Abstract
Interactions between genetic and environmental risk factors underlie a number of neuropsychiatric disorders, including schizophrenia (SZ) and autism (AD). Due to the complexity and multitude of the genetic and environmental factors attributed to these disorders, recent research strategies focus on elucidating the common molecular pathways through which these multiple risk factors may function. In this study, we examine the combined effects of a haplo-insufficiency of glutamate carboxypeptidase II (GCPII) and dietary folic acid deficiency. In addition to serving as a neuropeptidase, GCPII catalyzes the absorption of folate. GCPII and folate depletion interact within the one-carbon metabolic pathway and/or of modulate the glutamatergic system. Four groups of mice were tested: wildtype, GCPII hypomorphs, and wildtypes and GCPII hypomorphs both fed a folate deficient diet. Due to sex differences in the prevalence of SZ and AD, both male and female mice were assessed on a number of behavioral tasks including locomotor activity, rotorod, social interaction, pre-pulse inhibition, and spatial memory. Wildtype mice of both sexes fed a folic acid deficient diet showed motor coordination impairments and cognitive deficits, while social interactions were decreased only in males. GCPII mutant mice of both sexes also exhibited reduced social propensities. In contrast, all folate-depleted GCPII hypomorphs performed similarly to untreated wildtype mice, suggesting that reduced GCPII expression and folate deficiency are mutually protective. Analyses of folate and neurometabolite levels associated with glutamatergic function suggest several potential mechanisms through which GCPII and folate may be interacting to create this protective effect.
Keywords: gene-environment interactions, glutamate, glutamate carboxypeptidase II, folate, one-carbon metabolism
INTRODUCTION
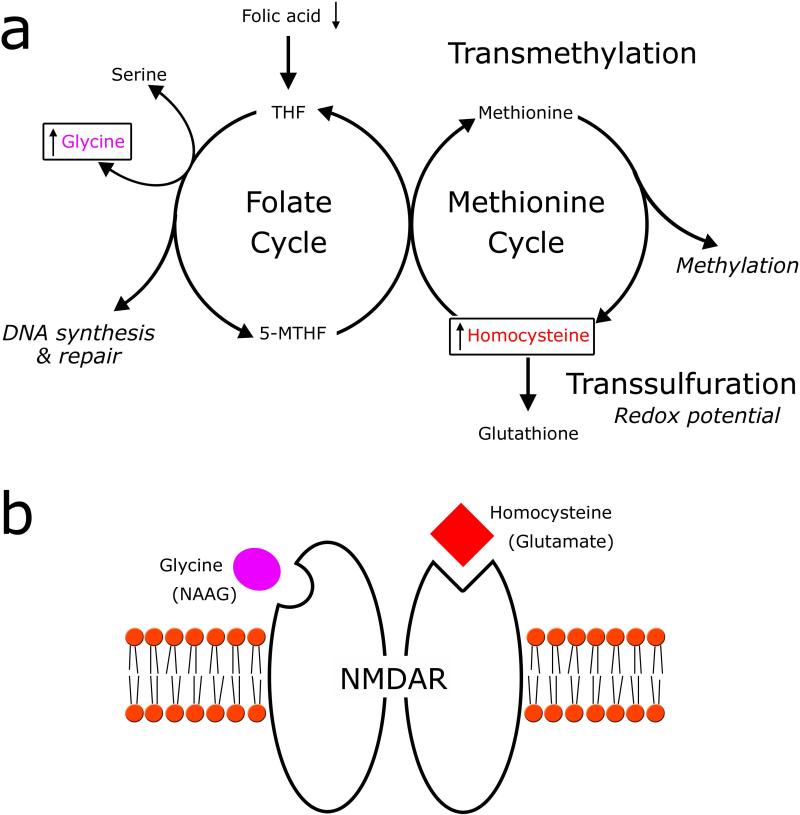
It is clear that numerous neuropsychiatric disorders, which are heterogeneous in origin, arise from complex interactions between genetic and environmental risk factors. Disorders with a developmental etiology, for example schizophrenia (SZ) and autism (AD), are particularly sensitive to these interactions during critical periods early in development and throughout the juvenile period (Bale et al., 2010). Recent reviews of the literature confirm that the number of genes and environmental factors that are associated with these neurodevelopmental disorders is staggering (for example Ross et al., 2006; El-Fishawy and State, 2010). Given the multitude of interacting factors, it is not surprising that recent studies focus on genetic and environmental factors that converge on specific metabolic and neurochemical pathways. Two intersecting pathways of interest in both SZ and AD are the one-carbon (C1) metabolic and the glutamatergic pathways (Figure 1a,b). C1 metabolism is comprised of three interconnected pathways, each of which mediates critical cellular functions including cellular redox balance (trans-sulfuration pathway), de novo nucleotide synthesis for DNA replication and repair (folate cycle), and methylation reactions (methionine cycle). These latter two cycles are collectively referred to as the transmethylation pathway (see Fox and Stover, 2008 for an in depth overview of C1 metabolism).
Figure 1. One-carbon cycle metabolites modulate glutamatergic neurotransmission.
(a) A simplified schematic of the three interconnected one-carbon cycle pathways. One-carbon metabolites, glycine and homocysteine (boxed), are hypothesized to be increased following folic acid deprivation and are also known to modulate glutamatergic neurotransmission. Tetrahydrofolate (THF) and 5-methyl-tetrahydrofolate (5-MTHF) are folate derivatives. (b) The binding sites of agonists glycine (purple) and homocysteine (red), from the one-carbon cycle, are depicted on the NMDA receptor. In addition, the binding sites of the excitatory neurotransmitter glutamate and the antagonist N-acetylaspartylglutamate (NAAG) are shown in parenthesis.
Although it is not always clear whether abnormalities in C1 metabolism are the primary cause of or secondary to other molecular changes, there is ample evidence to suggest that all three of these pathways contribute to disease pathology in SZ and AD (reviewed in Sugden, 2006; Deth et al., 2008). For example, an overabundance of reactive oxygen species and reduced production of antioxidants, through C1 metabolic intermediates, have been noted in a number of cases of SZ and AD (Do et al., 2000; James et al., 2006). The reduced capacity for DNA repair leads to genomic instability at fragile sites within genes that have been independently linked to these disorders (Smith et al., 2010). A decrease in the availability of methyl groups may both alter neurotransmitter degradation, as well as lead to epigenetic changes in gene expression within pathways implicated in these disorders (Mill et al., 2008; Nguyen et al., 2010). Epigenetics refers to heritable changes in gene expression that do not alter the DNA sequence and occur through the addition or removal of “marks” on DNA, including methyl groups supplied by C1 metabolism (Dupont et al., 2009).
One of the primary essential nutrients that act as a substrate for C1 metabolism is folate, or its synthetic form, folic acid. Following transport into the cell, folate is metabolized into a number of derivatives that mediate C1 metabolic reactions. Folate deficiency in humans is associated with neural tube deficits and spina bifida, and also with a number of neuropsychiatric disorders including depression, cognitive dysfunction, and epilepsy (Selhub et al., 2000; Morris et al., 2003; Ahrens et al., 2011; Mangold et al., 2011). Behavioral impairments associated with these disorders may be the result of abnormal neuronal function following changes to DNA methylation patterns, or could result directly from an alteration in the concentration of intermediates within the C1 metabolic cycle that also play important roles in glutamatergic neurotransmission (reviewed in Krebs et al., 2009). Depleted folate status increases glycine and homocysteine concentrations (Maloney et al., 2007) that can act as agonists at the N-methyl-d-aspartate (NMDA) subtype of glutamatergic receptor (Figure 1b) (Lipton et al., 1997; Yang and Svensson, 2008).
In a previous study, we investigated the behavior and biochemistry of a glutamate carboxypeptidase II (GCPII) heterozygote mouse (Han et al., 2009). GCPII and folate are connected through both C1 metabolism and glutamatergic neurotransmission. The gene for GCPII, folate hydrolase 1 (FOLH1), encodes an enzyme that catalyzes the removal of glutamate from two different substrates (Halsted et al., 1998). GCPII, also referred to as FOLH1, cleaves polyglutamates from dietary folate (Devlin et al., 2000) or from folate located within tissues to facilitate transport into or out of cells via folate transporters. Within the brain, GCPII, also referred to as N-acetylated alpha-linked acidic depeptidase 1 (NAALADase1) is localized to astrocytes and catalyzes the hydrolysis of N-acetylaspartylglutamate (NAAG) into N-acetylaspartate (NAA) and glutamate (Luthi-Carter et al., 1998; Berger et al., 1999). A mutation that reduces GCPII expression would reduce NMDAR activity through three putative mechanisms. First, extrasynaptic glutamate levels are decreased due to reduced hydrolysis of NAAG into NAA and glutamate. Second, increased NAAG concentrations act directly as a NMDAR antagonist, and third NAAG further reduces evoked glutamate release indirectly through its role as an agonist at the metabotropic glutamate receptor 3 (mGluR3) (reviewed in Coyle, 1997). Behavioral characterization of heterozygotic knockout (GCPII+/-) mice (null mice are embryonic lethal) suggests that a 50% reduction in GCPII expression leads to subtle deficits on a number of behavioral tasks (Han et al., 2009).
