Abstract
Reactive oxygen species (ROS) and antioxidants are essential to maintain a redox balance within tissues and cells. Intracellular ROS regulate key cellular functions such as proliferation, differentiation and apoptosis through cellular signaling, and response to injury. The redox environment is particularly important for stem/progenitor cells, as their self-renewal and differentiation has been shown to be redox sensitive. However, not much is known about ROS and antioxidant protein function in freshly isolated keratinocytes, notably the different keratinocyte subpopulations. Immunostaining of neonatal cutaneous sections revealed that antioxidant enzymes [catalase, SOD2, gluthatione peroxidase-1 (GPx)] and ROS are localized predominantly to the epidermis. We isolated keratinocyte subpopulations and found lower levels of SOD2, catalase and GPx, as well as decreased SOD and catalase activity in an epidermal side population with stem cell-like characteristics (EpSPs) compared to more differentiated (Non-SP) keratinocytes. EpSPs also exhibited less mitochondrial area, fewer peroxisomes and produced lower levels of ROS than Non-SPs. Finally, EpSPs were more resistant to UV radiation than their progeny. Together, our data indicate ROS and antioxidant levels are decreased in stem-like EpSPs.
Keywords: Keratinocytes, Stem cells, Anti-oxidant protein, Oxidative stress, Reactive oxygen species, Mouse
Introduction
The epidermis is a stratified cornified epithelium that is constantly shed into the environment. Keratinocytes in the basal layer of the epidermis proliferate and migrate upwards, flatten, lose their nuclei, and die. This program of terminal differentiation is executed throughout the lifetime of an organism, and is possible only because of the presence of undifferentiated epidermal stem cells or progenitor cells with high proliferative capacity (Potten and Morris 1988). In the skin, stem cells have been localized in the hair follicle bulge and in the basal layer of the epidermis (Cotsarelis et al. 1990; Bickenbach 1981; Li et al. 1998). Although biochemical markers to recognize epidermal stem cells have been identified (Tani et al. 2000; Trempus et al. 2003; Liu et al. 2003; Pellegrini et al. 2001; Jiao et al. 2004; Jaks et al. 2008), challenges remain to isolate live epidermal stem cells. We and others have previously shown that the use of fluorescent dyes in conjunction with flow cytometry allowed the isolation of a cellular population termed side population (SP) (Goodell et al. 1996) with epidermal stem cell characteristics (EpSPs) (Redvers et al. 2006; Trempus et al. 2003; Jiao et al. 2004; Dunnwald et al. 2001). Homeostasis of the epidermis is dependent on stem and progenitor cell renewal and their renewal and fate are influenced by numerous intrinsic and extrinsic factors, including the microenvironment (Xie and Spradling 2000; Li and Xie 2005). One aspect of the micro-environment is the presence of reactive oxygen species (ROS) that can directly affect cellular self-renewal and differentiation (Smith et al. 2000; Pervaiz et al. 2009; Juntilla et al. 2010).
ROS such as hydrogen peroxide and superoxide are primarily generated within the mitochondrial inner membrane, as some electrons leak from the mitochondrial electron transport chain and rapidly react with oxygen to form free radicals (Droge 2002; Moldovan and Moldovan 2004; Das et al. 1989). Under conditions of oxidative stress where levels of ROS are imbalanced with antioxidants, ROS can be detrimental to the cell itself. However, under homeostatic conditions, evidence suggests that ROS are critical to multiple signal transduction pathways by acting as second messengers (Monteiro and Stern 1996; Droge 2002).
Antioxidant proteins, such as superoxide dismutase (SOD), are one line of defense that cells use to regulate the amount of ROS within the cell. Manganese superoxide dismutase (SOD2 or MnSOD) is localized to mitochondria while copper-zinc superoxide dismutase (SOD1 or CuZnSOD) is primarily found in the cytoplasm (Zelko et al. 2002). These intracellular SODs convert superoxide into hydrogen peroxide. Catalase and glutathione peroxidase-1 (GPx), mainly located in the peroxisome and cytoplasm, respectively, reduce H2O2 into water and oxygen (Moreno et al. 1995). A decrease in antioxidant proteins or an increase in ROS can lead to conditions of oxidative stress (for review see Droge 2002). Oxidative stress has been linked to aging (Harman 1956) as well as many diseases and degenerative disorders such as diabetes (Giacco and Brownlee 2010), cancer (Oberley 2002), and amyotrophic lateral sclerosis (ALS) (Baillet et al. 2010).
