1097/00000637-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Resources IoLA. Guide for the care and use of laboratory animals / Institute of Laboratory Animals Resources, Commission on Life Sciences, National Research Council. 7th ed. Washington, DC: National Academy Press; 1996. p. 125. [Google Scholar]
- 23.Figueroa AA, Polley JW. Clinical controversies in oral and maxillofacial surgery: Part two. External versus internal distraction osteogenesis for the management of severe maxillary hypoplasia: external distraction. J Oral Maxillofac Surg. 2008;66:2598–2604. doi: 10.1016/j.joms.2008.05.371. [DOI] [PubMed] [Google Scholar]
- 24.Molina FM, Morales C, Taylor JA. Mandibular distraction osteogenesis in a patient with Melnick-Needles syndrome. J Craniofac Surg. 2008;19:277–279. doi: 10.1097/SCS.0b013e3181577aab. [DOI] [PubMed] [Google Scholar]
- 25.Rhee ST, Buchman SR. Pediatric mandibular distraction osteogenesis: the present and the future. J Craniofac Surg. 2003;14:803–808. doi: 10.1097/00001665-200309000-00040. [DOI] [PubMed] [Google Scholar]
- 26.Ilizarov GA. Clinical application of the tension-stress effect for limb lengthening. Clin Orthop Relat Res. 1990:8–26. [PubMed] [Google Scholar]
- 27.Turner CH. Measurement of the Young's modulus bending tests can be highly inaccurate. J Orthop Res. 1993;11:462–463. doi: 10.1002/jor.1100110321. [DOI] [PubMed] [Google Scholar]
- 28.Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 29.Cheung LK, Zheng LW. Effect of recombinant human bone morphogenetic protein-2 on mandibular distraction at different rates in an experimental model. J Craniofac Surg. 2006;17:100–108. doi: 10.1097/01.scs.0000188744.06723.1f. discussion 109–110, [DOI] [PubMed] [Google Scholar]
- 30.Elovic RP, Hipp JA, Hayes WC. A method for measuring the structural properties of the rat mandible. Arch Oral Biol. 1994;39:1029–1033. doi: 10.1016/0003-9969(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 31.Aronson J, Shen XC, Skinner RA, et al. Rat model of distraction osteogenesis. J Orthop Res. 1997;15:221–226. doi: 10.1002/jor.1100150210. [DOI] [PubMed] [Google Scholar]
- 32.Richards M, Goulet JA, Schaffler MB, et al. Temporal and spatial characterization of regenerate bone in the lengthened rabbit tibia. J Bone Miner Res. 1999;14:1978–1986. doi: 10.1359/jbmr.1999.14.11.1978. [DOI] [PubMed] [Google Scholar]
- 33.Richards M, Goulet JA, Weiss JA, et al. Bone regeneration and fracture healing. Experience with distraction osteogenesis model. Clin Orthop Relat Res. 1998:S191–S204. [PubMed] [Google Scholar]
- 34.Richards M, Waanders NA, Weiss JA, et al. Reduced gap strains induce changes in bone regeneration during distraction. J Biomech Eng. 1999;121:348–355. doi: 10.1115/1.2798331. [DOI] [PubMed] [Google Scholar]
- 35.Waanders NA, Richards M, Steen H, et al. Evaluation of the mechanical environment during distraction osteogenesis. Clin Orthop Relat Res. 1998:225–234. doi: 10.1097/00003086-199804000-00028. [DOI] [PubMed] [Google Scholar]
- 36.Huang CS, Ko WC, Lin WY, et al. Mandibular lengthening by distraction osteogenesis in children--a one-year follow-up study. Cleft Palate Craniofac J. 1999;36:269–274. doi: 10.1597/1545-1569_1999_036_0269_mlbdoi_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 37.Huisinga-Fischer CE, Vaandrager JM, Prahl-Andersen B. Longitudinal results of mandibular distraction osteogenesis in hemifacial microsomia. J Craniofac Surg. 2003;14:924–933. doi: 10.1097/00001665-200311000-00017. [DOI] [PubMed] [Google Scholar]
- 38.Ko EW, Hung KF, Huang CS, et al. Correction of facial asymmetry with multiplanar mandible distraction: a one-year follow-up study. Cleft Palate Craniofac J. 2004;41:5–12. doi: 10.1597/02-132. [DOI] [PubMed] [Google Scholar]
- 39.Kusnoto B, Figueroa AA, Polley JW. A longitudinal three-dimensional evaluation of the growth pattern in hemifacial microsomia treated by mandibular distraction osteogenesis: a preliminary report. J Craniofac Surg. 1999;10:480–486. doi: 10.1097/00001665-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Rachmiel A, Manor R, Peled M, et al. Intraoral distraction osteogenesis of the mandible in hemifacial microsomia. J Oral Maxillofac Surg. 2001;59:728–733. doi: 10.1053/joms.2001.24280. [DOI] [PubMed] [Google Scholar]
- 41.Shetye PR, Grayson BH, Mackool RJ, et al. Long-term stability and growth following unilateral mandibular distraction in growing children with craniofacial microsomia. Plast Reconstr Surg. 2006;118:985–995. doi: 10.1097/01.prs.0000232830.12603.eb. [DOI] [PubMed] [Google Scholar]
- 42.Chiapasco M, Lang NP, Bosshardt DD. Quality and quantity of bone following alveolar distraction osteogenesis in the human mandible. Clin Oral Implants Res. 2006;17:394–402. doi: 10.1111/j.1600-0501.2005.01247.x. [DOI] [PubMed] [Google Scholar]
- 43.Higuera S, Cole P, Stephenson JB, et al. Distraction rate and latency: factors in the outcome of paediatric maxillary distraction. J Plast Reconstr Aesthet Surg. 2008 doi: 10.1016/j.bjps.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 44.Kim UK, Chung IK, Lee KH, et al. Bone regeneration in mandibular distraction osteogenesis combined with compression stimulation. J Oral Maxillofac Surg. 2006;64:1498–1505. doi: 10.1016/j.joms.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 45.Goldstein SA. The mechanical properties of trabecular bone: dependence on anatomic location and function. J Biomech. 1987;20:1055–1061. doi: 10.1016/0021-9290(87)90023-6. [DOI] [PubMed] [Google Scholar]
- 46.Goldstein SA, Goulet R, McCubbrey D. Measurement and significance of three-dimensional architecture to the mechanical integrity of trabecular bone. Calcif Tissue Int. 1993;53(Suppl 1):S127–S132. doi: 10.1007/BF01673421. discussion S132-123, [DOI] [PubMed] [Google Scholar]
- 47.Chehade MJ, Pohl AP, Pearcy MJ, et al. Clinical implications of stiffness and strength changes in fracture healing. J Bone Joint Surg Br. 1997;79:9–12. doi: 10.1302/0301-620x.79b1.6324. [DOI] [PubMed] [Google Scholar]
- 48.Shefelbine SJ, Simon U, Claes L, et al. Prediction of fracture callus mechanical properties using micro-CT images and voxel-based finite element analysis. Bone. 2005;36:480–488. doi: 10.1016/j.bone.2004.11.007. [DOI] [PubMed] [Google Scholar]