Both folate and GCPII are capable of interacting within the C1 metabolic pathway as well as directly in modulating glutamatergic neurotransmission. However, it is still largely unknown how specific genotypes modulate the response to adverse environmental factors. Therefore, this study aims to model the interactions between risk factors relevant to a number of neurodevelopmental disorders by understanding the interaction between folate deficiency, an environmental risk factor, and a mutation resulting in reduced GCPII expression on behavior in mice. In both SZ and AD, males are more susceptible than females to developing the disorder. Thus, we assessed behavior in both male and female mice to determine if this genetic and environmental factor differentially affects the two sexes. Within each sex, four groups of mice, wildtype, wildtype mice fed a diet deficient in folic acid, GCPII hypomorph, and GCPII hypomorph mice fed a folate deficient diet, were tested on behavioral tasks that assess motor, sensory-motor gating, social and cognitive function.
METHODS
GCPII Mouse Model and Folate Deficient Diet
All experiments were conducted on GCPII+/- mice bred on a C57BL/6 background for >10 generations (Han et al., 2009) with procedures approved by the Wellesley College Institutional Animal Care and Use Committee, conforming to the standards set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. At weaning, pups were housed in cages with up to 5 same sex littermates. Mice were maintained on a 12 h light/dark cycle with lights on at 0700 and food and water provided ad libitum. GCPII+/- dams were mated to C57BL/6 male mice and birth was considered postnatal day 1 (PN 1). A total of 90 offspring, used in this study, were randomly placed on either a control diet (Harlan Teklad; USA) containing 2 mg/kg folate or a folate deficient diet (Harlan Teklad; USA) containing 0.3 mg/kg folate and 1% succinyl sulfathiazole at PN 25. Male and female mice from each of the following four groups were utilized: wildtype control (WTC), wildtype folate-deficient (WTFD), GCPII+/-control (GC), and GCPII+/- folate-deficient (GFD).
Behavioral Assays
An observer blind to the mouse's genotype performed and analyzed all behavioral tests. All mice underwent an identical battery of tests at the approximate postnatal ages indicated. Dark-cycle locomotor activity (PN 90): Baseline locomotion of the mouse was measured over the 12 hr dark-cycle as described in (Han et al., 2009). The average number of movements for each hour and across the 12 hours were compared between groups. Accelerating rotor-rod (PN 91): Balance and motor coordination were measured on an accelerating rotor-rod (San Diego Instruments, San Diego, CA) as described in (Nag and Berger-Sweeney, 2007). The average of three successive trails were used for statistical analysis. Pre-pulse Inhibition (PN 110): Startle chambers from San Diego Instruments (San Diego, CA) were used as in (Han et al., 2009). Briefly, a 70 dB white background noise was presented for 5 min to acclimate the mouse to the chamber and continued throughout test session. Fourteen blocks of trials were presented with variable interstimulus intervals averaging 15 sec. Blocks 1 and 14 consisted of six pulse-alone (40 msec long 120-dB broadband burst) trials. Blocks 2 through 13 each contained five trials presented in a pseudorandomized order comprising: a 120-dB pulse alone (as in Blocks 1 and 14), a 120-dB pulse preceded 50 msec by 20 msec-long prepulses of 73, 76, or 82-dB (3, 6, and 12 dB above background), and a no-stimulus trial. Social approach (PN 115): Mice were tested for sociability using a three-chambered paradigm originally described in (Moy et al., 2004). The test consisted of two parts. Habituation: The subject mouse was placed into the center compartment for 5 min with the doorways to side compartments closed. Sociability: Five min after the completion of the habituation phase, an unfamiliar age-matched C57BL/6 was enclosed in a small wire cage (Galaxy Cup, Spectrum Diversified Designs, Streetsboro, OH) in either the left or the right chamber and allowed to habituated for 1 minute. The location of the unfamiliar mouse was counterbalanced between subjects. The subject was then returned to the center compartment, with both doorways open, and allowed to explore for 10 min. The sociability phase was videotaped and the time the subject mouse spent in each compartment as well as the number of entries into each chamber were determined. Morris Water Maze (PN 120 – 132): The water maze uses the Water 2020 (HVS Imaging, UK) video tracking system and the following procedure. Learning: Each mouse was given four trials per day, spaced 10 minutes apart, over eight consecutive days to learn the location of a hidden platform. During each trial the mouse was placed in the pool at one of four defined compass points around the edge of the pool and given 60 sec to find the platform. Upon reaching the platform, the mouse was left on the platform for 5 sec before being return to its home cage. Latency to the platform was used to assess the mouse's spatial learning ability. Probe: On the ninth day, the platform was removed and the mouse was given 60 sec to search for the platform, before being removed by the experimenter. The percent time spent in the platform quadrant versus other quadrants was used as a measure of the mouse's spatial memory.
Tissue collection
Blood was collected from the submandibular jaw vein for folate measurements the day after rotorod testing. Following the completion of behavioral testing, mice were overdosed with CO2. Trunk blood was collected for homocysteine measurements. The cortex and striatum were rapidly dissected, snap frozen on dry ice, and stored at -80°C for 1H-NMR analysis and glutathione assay.
Folate and homocysteine measurements
Plasma and liver folate were assayed by microbiological assay as described by Horne and Patterson (1988) and homocysteine was assayed as described in Araki and Sako (1987).
1H-NMR analysis of neurometabolites
To prepare samples for NMR spectroscopy, the frozen cortical tissue was processed and water-soluble metabolites extracted as described in Ward, et al. (2009). Solutions for 1H NMR spectroscopy were prepared by dissolving the dried water-soluble extracts in 500μL of 99% D2O (Wilmad) (pH = 7.8 ± 0.2). A coaxial insert containing trimethylsilyl propionate (TSP – 0.01 M in D2O) served as the chemical shift reference. Spectra were acquired using the pulse program zg30 (TR = 2.00 ms, NS = 400) on a 7.05 T Bruker Avance NMR spectrometer. Data collection and analysis were performed genotype blind. NMR spectra were analyzed using 1D WINNMR (Bruker). The free-induction decay was processed with a Lorentz-Gaussian Convert resolution-enhancing apodization function (LB = -2.1 Hz) to facilitate curve fitting. Peak integrals were obtained for glutamate (Glu), glutamine (Gln), (NAA+NAAG) (overlapping peaks), and creatine (CR3). Published chemical shifts (Govindaraju 2000) and spiking studies with Glu and Gln were used in the identification of peaks corresponding to the metabolites of interest. Normalized (i.e. absolute peak area divided by the number of protons producing the peak area) brain metabolite ratios relative to CR singlet at 3 ppm were calculated for Glu, Gln, and (NAA+NAAG).
Glutathione assay
Defrosted striatal samples were weighed and homogenized in 5% metaphosphoric acid at a concentration of 20 mL/gram tissue. Following centrifugation, the supernatant was collected for the assay. Total and oxidized glutathione were measured using a Glutathione Assay Kit (Cayman Chemicals; Ann Arbor, MI) following manufacturer's instructions. All samples were measured in triplicate.
Statistical Analysis
All data were analyzed separately for males and females using two-way analysis of variance (ANOVAs) using genotype and diet as the between group factors. For the social interaction task, student's t-tests were used to determine whether mice within an experimental group exhibited a preference for a social over a non-social stimulus. Data collected over multiple days in the morris water maze task were analyzed using two-way repeated-measures ANOVAs with day as the repeated measure. Post hoc bonferroni analyses (minimizes error due to multiple comparisons) were used to examine differences between groups following ANOVAs. All analysis were performed using SPSS software (SPSS Inc. Chicago, IL, USA) with p < 0.05 considered significant.
RESULTS
Folate levels are reduced and homocysteine is increased in folate-deprived experimental groups
Analysis of folate levels in blood plasma before the start of behavioral testing revealed a significant effect of DIET (ANOVA Males: [F(1,29) = 43.1, p < 0.001]; Females: [F(1,23) = 42.307, p < 0.001]). The levels of folate were significantly decreased in both wildtype and GCPII+/- folate deficient groups compared to wildtype and GCPII+/- control mice for both sexes (Males p's < 0.03; Females p's < 0.05), indicating that the diet deficient in folic acid effectively reduced folate (Table 1). Interestingly, there was a significant GENOTYPE × DIET interaction (ANOVA: Males: [F(1,29) = 6.201, p = 0.02]; Females: [F(1,23) = 6.292, p = 0.021]). Post-hoc analysis revealed that the levels of folate in GCPII+/- control mice were significantly higher than wildtype control mice for both sexes (Males: p = 0.035; Females: p = 0.05) suggesting that, in addition to the diet, the GCPII also significantly affects plasma folate levels. Analysis of liver folate levels in males following the completion of behavioral testing revealed a significant effect of DIET (ANOVA Males: [F(1,25) = 265.1, p < 0.001]). Similar to plasma, folate levels in the liver were significantly decreased in both wildtype and GCPII+/- folate deficient groups compared to wildtype and GCPII+/- control mice (p's < 0.001). Unlike plasma, folate levels in the liver were not significantly increased in GCPII+/-control compared to wildtype control mice. In addition, there was a slight but significant increase in folate levels in liver of GCPII+/- folate deficient mice compared to wildtype folate deficient mice (p = 0.047). Together, these data indicate that folate levels were significantly reduced in folate-deprived groups compared to control groups, but also that the GCPII mutation increases plasma and liver folate levels.