There have been numerous studies on oxidative stress and skin regarding exposure to chemical and environmental pollutants and how ROS may contribute to multiple skin diseases and conditions (Trouba et al. 2002). However, very few studies have evaluated the normal homeostatic levels of ROS and antioxidant proteins in healthy (or non-diseased) epidermis (Hornig-Do et al. 2007) and ROS in the context of stem/progenitor cells (Stern and Bickenbach 2007). Therefore, the aim of our study is to determine the redox microenvironment of freshly isolated (non-cultured) epidermal progenitor cells and their more differentiated progeny obtained from murine epidermis. We have previously defined our EpSPs and Non-SPs cell populations and showed that the EpSPs have characteristics of undifferentiated cells, while Non-SPs have characteristics of more differentiated progenitor cells (Jiao et al. 2004; Oberley et al. 2008; Dunnwald et al. 2003). The results of our study presented here show that EpSPs have decreased antioxidant protein levels and enzymatic activity and produce less ROS compared to their progeny. In addition to these characteristics, EpSPs possess less mitochondrial area and fewer peroxisomes than Non-SPs.
Materials and methods
Isolation and culture of epidermal cells
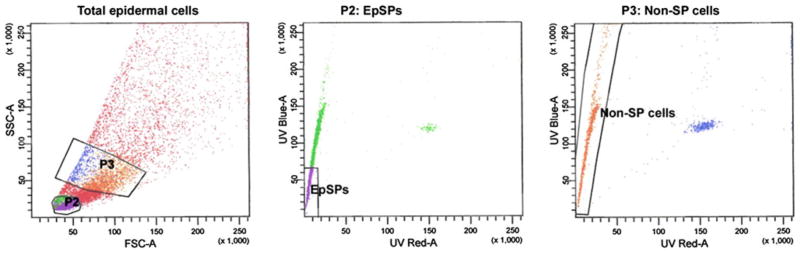
Murine epidermal cells and subpopulations were isolated as previously described (Oberley et al. 2008; Jiao et al. 2004). Briefly, back skin from 1- to 2-day-old C57BL/6J mice was removed and incubated in trypsin 0.25% at 4°C overnight. The epidermis was then separated from the dermis, and epidermal cells obtained by gentle agitation in culture medium. This cellular population will be referred as unsorted. Unsorted cells are incubated in Hoechst (2.5 μg/ml; Sigma, St-Louis, MO) for 90 min at 37°C. The cellular suspension was spun down and cells resuspended in propidium iodide (0.5 μg/ml; Sigma). Cells are separated into EpSPs and Non-SP populations using a Becton–Dickinson FACSDiVa cell sorter with band filter settings previously described (Jiao et al. 2004) and shown in Fig. 1. The smallest 25th percentile of the cells are gated in P2 (Fig. 1), and the verapamil-sensitive population containing low Hoechst red and blue sorted as EpSPs (Jiao et al. 2004). EpSPs represent about 1–5% of the total epidermal population. The Non-SP cells are bigger cells (P3 gate) with low Hoechst red content and represent about 15–20% of the epidermal population (Fig. 1).
Fig. 1.
Flow sorting of epidermal cells into EpSPs and Non-SP cells. The figure shows gates for EpSPs and Non-SPs as described in “Material and methods” and previously (Jiao et al. 2004)
For colony forming efficiency assay, EpSPs and Non-SP cells were grown as previously described (Oberley et al. 2008). One thousand keratinocytes in passage one were plated per 60 mm dishes in the presence of 3 × 105 mitomycin C-treated J2 3T3 fibroblasts. Twenty-four hours later, dishes were exposed to 5 J or 10 J of UV radiation, or left untreated. The media was changed bi-weekly and the cells were fixed and stained after 14 days as previously reported (Rheinwald and Green 1975). The number of colonies was counted and colony forming efficiency calculated.