TABLE 1.
General Health and One-Carbon Metabolite Measurements
| WTC | WTFD | GC | GFD | |
|---|---|---|---|---|
| Weight | ||||
| Males | 26.95 ± 0.60 | 27.42 ± 0.83 | 27.07 ± 0.72 | 27.48 ± 0.44 |
| Females | 21.81 ± 0.68 | 21.54 ± 0.50 | 21.74 ± 0.51 | 21.10 ± 0.35 |
| Plasma Folate (ng/ml) | ||||
| Males | 90.5 ± 10.4 | 36.8 ± 7.2* | 146.0 ± 22.7† | 26.6 ± 6.1* |
| Females | 74.5 ± 11.0 | 37.3 ± 12.1 | 111.9 ± 9.8† | 28.0 ± 3.4* |
| Liver Folate (μg/g) | ||||
| Males | 72.4 ± 2.3 | 33.4 ± 1.3* | 70.4 ± 4.5 | 37.1 ± 0.8*‡ |
| Homocysteine (μM) | ||||
| Females | 5.0 ± 1.2 | 10.2 ± 1.5¥ | 8.1 ± 1.4 | 9.6 ± 1.9¥ |
All values represent the average ± SEM.
values significant less than WTC
values significantly larger than WTC
values significant larger than WTFD.
folate deficient groups combined significantly larger than control groups combined.
Weight: n's = 9 – 15 / group. Plasma and Liver Folate: n's = 4 – 9 / group. Homocysteine: n's = 5 – 6 / group.
Abbreviations: WTC, wildtype control; WTFD, wildtype folate deficient; GC, GCPII+/- control; GFD, GCPII+/- folate deficient.
Folate deprivation has previously been shown to result in increased levels of homocysteine (Ernest et al., 2006; MacFarlane et al., 2009), a NMDAR agonist. In our mice, there was a significant effect of DIET on levels of homocysteine in the blood following behavioral testing (ANOVA Females: [F(1,21) = 59.28, p = 0.045]). Posthoc analysis revealed a significant increase in homocysteine levels in wildtype and GCPII+/- folate deficient mice combined compared to wildtype and GCPII+/- control mice combined (Table 1). No further significant differences between groups were noted.
GCPII+/- and wildtype folate deficient mice exhibit a number of behavioral impairments
Postnatal folate deficiency may lead to overt health problems, including decreased body weight, which may affect performance on behavioral tasks (Gospe et al., 1995). Visual observation of the mice revealed no obvious general health issues or poor coat maintenance in the GCPII+/-control, wildtype folate deficient or GCPII+/- folate deficient mice. In addition, body weight, measured after the completion of behavioral tasks at PN 145, was not significantly different between the groups (all p's > 0.5; Table 1).
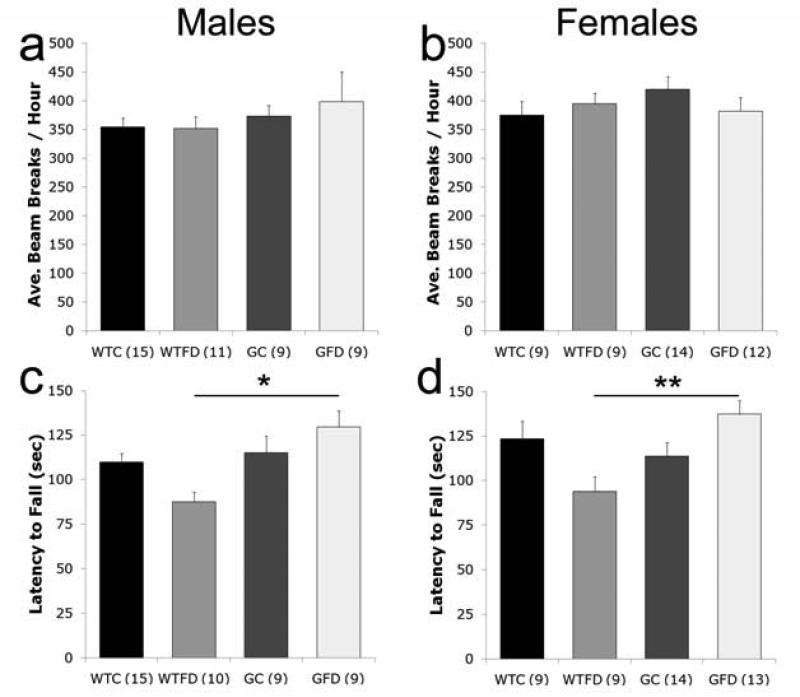
Mice were tested on five behavioral tasks to assess a number of aspects of behavior associated with folate-deficiency or glutamatergic dysfunction (i.e. motor activity, rotorod, social approach, pre-pulse inhibition, spatial water maze tasks). We measured general activity over the 12-h dark cycle. There were no significant differences in the average total activity level over the 12-h period by GENOTYPE or DIET for either the male or the female mice (Figure 2a,b), nor in the pattern of activity across the 12 hours (data not shown). However, motor coordination, assessed on the rotorod, was altered. There was a significant effect of GENOTYPE in males (ANOVA: [F(1,39) = 6.572, p = 0.014]) and a significant GENOTYPE × DIET interaction in females (ANOVA: [F(1,41) = 9.819, p = 0.003]). Post hoc analyses indicate that, motor coordination is only significantly impaired in both wildtype folate deficient males and females compared to GCPII+/- folate deficient mice in both sexes (Males: p < 0.03; Females p < 0.01; Fig 2c,d).
Figure 2. Motor activity and coordination.
Motor activity (a,b) was recorded in 1 hour intervals over a 12-h dark cycle. The average number of beam breaks across intervals is not significantly different between groups in male (a) or female (b) mice. Motor coordination, assessed on the rotorod, is impaired in wildtype folate deficient (WTFD) mice compared to GCPII+/- folate deficient (GFD) mice in both males (c) and females (d). All values are average ± SEM. n's per group are given in parenthesis next to each experimental group; * p < 0.05, ** p < 0.01. Abbreviations: WTC, wildtype control; WTFD, wildtype folate deficient; GC, GCPII+/- control; GFD, GCPII+/- folate deficient.
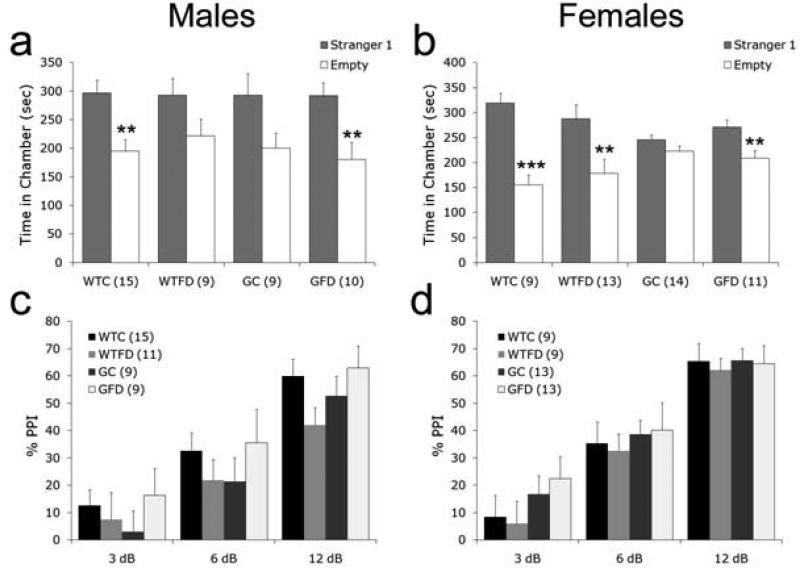
Social withdrawal was assessed with a simple social approach task, which relies on a mouse's innate tendency to investigate another mouse over an inanimate object (Moy et al., 2004). Wildtype control mice of both sexes exhibited a normal preference for spending more time with an unfamiliar mouse than with an empty cage (Student's t-test: Males: p < 0.002; Females: p < 0.001; Fig 3a,b). In addition, both male and female GCPII+/- folate deficient mice spent significantly more time with the mouse versus an empty cage (Student's t-test: p's < 0.01). Consistent with our previous study (Han et al., 2009), neither male nor female GCPII+/- control mice spent significantly more time investigating the mouse over the empty cage (Student's t-test Males: p = 0.07; Females: p = 0.19). Interestingly, male wildtype folate deficient mice showed no preference (p = 0.11), while female wildtype folate deficient mice showed a normal preference for spending time with another mouse vs. an empty cage (p < 0.01).
Figure 3. Social behavior and sensorimotor gating.
Interest in social interactions (a,b) was measured by comparing the amount of time a mouse spent investigating another mouse vs. an empty cage. Social approach was reduced in both male (a) and female (b) GC mice as well as male wildtype folate deficient (WTFD) mice. Sensorimotor gating (c,d), as assessed by PPI, was normal in all male (c) and all female (d) mice. All values are average ± SEM. n's per group are given in parenthesis next to each experimental group; ** p < 0.01, *** p < 0.001. Abbreviations: WTC, wildtype control; WTFD, wildtype folate deficient; GC, GCPII+/- control; GFD, GCPII+/- folate deficient.
Pre-pulse inhibition (PPI) of the acoustic startle reflex measures sensorimotor gating. PPI has previously been shown to be sensitive to pharmacological inhibition of GCPII (Takatsu et al., 2011). There were no significant differences in response to the 120 dB stimulus with pre-pulses 3 dB, 6 dB, or 12 db above background in either males or females (Fig 3c,d). Interpretable results of pre-pulse inhibition are highly dependent on similar baseline startle responses between groups (Yee et al., 2005). Response to the 120 dB stimulus alone was similar between all groups of males (wildtype control: 288 ± 34; wildtype folate deficient: 319 ± 52; GCPII+/- control: 320 ± 65; GCPII+/- folate deficient: 215 ± 25). In females, there was a significant effect of DIET on baseline startle response to the 120 dB stimulus [F(1,44) = 5.188, p <0.03]. Post-hoc analysis revealed that despite numerically lower startle responses in wildtype and GCPII+/- folate deficient mice combined, there were no significant differences between groups (wildtype control: 209 ± 26; wildtype folate deficient: 122 ± 17; GCPII+/- control: 243 ± 43; GCPII+/- folate deficient: 174 ± 34).
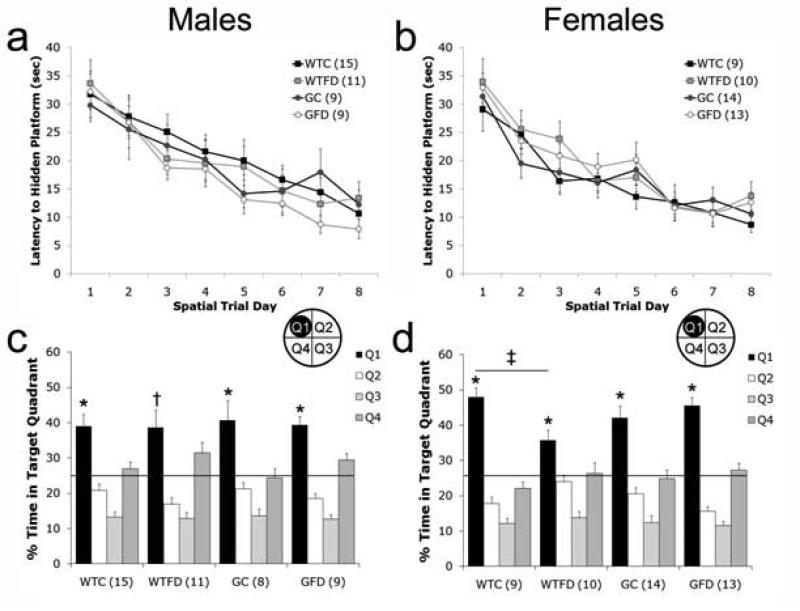
Impaired cognitive performance is associated with both folate deficiency (Selhub et al., 2000) and GCPII polymorphisms (Halsted et al., 2007) in humans. Therefore, we chose to examine cognitive performance on a water maze task. Male and female mice learned the location of the hidden platform during the acquisition phase of spatial learning (Fig 4a,b). Latency to find the platform decreased significantly across testing days (Repeated-measures ANOVA: Males: [F(7,33) = 24.825, p < 0.001]; Females [F(7,36) = 23.154, p < 0.001] indicating that the mice learned the location of the platform. All groups learned the location of the platform equally quickly as there were no significant effects of GENOTYPE, DIET or GENOTYPE × DIET interactions on latency.
Figure 4. Performance on Spatial Water Maze Task.
Latency to find a hidden platform is similar in all males (a) and all females (a) across the 8 days of learning. When mice were tested on Day 9 for memory of the platform location in a 60 sec probe trial, both male (c) and female (d) wildtype folate deficient (WTFD) showed impairments. All values are average ± SEM. n's per group are given in parenthesis next to each experimental group; * signifies p < 0.05 between Q1 and Q2 – Q4. † signifies p < 0.05 between Q1 and Q2 – Q3, but not Q4. ‡ p < 0.05. Abbreviations: WTC, wildtype control; WTFD, wildtype folate deficient; GC, GCPII+/- control; GFD, GCPII+/- folate deficient.
On Day 9, memory for the hidden platform location was assessed during a 60 sec probe trial. During the probe trial (Fig 4c,d), both male and female wildtype folate deficient mice exhibited an impaired memory for the hidden platform location (Q1) that manifest itself in subtly different ways. In males, there were no significant effects of GENOTYPE, DIET, or GENOTYPE x DIET on % time in the four quadrants; however, there was a significant effect of quadrant within groups. Further analysis revealed that wildtype control, GCPII+/- control, and GCPII+/- folate deficient mice all spent significantly more time in the target quadrant (Q1) than the other 3 quadrants (all p's < 0.01). wildtype folate deficient mice, on the other hand, spent significantly more time in Q1 than Q2 and Q3 (p's < 0.001), but not in the adjacent quadrant, Q4 (p = 0.33). In females, there was a significant GENOTYPE x DIET interaction on % time in the four quadrants [F(4,38) = 2.973, p < 0.03]. Post-hoc analysis comparing the % time in each quadrant showed that wildtype control females spent significantly more time than wildtype folate deficient mice in the target quadrant, Q1 (p < 0.05). However, unlike the males, all four groups spent significantly more time in Q1 than the other 3 quadrants (all p's < 0.05). To ensure that memory deficits on the probe trial were not associated with motor coordination impairments in wildtype folate deficient (displayed during rotorod testing), swim speed was compared between groups. Swim speed (m/sec) was not significantly different between groups for either the males (wildtype control: 0.21 ± 0.02, wildtype folate deficient: 0.20 ± 0.04, GCPII+/- control: 0.21 ± 0.01, GCPII+/- folate deficient: 0.20 ± 0.03) or the females (wildtype control: 0.21 ± 0.01, wildtype folate deficient: 0.21 ± 0.02, GCPII+/- control: 0.21 ± 0.02, GCPII+/- folate deficient: 0.21 ± 0.02).
Together these data indicate that GCPII+/- control and wildtype folate deficient mice are each impaired on a subset of behavioral tasks (GCPII+/- control, social approach; wildtype folate deficient, rotorod, social approach, and spatial memory); however, GCPII+/- folate deficient mice exhibit no behavioral abnormalities.
Abnormal neurometabolite concentrations in GCPII+/- control and wildtype folate deficient mice
1H-NMR spectroscopy is a useful method to determine the concentrations of metabolites associated with glutamatergic function. Studies have thoroughly characterized a number of metabolites in rodent studies with NMDAR dysfunction (Kondziella et al., 2006; Iltis et al., 2009). In this study, we measured neurometabolites in ex vivo cortical samples, which allowed fine resolution of glutamate and glutamine levels not possible with in vivo NMR (Table 2). The cortex was chosen because it is a region important in cognitive and social function, the main behavioral abnormalities noted in this study. There were no significant effects of GENOTYPE, DIET, or GENOTYPE × DIET interactions on the concentrations of glutamate (Glu:CR3), glutamine (Gln:CR3), total concentration of glutamate + glutamine (Glu+Gln:CR3), or NAA (NAA:CR3). However, there was a significant effect of GENOTYPE on the ratio of glutamate to glutamine (Glu:Gln; ANOVA [F(1,36) = 4.190, p = 0.049]). Posthoc analysis showed that Glu:Gln was increased significantly by 15% in GCPII+/- control mice compared to wildtype control mice (p = 0.009) with no other significant differences between groups. An increase in the ratio of glutamate to glutamine in GCPII+/- control mice appears to be due primarily to a decrease in glutamine levels rather than a change in glutamate levels.
TABLE 2.
Neurometabolite Levels in Brain Extracts
| WTC | WTFD | GC | GFD | |
|---|---|---|---|---|
| Glutamate (Glu:Cr3)† | 0.98 ± 0.02 | 1.05 ± 0.05 | 1.02 ± 0.02 | 1.05 ± 0.04 |
| Glutamine (Gln:Cr3)† | 0.64 ± 0.04 | 0.63 ± 0.03 | 0.54 ± 0.01 | 0.62 ± 0.06 |
| Glu + Gln:Cr3† | 1.59 ± 0.04 | 1.68 ± 0.07 | 1.58 ± 0.04 | 1.67 ± 0.08 |
| Glu:Gln† | 1.62 ± 0.07 | 1.67 ± 0.10 | 1.87 ± 0.04* | 1.77 ± 0.14 |
| NAA+NAAG:Cr3† | 0.75 ± 0.03 | 0.74 ± 0.01 | 0.73 ± 0.005 | 0.71 ± 0.01 |
| Total Glutathione‡ (nmol/mg protein) | 1864 ± 32 | 1942 ± 62* | 1970 ± 102* | 1900 ± 66 |
All values represent average ± SEM.
measured with 1H-NMRS in cortex (n's = 8-10/group)
measured with a colorimetric assay in striatum (n = 8/group)
values significantly different from WTC levels (p < 0.05).