Western blot analysis and quantitative RT-PCR
Protein from EpSPs and Non-SP pellets was extracted in 0.05 phosphate buffer saline solution, pH 7.8. Twenty micrograms of protein was loaded on 10% acrylamide gel and transferred to PVDF membrane. Immunodetection was performed using enhanced chemiluminescence detection reagents (Amersham, Piscataway, NJ). Antibodies used in the studies are: rabbit anti-SOD2 1:1,000 (UpState, Lake Placid, NY); rabbit anti-SOD1 1:2,000 (Upstate, Lake Placid, NY); rabbit anti-glutathione 1 (GPx) 1:2,000 (LabFrontier, Seoul, Korea); rabbit anti-catalase 1:2,000 (LabFrontier); anti-β-actin 1:2,000 (Sigma) as a loading control for western blot analysis; secondary horseradish peroxidase-conjugated goat anti-rabbit IgG (1:10,000, Chemicon, Temecula, CA).
Messenger RNA was extracted from EpSPs and Non-SP pellets using a RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions and as previously described (Oberley-Deegan et al. 2010). The TaqMan RNA-to-CT 1-Step Kit (Applied Biosystems (ABI), Foster City, CA, USA) was used according to the manufacturer’s instructions with ABI TaqMan primers for Sod1, Sod2, Catalase, GPx and Gapdh. Relative SOD1, SOD2, catalase and GPx mRNA levels were assessed by the comparative quantitation method and calculated differences in mRNA expression were determined according to the manufacturer’s User Bulletin 2 (October 2001, ABI).
Immunodetection
Immunofluorescence was performed as previously described using frozen sections of unfixed 1- to 2-day-old murine back skin (Michel et al. 1996). Frozen cross-sections (5 μm) from neonatal back skin were incubated in the same primary antibodies as described above, with different working dilutions (rabbit anti-SOD2 1:500 (UpState, Lake Placid, NY); rabbit anti-GPx 1:500 (LabFrontier, Seoul, Korea), or rabbit anti-catalase 1:500 (LabFrontier). Detection was performed using secondary Alexa 568-conjugated goat anti-rabbit IgG (1:200, Molecular Probe, Eugene, OR). Nuclear DNA was labeled with DAPI. Slides were viewed under an E800 Eclipse Epi-fluorescence Nikon microscope equipped with a RT-Slider camera and pseudocolorized.
SOD and catalase activity gel
Sorted cells were isolated and protein was extracted as described above. EpSPs and Non-SPs cellular extracts were diluted 1:1 in loading gel buffer. The SOD activity gel assay was performed as described previously (Weydert and Cullen 2010). SOD activity gels cannot distinguish between SOD1 and SOD2 activity, therefore we will use an undefined terminology and use ‘SOD activity’ with the understanding that it could refer to SOD1, SOD2 or both activities. Briefly, proteins were natively electrophoresed on a 10% Tris/HCl gel at 100 V for 3 h. The gel was incubated in 2.34 mM nitro blue tetrazolium, 28 mM TE-MED and 2.8 × 10−5 riboflavin for 20 min in the dark. The gel was washed excessively in water and then exposed to light until achromatic bands formed. The native catalase activity gel assay was performed as previously described (Du et al. 2006). Samples were run in 8% native bisacrylamide and 5% acrylamide stacking gels at 40 mA for 3 h at 4°C in pre-electrophoresis running buffer, then 2–3 h in electrophoresis buffer as originally described (Beers and Sizer 1952). The gel was washed in distilled water for 10 min, then incubated in 0.003% H2O2 in double distilled water for 10 min, washed again in dd H2O, and stained with 2% ferric chloride and 2% potassium ferricyanide. Densitometry of the gel was performed using ImageJ NIH software.
Transmission electron microscopy and morphometric analysis
For the visualization of the mitochondria, pellets of EpSPs and Non-SPs were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate overnight at 4°C and embedded in Epon as previously described (Michel et al. 1999). Seventy microns thick sections were visualized using a Hitachi TEM JEOL 1230. Pictures were taken at 1,000× magnification. Using ImageJ NIH software, cellular area, mitochondrial area and nuclear area were determined. Nuclear area was subtracted from the cellular area to obtain the cytoplasmic area. Percentage of mitochondrial area per cytoplasmic area was calculated.
For the visualization of the peroxisome, pellets of EpSPs and Non-SPs were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate overnight at 4°C, washed, then incubated in 0.25% diaminobenzidine for 1 h. Pellets were fixed in osmium and embedded in Epon as described above. Seventy microns thick sections were visualized using a Hitachi TEM JEOL 1230. Sections were collected at 20 μm intervals to insure that different cells were analyzed. The number of peroxisomes per cell in each group was counted using low power digital images. About 120 cells (2 or 3 independent experiments) per group were analyzed.