Abbreviations: WTC, wildtype control; WTFD, wildtype folate deficient; GC, GCPII+/- control; GFD, GCPII+/- folate deficient.
In addition, we measured glutathione (GSH), the major intracellular antioxidant in the brain (Aoyama et al., 2008). GSH requires precursors from C1 metabolism, namely homocysteine, for production and levels of GSH are also regulated by glutamatergic function. The levels of reduced, oxidized and total glutathione in our four groups were measured. Reduced and oxidized levels of glutathione were not significantly different between groups (data not shown); however, there was a significant GENOTYPE × TREATMENT effect for concentration of total glutathione (ANOVA: [F(1,27) = 8.42, p = 0.007]. Further analysis showed that GSH is significantly elevated in wildtype folate deficient [p > 0.01] and in GCPII+/- control [p > 0.05] in comparison to wildtype control mice. GSH levels are not significantly different between wildtype control and GCPII+/- folate deficient mice.
DISCUSSION
These data reveal several interactions between a mutation in GCPII and altered levels of one of GCPII's substrates, folic acid, on behavior and associated metabolic and neurotransmitter function. We investigated this interaction by comparing mice with low dietary folic acid and/or the GCPII mutation with wildtype nutritionally intact mice. Behaviorally, mice with reduced GCPII expression or with folate-deficiency alone exhibit a number of phenotypic abnormalities. Similar to our previous findings, GCPII+/- control mice are less social than wildtype control mice (Han et al., 2009). Similar to studies in other laboratories, our wildtype folate deficient mice have impaired motor coordination and spatial memory (Troen et al., 2008; Chang et al., 2009). Additionally, we show that folate-deficient male mice exhibit decreased sociability, similar to the GCPII heterozygotes. The fact that only male wildtype folate deficient mice exhibit social deficits supports recent speculation that hormonal regulation of methyltransferases, enzymes that function to add methyl groups, can affect the function of neurotransmitter systems. Functional differences such as these may underlie a sexually dimorphic predisposition to neuropsychiatric disorders (Harrison and Tunbridge, 2008). In contrast to our original hypothesis that this gene-environment combination would be additive, male and female mice with both the GCPII mutation and folate-deficiency performed normally on all behavioral tasks. These data suggest that the GCPII mutation protects against folate-deficiency and/or conversely that folate-deficiency protects against reduced GCPII enzyme activity. This is a finding that we did not predict originally.
We saw further evidence that the GCPII mutation may protect against a folate-deficient diet. Prenatal folate deprivation in the mother caused physical abnormalities in the offspring (including small size, as well as eye deformities and rectal prolapse). These physical abnormalities in the offspring of folate-deprived mothers prevented us from testing the offspring behaviorally. In contrast, pups with combined GCPII mutation and folate-deficiency appeared normal (unpublished observations LS, JBS). The discovery of a mutually protective effect from the concomitant presence of two otherwise pathogenic factors, begs questions regarding the metabolic pathways that might be involved in conferring protection. GCPII and folate interact both within C1 metabolism, as well as directly on the glutamatergic function.
Dietary folic acid and GCPII are important regulators of C1 metabolism. Folic acid supplies the main substrate for the metabolic cycle, while GCPII regulates the folate levels within the cell to ensure normal C1 metabolic function. As we expected, plasma and liver folate levels were significantly reduced in folate-deficient mice compared to wildtype control mice. In contrast, we found that plasma folate levels in GCPII+/- mice were significantly elevated compared to control folate levels. Studies in human populations show that a specific polymorphism of GCPII (1561C→T) is associated with a 53% reduction in activity (Devlin et al., 2000) and higher plasma folate levels (Halsted et al., 2007). These findings suggest that GCPII haplo-insufficiency has similar effects on folate levels in both humans and mice. Based on GCPII's role in catalyzing the removal of glutamate moieties from folate, elevated plasma concentrations of folate are not entirely unexpected.
Transport of folates into or out of the cell by folate transporters can only occur when folate is monoglutamated (Gangjee et al., 2002). Following entry into the cell, folates are rapidly polyglutamated through the addition of 4-8 glutamate residues. Polyglutamation functions both to maintain high levels of folate intracellularly and to increase the affinity of folates for enzymes within C1 metabolic cycle (Shane, 1989). Synthetic folic acid, the only dietary source of folate for our mice, contains only one glutamate residue and therefore can readily be transported into plasma, liver, or even brain where it is converted into folate derivatives that are polyglutamated. Reduced GCPII enzymatic activity likely leads to cleavage of fewer glutamates and thus to a higher concentration of folates within the cell. If brain concentrations of folates are excessively high in GCPII+/- control mice, C1 metabolic function may be adversely affected, which could explain the reduced propensity for social interaction in these mice. Interestingly, questions have been raised as to whether the recent excess in folic acid supplementation, both from food fortification programs and vitamin intake, especially in utero, is associated with the rapid increase in prevalence of autism during this same time period (Rogers, 2008; Beard et al., 2011). Further analysis of the distribution of folates in the brains of GCPII+/- control mice and further behavioral testing may potentially provide some important clues to answer this question.
In contrast, an enhanced ability to maintain intracellular folate levels by reduced GCPII activity may be protective during folic acid deficiency. While folate levels in GCPII+/- folate deficient mice were not increased in the plasma compared to wildtype folate deficient mice, the liver exhibited a slight elevation in folate during depletion. In general, studies in rodents show that the brain is more protected against folate deficiency than other tissues (Chen et al., 2001) suggesting that depletion of folates in the brain may not be as severe as that measured in the liver. Thus, it is possible that brain folate is more amenable to normalization than plasma or liver folate levels in the presence of reduced GCPII function. Therefore, one mechanism through which reciprocal behavioral protection could have occurred in the current study is through the normalization of brain folate concentrations.
Folate depletion and GCPII mutations are also thought to affect glutamatergic neurotransmission; therefore, we examined several metabolites in the glutamatergic pathway using 1H-NMR spectroscopy to determine whether the GCPII mutation alone affects NMDAR function and to what extent, if any, dietary folate deficiency and GCPII may interact directly on the glutamatergic system. Contrary to our expectation, GCPII+/- mice on a control diet exhibit no change in glutamate levels, which may reflect the fact that 1H-NMR cannot distinguish between metabolic and neurotransmitter glutamate levels. Despite a lack of change in glutamate concentrations in GCPII+/- mice, there was a significant increase in the glutamate/glutamine (Glu/Gln) ratio due primarily to a reduction in glutamine. Glutamine is primarily synthesized in astrocytes from glutamate taken up from the synaptic cleft. An increase in the Glu/Gln likely reflects reduced glutamatergic neurotransmission (Yuksel 2010). Similar alterations in the glutamate-glutamine cycle have also been reported in rodents administered NMDAR antagonists PCP (Iltis et al., 2009), MK-801 (Brenner et al., 2005; Kondziella et al., 2006) and ketamine (Kim et al., 2011). We were unable to detect any differences in Glu/Gln in wildtype folate deficient mice. This is not unexpected as low folate concentrations and an increase in homocysteine should directly modulate NMDARs without altering glutamatergic neurotransmission (Lipton et al., 1997). While changes in neurometabolites only provide an indirect measure of NMDAR activity, the 1H-NMR data are consistent with the hypothesis that GCPII haplo-insufficiency is associated with decreased glutamate neurotransmission, while the negative effects of folate deficiency are more likely associated with direct modulation of the NMDAR.
We also examined levels of glutathione (GSH) in the brains of the four experimental groups of mice. GSH utilizes homocysteine, from the methionine cycle, as a precursor for synthesis (Reed et al., 2008; Lu, 2009) and can be released from the cell in an NMDAR-dependent manner (Wallin et al., 1999). Thus, GSH content within the brain may reflect changes in C1 metabolic function, release, or both. Unexpectedly, we found that both folate-deficiency in wildtype mice and GCPII haploinsufficiency led to significant increases in GSH. Elevated GSH levels in wildtype folate deficient may indicate mild chronic neurotoxicity. Others have shown that mild excitotoxicity associated with increased NMDAR activation during folate depletion increases antioxidant defense and therefore GSH content (Shea et al., 2004; Tchantchou et al., 2004; Kronenberg et al., 2008). Conversely, elevated GSH in GCPII+/- control mice may instead reflect the maintenance of a larger pool of GSH within the neurons due to reduced NMDAR activation. Increased GSH in GCPII+/-control mice is consistent with elevated levels reported in rodents administered NMDAR antagonists MK-801 and PCP (Brenner et al., 2005; Kondziella et al., 2006).
Based on the neurochemical data, we propose the following mechanism for reciprocal behavioral rescue within the glutamatergic system (see Figure 5). In wildtype and GCPII+/- folate deficient mice, folate deficiency leads to increased homocysteine levels. We hypothesize that hyper-homocysteinemia leads to elevated activation of NMDARs in both wildtype and GCPII+/- folate deficient mice. In GCPII+/- mice, on the other hand, NMDAR hypofunction is due to a combination of reduced glutamate release and increased NMDAR antagonism by NAAG. In the GCPII+/- folate deficient mice, activation of NMDARs is normalized. The precise mechanism through which this occurs is unclear; however, based on the binding affinities of NAAG and homocysteine, the following mechanism seems most probable. Homocysteine is a relatively high-affinity NMDAR agonist (Lipton et al., 1997), whereas NAAG binds with relatively low-affinity to NMDARs (Valivullah et al., 1994) and with much higher affinity to mGluR3s (Wroblewska et al., 1997). In the presence of high levels of homocysteine and NAAG, homocysteine could increase activation of NMDARs, while glutamate levels are decreased both through reduced glutamate production and through an increase in mGluR3 agonism by NAAG that further inhibits presynaptic glutamate release. In support of this hypothesis, studies show that mGluR2/3 agonists alone can induce behavioral deficits in rodents, but are also capable of attenuating behavioral deficits that are the result of increased glutamate release (for recent examples, Schlumberger et al., 2009; Pozzi et al., 2011). Together, these experiments underscore the importance of maintaining precise levels of activity to prevent behavioral impairments. Examination of folate levels as well as indirect measures of NMDAR function suggest that GCPII and folate may be interacting in opposite manners within both of these pathways and provide two potential mechanisms through which the combined mutation and deficiency may mutually protect against associated behavioral impairments.
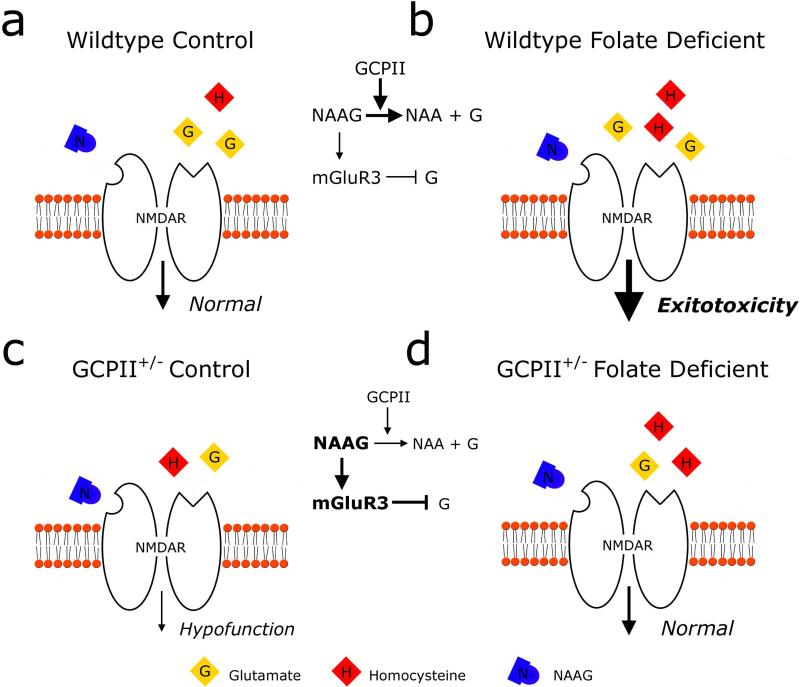
Figure 5. Proposed NMDA receptor mediated mechanism of reciprocal behavioral protection.
A model showing the relative levels of NMDAR agonists, glutamate (G) and homocysteine (H), and antagonist NAAG (N) in the synaptic cleft during glutamatergic neurotransmission in the four experimental groups. In wildtype mice on a control diet (a) or folate deficient diet (b), GCPII hydrolyzes NAAG into NAA and glutamate similarly; therefore, both groups exhibit similar levels of glutamate and NAAG. In wildtype folate deprived mice (b), increased levels of homocysteine can additionally activate NMDAR's and cause excitotoxicity. Reduced function of GCPII in heterozygous mice on both a control diet (c) and a folate deficient diet (d) increases the levels of NAAG and decreases NAA and glutamate levels. NAAG likely predominately activates presynaptic mGluR3 receptors to further inhibit glutamate release. Thus, both GCPII heterozygous groups exhibit a similar decrease in levels of glutamate compared to wildtype groups. In GCPII mice on a control diet (c), reduced glutamate results in NMDAR hypofunction whereas in GCPII folate deficient mice (d), decreased levels of glutamate are paired with increased homocysteine that results in overall normal levels of activity.
The current study raises the interesting conjecture that maintenance of hypofunctioning mutations of GCPII in the gene pool could be preserved by reduced availability of its substrate, folic acid. Folic acid deficiency is not uncommon in times of famine and was likely much more common in the past than it is in currently in the developed world. Shielding the organism from this dietary deficiency would be an important protective mechanism. It is reasonable to assume that protection could come from mutations within the same molecular pathway that would return the pathway's function to normal. In line with this conjecture, recent fortification of foods with folic acid has led to increases in the population frequency of another mutation within the C1 metabolic pathway (Munoz-Moran et al., 1998; Agodi et al., 2011), which is also a risk factor for both SZ (Hill et al., 2011) and AD (James et al., 2006). Selection for mutations in key genes, therefore, could provide a protective mechanism at a population level, but may also place individuals carrying the mutation at a higher risk for metabolic and behavioral dysfunction at normal dietary levels of folate.
Mutations in GCPII and folate deficiency are independent risk factors for neuropsychiatric disorders. GCPII expression is reduced in the prefrontal cortex and hippocampus of patients with SZ (Tsai et al., 1995; Guilarte et al., 2008; Ghose et al., 2009), while folate deficiency in utero has been linked to an increased risk in the offspring for SZ (reviewed in Picker and Coyle, 2005; McGrath et al., 2010) and AD (Schmidt et al., 2011). There are a number of putative mechanisms through which GCPII and folic acid may function. The current experiments provide clues as to two potential mechanisms through which this genetic and environmental risk factor may independently function to alter the behavioral phenotype. This study demonstrates how the shift in focus to understanding gene-environment interactions may point to the next important avenues of investigation to understand the complex etiology of disorders such as schizophrenia and autism.
Acknowledgements
The authors would like to thank Hillary Chu and Carla Lopez for their help with behavioral testing and analysis, Urs Berger for genotyping the mice, and Pat Carey and Valerie LePage for taking excellent care of the mice. Research was supported by NIH grant DK42033 to BS and R01MH05190 and P50MH0G0450 to JTC.
ABBREVIATIONS
- AD
autism disorder
- C1 metabolism
one-carbon metabolism
- CR3
creatine
- GC
GCPII+/- control diet
- GFD
GCPII+/- folate-deprived
- GCPII
glutamate carboxypeptidase II
- Glu
glutamate
- Gln
glutamine
- GSH
glutathione
- mGluR3
metabotropic glutamate receptor 3
- NAA
N-acetylaspartate
- NAAG
N-acetylaspartylglutamate
- NMDAR
N-methyl-d-aspartate receptor
- NMR
nuclear magnetic resonance
- PCP
phencyclidine
- SZ
schizophrenia
- WTC
wildtype control diet
- WTFD
wildtype folate-deprived
Footnotes
Conflict of Interest: The authors are unaware of any conflicts of interest.