Reactive oxygen species detection by dihydroethidium (DHE)
The oxidation of dihydroethidium by ROS generates a red fluorescent compound that can be visualized under a 570 nm fluorescent light. Frozen neonate skin sections and freshly isolated cells obtained as described above were incubated in 1 μM DHE for 15 min at room temperature in the dark. After washing the slides for 2–3 min in PBS, cells and tissue sections were quickly rinsed in PBS and fluorescence was observed using an E800 Eclipse Epifluorescence Nikon microscope equipped with a RT-Slider camera. Images were acquired in black and white and taken at the same exposure time. Fluorescent intensity of the digital images was quantified using ImageJ NIH software. Three experiments with technical and biological replicates were performed and a total of over 500 cells per group were scored.
Statistical analysis
Statistical evaluation was determined using Student’s t test with Welch’s correction with a P-value smaller than 0.05 for two data sets.
Results
ROS and antioxidant proteins are more abundant in the epidermis than the dermis
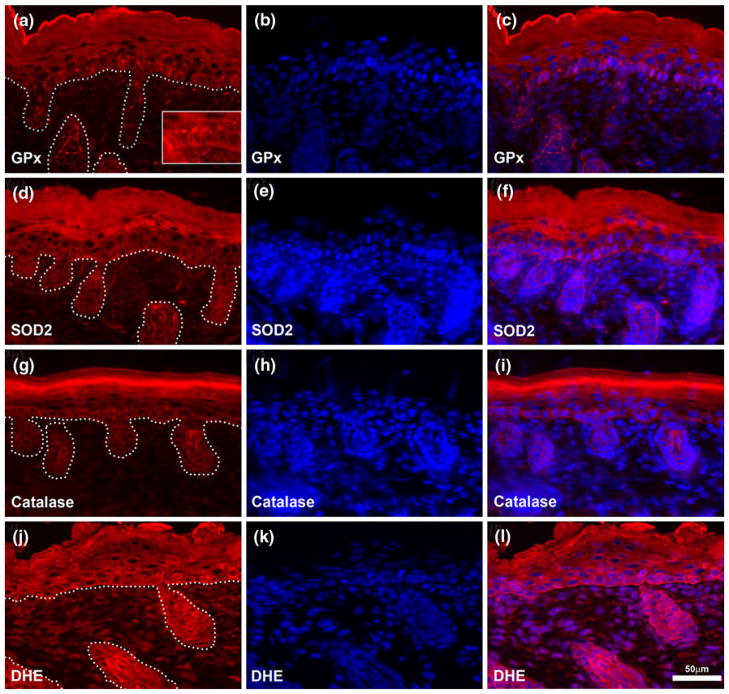
In order to evaluate the localization of antioxidant proteins in murine skin under normal homeostatic conditions, we performed immunostaining of frozen unfixed neonatal cutaneous sections for glutathione peroxidase-1 (GPx), catalase and SOD2 (Fig. 2). All of these antioxidant proteins were predominantly expressed in epidermal cells (interfollicular epidermis and hair follicle) with a reduced or undetectable level of expression in the dermal compartment of the skin, including fibroblasts and the micro-vasculature. GPx was detected throughout all the epidermal layers, with reduced expression in the dermis (Fig. 2a–c). In the basal layer of the epidermis, the protein was concentrated on the apical side of the cells (Fig. 2a, insert). SOD2 was limited to the basal layer of the epidermis (Fig. 2d–f). Catalase was restricted to the epidermal compartment with intense staining in the granular layer (Fig. 2g–i). We did not detect the presence of SOD1 on these sections (data not shown).
Fig. 2.
Antioxidant proteins and reactive oxygen species are predominantly localized to the epidermis. Unfixed frozen neonatal murine skin sections were immunostained for antioxidant proteins [GPx-1 (a–c), SOD2 (d–f), and catalase (g–i)] or incubated with dihydroethidium (DHE, j–l) to detect reactive oxygen species production in situ. Nuclear DNA is labeled with DAPI (blue) and overlay of protein signal (red) presented in the far right column. Dotted line in the left column delineates the junction between hair follicle or interfollicular epidermis and the dermal compartment. Insert in (a) allows better visualization of apical localization of GPx-1. Scale bar 50 μm
In situ detection of ROS by fluorescent microscopy of oxidized dihydroethidium showed signal in both dermal and epidermal compartment, with apparent stronger signal in keratinocytes compared to fibroblasts (Fig. 2j–l).
Together, these data show that under normal homeostatic conditions, antioxidant proteins of the skin are mainly localized in epidermal cells. Furthermore, ROS appeared produced at higher levels in the epidermal tissue than in the dermal extracellular matrix. Thus, our results indicate more active redox biology in the epidermal compartment than the dermal compartment.
Antioxidant protein levels are lower in EpSPs than more differentiated keratinocytes
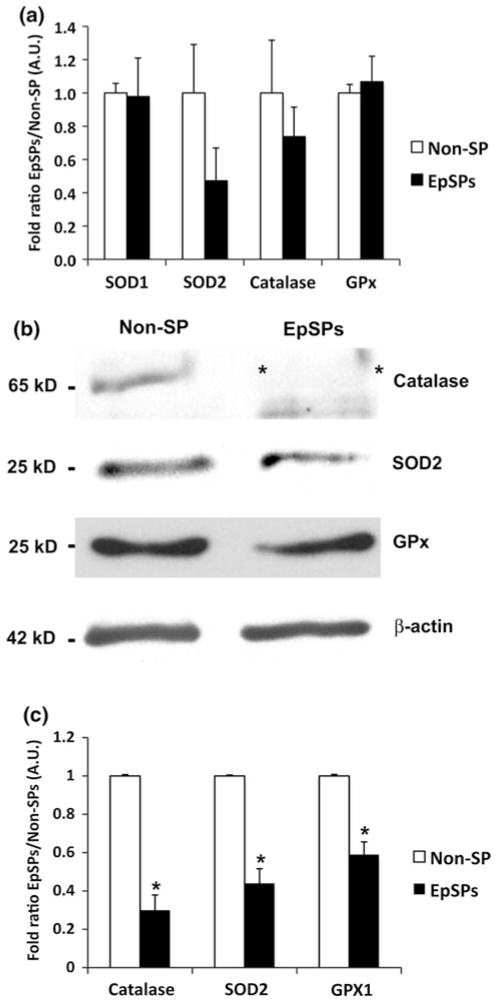
To gain insights at the level of antioxidant proteins in different epidermal subpopulations, we flow-sorted epidermal side population (EpSPs) keratinocytes from their more differentiated progeny (Non-SP cells). Real-time PCR indicated the presence of SOD1, SOD2, catalase and GPx mRNA in both populations. No statistical significant difference among EpSPs and Non-SP cells were detected, although a trend toward lower amount of SOD2 and catalase in EpSPs were observed (Fig. 3a). Western blot analysis confirmed the presence of GPx, catalase and SOD2 in total unsorted epidermal extracts (data not shown), while we could not detect SOD1 with our antibody. Levels of all three antioxidant proteins were lower in the EpSPs compared to Non-SP cells (Fig. 3b, c). Particularly, catalase was almost undetectable in the EpSPs compared to Non-SPs. These data indicate that, overall, EpSPs have lower levels of antioxidant proteins than their differentiated progeny.
Fig. 3.