References
- Agodi A, Barchitta M, Valenti G, Marzagalli R, Frontini V, Marchese AE. Increase in the prevalence of the MTHFR 677 TT polymorphism in women born since 1959: potential implications for folate requirements. European journal of clinical nutrition. 2011 doi: 10.1038/ejcn.2011.125. [DOI] [PubMed] [Google Scholar]
- Ahrens K, Yazdy MM, Mitchell AA, Werler MM. Folic Acid intake and spina bifida in the era of dietary folic Acid fortification. Epidemiology. 2011;22:731–737. doi: 10.1097/EDE.0b013e3182227887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama K, Watabe M, Nakaki T. Regulation of neuronal glutathione synthesis. Journal of pharmacological sciences. 2008;108:227–238. doi: 10.1254/jphs.08r01cr. [DOI] [PubMed] [Google Scholar]
- Araki A, Sako Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. Journal of chromatography. 1987;422:43–52. doi: 10.1016/0378-4347(87)80438-3. [DOI] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nemeroff CB, Reyes TM, Simerly RB, Susser ES, Nestler EJ. Early life programming and neurodevelopmental disorders. Biological psychiatry. 2010;68:314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard CM, Panser LA, Katusic SK. Is excess folic acid supplementation a risk factor for autism? Medical hypotheses. 2011;77:15–17. doi: 10.1016/j.mehy.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Berger UV, Luthi-Carter R, Passani LA, Elkabes S, Black I, Konradi C, Coyle JT. Glutamate carboxypeptidase II is expressed by astrocytes in the adult rat nervous system. The Journal of comparative neurology. 1999;415:52–64. doi: 10.1002/(sici)1096-9861(19991206)415:1<52::aid-cne4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Brenner E, Kondziella D, Haberg A, Sonnewald U. Impaired glutamine metabolism in NMDA receptor hypofunction induced by MK801. Journal of neurochemistry. 2005;94:1594–1603. doi: 10.1111/j.1471-4159.2005.03311.x. [DOI] [PubMed] [Google Scholar]
- Chang N, Kim E, Kim KN, Kim H, Kim SY, Jeong BS. Folate nutrition is related to neuropsychological functions in the elderly. Nutrition research and practice. 2009;3:43–48. doi: 10.4162/nrp.2009.3.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Karaplis AC, Ackerman SL, Pogribny IP, Melnyk S, Lussier-Cacan S, Chen MF, Pai A, John SW, Smith RS, Bottiglieri T, Bagley P, Selhub J, Rudnicki MA, James SJ, Rozen R. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Human molecular genetics. 2001;10:433–443. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- Coyle JT. The nagging question of the function of N-acetylaspartylglutamate. Neurobiology of disease. 1997;4:231–238. doi: 10.1006/nbdi.1997.0153. [DOI] [PubMed] [Google Scholar]
- Deth R, Muratore C, Benzecry J, Power-Charnitsky VA, Waly M. How environmental and genetic factors combine to cause autism: A redox/methylation hypothesis. Neurotoxicology. 2008;29:190–201. doi: 10.1016/j.neuro.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Devlin AM, Ling EH, Peerson JM, Fernando S, Clarke R, Smith AD, Halsted CH. Glutamate carboxypeptidase II: a polymorphism associated with lower levels of serum folate and hyperhomocysteinemia. Human molecular genetics. 2000;9:2837–2844. doi: 10.1093/hmg/9.19.2837. [DOI] [PubMed] [Google Scholar]
- Do KQ, Trabesinger AH, Kirsten-Kruger M, Lauer CJ, Dydak U, Hell D, Holsboer F, Boesiger P, Cuenod M. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. The European journal of neuroscience. 2000;12:3721–3728. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- Dupont C, Armant DR, Brenner CA. Epigenetics: definition, mechanisms and clinical perspective. Seminars in reproductive medicine. 2009;27:351–357. doi: 10.1055/s-0029-1237423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Fishawy P, State MW. The genetics of autism: key issues, recent findings, and clinical implications. The Psychiatric clinics of North America. 2010;33:83–105. doi: 10.1016/j.psc.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernest S, Carter M, Shao H, Hosack A, Lerner N, Colmenares C, Rosenblatt DS, Pao YH, Ross ME, Nadeau JH. Parallel changes in metabolite and expression profiles in crooked-tail mutant and folate-reduced wild-type mice. Human molecular genetics. 2006;15:3387–3393. doi: 10.1093/hmg/ddl415. [DOI] [PubMed] [Google Scholar]
- Fox JT, Stover PJ. Folate-mediated one-carbon metabolism. Vitamins and hormones. 2008;79:1–44. doi: 10.1016/S0083-6729(08)00401-9. [DOI] [PubMed] [Google Scholar]
- Gangjee A, Dubash NP, Zeng Y, McGuire JJ. Recent advances in the chemistry and biology of folypoly-gamma-glutamate synthetase substrates and inhibitors. Current medicinal chemistry. Anti-cancer agents. 2002;2:331–355. doi: 10.2174/1568011024606352. [DOI] [PubMed] [Google Scholar]
- Ghose S, Chin R, Gallegos A, Roberts R, Coyle J, Tamminga C. Localization of NAAG-related gene expression deficits to the anterior hippocampus in schizophrenia. Schizophrenia research. 2009;111:131–137. doi: 10.1016/j.schres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospe SM, Jr., Gietzen DW, Summers PJ, Lunetta JM, Miller JW, Selhub J, Ellis WG, Clifford AJ. Behavioral and neurochemical changes in folate-deficient mice. Physiology & behavior. 1995;58:935–941. doi: 10.1016/0031-9384(95)00156-d. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Hammoud DA, McGlothan JL, Caffo BS, Foss CA, Kozikowski AP, Pomper MG. Dysregulation of glutamate carboxypeptidase II in psychiatric disease. Schizophrenia research. 2008;99:324–332. doi: 10.1016/j.schres.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsted CH, Ling EH, Luthi-Carter R, Villanueva JA, Gardner JM, Coyle JT. Folylpoly-gamma-glutamate carboxypeptidase from pig jejunum. Molecular characterization and relation to glutamate carboxypeptidase II. The Journal of biological chemistry. 1998;273:20417–20424. doi: 10.1074/jbc.273.32.20417. [DOI] [PubMed] [Google Scholar]
- Halsted CH, Wong DH, Peerson JM, Warden CH, Refsum H, Smith AD, Nygard OK, Ueland PM, Vollset SE, Tell GS. Relations of glutamate carboxypeptidase II (GCPII) polymorphisms to folate and homocysteine concentrations and to scores of cognition, anxiety, and depression in a homogeneous Norwegian population: the Hordaland Homocysteine Study. The American journal of clinical nutrition. 2007;86:514–521. doi: 10.1093/ajcn/86.2.514. [DOI] [PubMed] [Google Scholar]
- Han L, Picker JD, Schaevitz LR, Tsai G, Feng J, Jiang Z, Chu HC, Basu AC, Berger-Sweeney J, Coyle JT. Phenotypic characterization of mice heterozygous for a null mutation of glutamate carboxypeptidase II. Synapse. 2009;63:625–635. doi: 10.1002/syn.20649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Tunbridge EM. Catechol-O-methyltransferase (COMT): a gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:3037–3045. doi: 10.1038/sj.npp.1301543. [DOI] [PubMed] [Google Scholar]
- Hill M, Shannahan K, Jasinski S, Macklin EA, Raeke L, Roffman JL, Goff DC. Folate supplementation in schizophrenia: a possible role for MTHFR genotype. Schizophrenia research. 2011;127:41–45. doi: 10.1016/j.schres.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Horne DW, Patterson D. Lactobacillus casei microbiological assay of folic acid derivatives in 96-well microtiter plates. Clinical chemistry. 1988;34:2357–2359. [PubMed] [Google Scholar]
- Iltis I, Koski DM, Eberly LE, Nelson CD, Deelchand DK, Valette J, Ugurbil K, Lim KO, Henry PG. Neurochemical changes in the rat prefrontal cortex following acute phencyclidine treatment: an in vivo localized (1)H MRS study. NMR in biomedicine. 2009;22:737–744. doi: 10.1002/nbm.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SJ, Melnyk S, Jernigan S, Cleves MA, Halsted CH, Wong DH, Cutler P, Bock K, Boris M, Bradstreet JJ, Baker SM, Gaylor DW. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. American journal of medical genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2006;141B:947–956. doi: 10.1002/ajmg.b.30366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Lee H, Kim HJ, Bang E, Lee SH, Lee DW, Woo DC, Choi CB, Hong KS, Lee C, Choe BY. In vivo and ex vivo evidence for ketamine-induced hyperglutamatergic activity in the cerebral cortex of the rat: Potential relevance to schizophrenia. NMR in biomedicine. 2011 doi: 10.1002/nbm.1681. [DOI] [PubMed] [Google Scholar]
- Kondziella D, Brenner E, Eyjolfsson EM, Markinhuhta KR, Carlsson ML, Sonnewald U. Glial-neuronal interactions are impaired in the schizophrenia model of repeated MK801 exposure. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:1880–1887. doi: 10.1038/sj.npp.1300993. [DOI] [PubMed] [Google Scholar]
- Krebs MO, Bellon A, Mainguy G, Jay TM, Frieling H. One-carbon metabolism and schizophrenia: current challenges and future directions. Trends in molecular medicine. 2009;15:562–570. doi: 10.1016/j.molmed.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Harms C, Sobol RW, Cardozo-Pelaez F, Linhart H, Winter B, Balkaya M, Gertz K, Gay SB, Cox D, Eckart S, Ahmadi M, Juckel G, Kempermann G, Hellweg R, Sohr R, Hortnagl H, Wilson SH, Jaenisch R, Endres M. Folate deficiency induces neurodegeneration and brain dysfunction in mice lacking uracil DNA glycosylase. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:7219–7230. doi: 10.1523/JNEUROSCI.0940-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA, Kim WK, Choi YB, Kumar S, D'Emilia DM, Rayudu PV, Arnelle DR, Stamler JS. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:5923–5928. doi: 10.1073/pnas.94.11.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC. Regulation of glutathione synthesis. Molecular aspects of medicine. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi-Carter R, Barczak AK, Speno H, Coyle JT. Hydrolysis of the neuropeptide N-acetylaspartylglutamate (NAAG) by cloned human glutamate carboxypeptidase II. Brain research. 1998;795:341–348. doi: 10.1016/s0006-8993(98)00244-3. [DOI] [PubMed] [Google Scholar]
- MacFarlane AJ, Perry CA, Girnary HH, Gao D, Allen RH, Stabler SP, Shane B, Stover PJ. Mthfd1 is an essential gene in mice and alters biomarkers of impaired one-carbon metabolism. The Journal of biological chemistry. 2009;284:1533–1539. doi: 10.1074/jbc.M808281200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney CA, Hay SM, Rees WD. Folate deficiency during pregnancy impacts on methyl metabolism without affecting global DNA methylation in the rat fetus. The British journal of nutrition. 2007;97:1090–1098. doi: 10.1017/S0007114507670834. [DOI] [PubMed] [Google Scholar]
- Mangold S, Blau N, Opladen T, Steinfeld R, Wessling B, Zerres K, Hausler M. Cerebral folate deficiency: A neurometabolic syndrome? Molecular genetics and metabolism. 2011 doi: 10.1016/j.ymgme.2011.06.004. [DOI] [PubMed] [Google Scholar]
- McGrath J, Brown A, St Clair D. Prevention And Schizophrenia--The Role of Dietary Factors. Schizophrenia bulletin. 2010 doi: 10.1093/schbul/sbq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill J, Tang T, Kaminsky Z, Khare T, Yazdanpanah S, Bouchard L, Jia P, Assadzadeh A, Flanagan J, Schumacher A, Wang SC, Petronis A. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. American journal of human genetics. 2008;82:696–711. doi: 10.1016/j.ajhg.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MS, Fava M, Jacques PF, Selhub J, Rosenberg IH. Depression and folate status in the US Population. Psychotherapy and psychosomatics. 2003;72:80–87. doi: 10.1159/000068692. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes, brain, and behavior. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Munoz-Moran E, Dieguez-Lucena JL, Fernandez-Arcas N, Peran-Mesa S, Reyes-Engel A. Genetic selection and folate intake during pregnancy. Lancet. 1998;352:1120–1121. doi: 10.1016/s0140-6736(05)79761-0. [DOI] [PubMed] [Google Scholar]
- Nag N, Berger-Sweeney JE. Postnatal dietary choline supplementation alters behavior in a mouse model of Rett syndrome. Neurobiology of disease. 2007;26:473–480. doi: 10.1016/j.nbd.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Nguyen A, Rauch TA, Pfeifer GP, Hu VW. Global methylation profiling of lymphoblastoid cell lines reveals epigenetic contributions to autism spectrum disorders and a novel autism candidate gene, RORA, whose protein product is reduced in autistic brain. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:3036–3051. doi: 10.1096/fj.10-154484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker JD, Coyle JT. Do maternal folate and homocysteine levels play a role in neurodevelopmental processes that increase risk for schizophrenia? Harvard review of psychiatry. 2005;13:197–205. doi: 10.1080/10673220500243372. [DOI] [PubMed] [Google Scholar]
- Pozzi L, Baviera M, Sacchetti G, Calcagno E, Balducci C, Invernizzi RW, Carli M. Attention deficit induced by blockade of N-methyl D-aspartate receptors in the prefrontal cortex is associated with enhanced glutamate release and cAMP response element binding protein phosphorylation: role of metabotropic glutamate receptors 2/3. Neuroscience. 2011;176:336–348. doi: 10.1016/j.neuroscience.2010.11.060. [DOI] [PubMed] [Google Scholar]
- Reed MC, Thomas RL, Pavisic J, James SJ, Ulrich CM, Nijhout HF. A mathematical model of glutathione metabolism. Theoretical biology & medical modelling. 2008;5:8. doi: 10.1186/1742-4682-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EJ. Has enhanced folate status during pregnancy altered natural selection and possibly Autism prevalence? A closer look at a possible link. Medical hypotheses. 2008;71:406–410. doi: 10.1016/j.mehy.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Schlumberger C, Schafer D, Barberi C, More L, Nagel J, Pietraszek M, Schmidt WJ, Danysz W. Effects of a metabotropic glutamate receptor group II agonist LY354740 in animal models of positive schizophrenia symptoms and cognition. Behavioural pharmacology. 2009;20:56–66. doi: 10.1097/FBP.0b013e3283242f57. [DOI] [PubMed] [Google Scholar]
- Schmidt RJ, Hansen RL, Hartiala J, Allayee H, Schmidt LC, Tancredi DJ, Tassone F, Hertz-Picciotto I. Prenatal Vitamins, One-carbon Metabolism Gene Variants, and Risk for Autism. Epidemiology. 2011;22:476–485. doi: 10.1097/EDE.0b013e31821d0e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selhub J, Bagley LC, Miller J, Rosenberg IH. B vitamins, homocysteine, and neurocognitive function in the elderly. The American journal of clinical nutrition. 2000;71:614S–620S. doi: 10.1093/ajcn/71.2.614s. [DOI] [PubMed] [Google Scholar]
- Shane B. Folylpolyglutamate synthesis and role in the regulation of one-carbon metabolism. Vitamins and hormones. 1989;45:263–335. doi: 10.1016/s0083-6729(08)60397-0. [DOI] [PubMed] [Google Scholar]
- Shea TB, Ortiz D, Rogers E. Differential susceptibity of transgenic mice lacking one or both apolipoprotein alleles to folate and vitamin E deprivation. Journal of Alzheimer's disease : JAD. 2004;6:269–273. doi: 10.3233/jad-2004-6307. [DOI] [PubMed] [Google Scholar]
- Smith CL, Bolton A, Nguyen G. Genomic and epigenomic instability, fragile sites, schizophrenia and autism. Current genomics. 2010;11:447–469. doi: 10.2174/138920210793176001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden C. One-carbon metabolism in psychiatric illness. Nutrition research reviews. 2006;19:117–136. doi: 10.1079/NRR2006119. [DOI] [PubMed] [Google Scholar]
- Takatsu Y, Fujita Y, Tsukamoto T, Slusher BS, Hashimoto K. Orally active glutamate carboxypeptidase II inhibitor 2-MPPA attenuates dizocilpine-induced prepulse inhibition deficits in mice. Brain research. 2011;1371:82–86. doi: 10.1016/j.brainres.2010.11.048. [DOI] [PubMed] [Google Scholar]
- Tchantchou F, Graves M, Ashline D, Morin A, Pimenta A, Ortiz D, Rogers E, Shea TB. Increased transcription and activity of glutathione synthase in response to deficiencies in folate, vitamin E, and apolipoprotein E. Journal of neuroscience research. 2004;75:508–515. doi: 10.1002/jnr.10867. [DOI] [PubMed] [Google Scholar]
- Troen AM, Chao WH, Crivello NA, D'Anci KE, Shukitt-Hale B, Smith DE, Selhub J, Rosenberg IH. Cognitive impairment in folate-deficient rats corresponds to depleted brain phosphatidylcholine and is prevented by dietary methionine without lowering plasma homocysteine. The Journal of nutrition. 2008;138:2502–2509. doi: 10.3945/jn.108.093641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai G, Passani LA, Slusher BS, Carter R, Baer L, Kleinman JE, Coyle JT. Abnormal excitatory neurotransmitter metabolism in schizophrenic brains. Archives of general psychiatry. 1995;52:829–836. doi: 10.1001/archpsyc.1995.03950220039008. [DOI] [PubMed] [Google Scholar]
- Valivullah HM, Lancaster J, Sweetnam PM, Neale JH. Interactions between N-acetylaspartylglutamate and AMPA, kainate, and NMDA binding sites. Journal of neurochemistry. 1994;63:1714–1719. doi: 10.1046/j.1471-4159.1994.63051714.x. [DOI] [PubMed] [Google Scholar]
- Wallin C, Weber SG, Sandberg M. Glutathione efflux induced by NMDA and kainate: implications in neurotoxicity? Journal of neurochemistry. 1999;73:1566–1572. doi: 10.1046/j.1471-4159.1999.0731566.x. [DOI] [PubMed] [Google Scholar]
- Ward BC, Kolodny NH, Nag N, Berger-Sweeney JE. Neurochemical changes in a mouse model of Rett syndrome: changes over time and in response to perinatal choline nutritional supplementation. Journal of neurochemistry. 2009;108:361–371. doi: 10.1111/j.1471-4159.2008.05768.x. [DOI] [PubMed] [Google Scholar]
- Wroblewska B, Wroblewski JT, Pshenichkin S, Surin A, Sullivan SE, Neale JH. N-acetylaspartylglutamate selectively activates mGluR3 receptors in transfected cells. Journal of neurochemistry. 1997;69:174–181. doi: 10.1046/j.1471-4159.1997.69010174.x. [DOI] [PubMed] [Google Scholar]
- Yang CR, Svensson KA. Allosteric modulation of NMDA receptor via elevation of brain glycine and D-serine: the therapeutic potentials for schizophrenia. Pharmacology & therapeutics. 2008;120:317–332. doi: 10.1016/j.pharmthera.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Yee BK, Chang T, Pietropaolo S, Feldon J. The expression of prepulse inhibition of the acoustic startle reflex as a function of three pulse stimulus intensities, three prepulse stimulus intensities, and three levels of startle responsiveness in C57BL6/J mice. Behavioural brain research. 2005;163:265–276. doi: 10.1016/j.bbr.2005.05.013. [DOI] [PubMed] [Google Scholar]