Antioxidant protein levels are decreased in EpSPs compared to Non-SP cells. (a) Quantitative RT-PCR of Non-SP and EpSPs extracts for SOD1, SOD2, catalase and GPx with results expressed as fold ratio EpSPs/Non-SP. (b) Western blot analysis of Non-SP cellular extracts and EpSP cellular extracts probed with catalase, SOD2, GPx, and β-actin. (c) Bar graph representing fold ratio EpSPs/Non-SPs. All experiments were run at least three times with technical duplicates. Note the strong decrease of catalase denoted by two asterisks in b. Data ± SEM. *P <0.05 by unpaired t test
SOD and catalase activities are lower in EpSPs than other keratinocytes
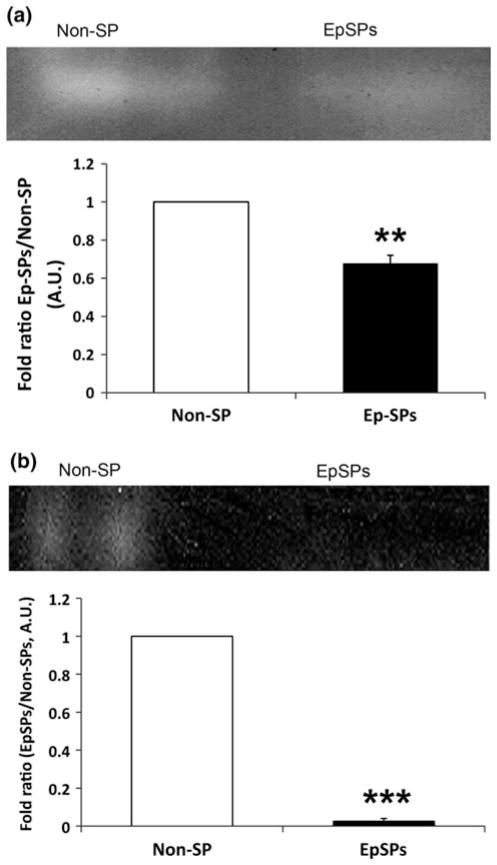
Western blot analysis showed about three- to fourfold decreased catalase and SOD2 protein levels in EpSPs compared to unsorted keratinocytes (Fig. 3). Though both antioxidant protein levels were decreased, we needed to determine their enzymatic activity. We saw decreased SOD and no catalase activity in the EpSPs compared to Non-SP cells (Fig. 4a, b). These data demonstrate that both enzymatic activities were low in undifferentiated keratinocytes. Together with our western blot results, our findings demonstrate that SOD2 and catalase protein levels and SOD and catalase enzymatic activities were highly decreased in EpSP cells compared to more differentiated keratinocytes.
Fig. 4.
Decreased SOD and catalase enzymatic activity in EpSPs. (a) SOD and (b) catalase activity gel assays were performed with Non-SPs and EpSPs cellular extracts. For each enzyme, a representative image of three experiments is shown. Densitometry chart of three activity gels expressed as fold ratio (EpSPs/Non-SPs) ± SEM. *P <0.05 by t test
The cytoplasm of EpSPs contains less mitochondrial area compared to more differentiated Non-SP cells
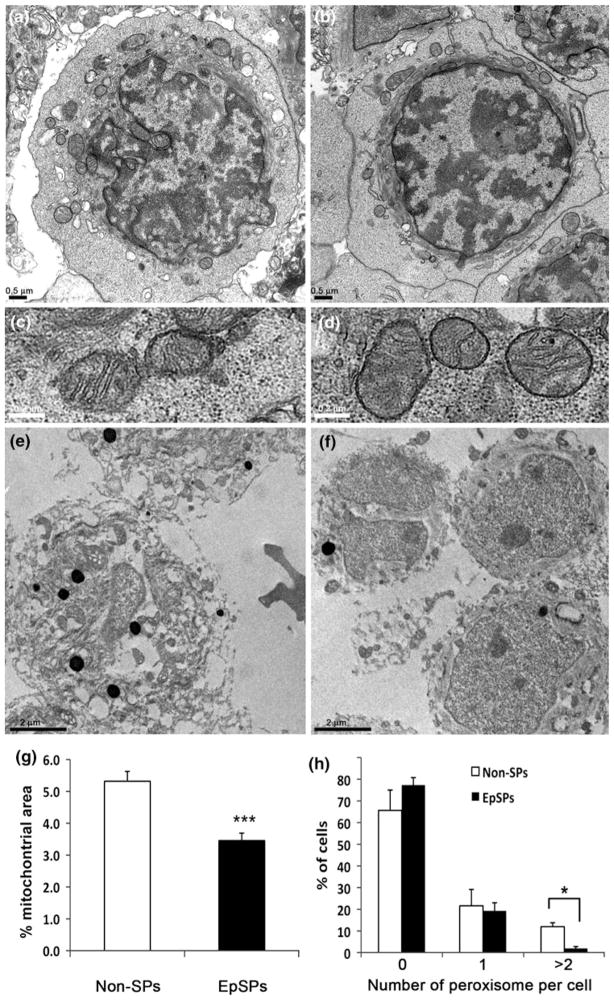
As SOD2 is only expressed in mitochondria (Weisiger and Fridovich 1973), and has lower expression in EpSPs compared to Non-SPs (Fig. 3), we decided to evaluate the area of mitochondria within the cytoplasm of a cell. Pellets of EpSPs and Non-SPs were fixed for transmission electron microscopy and sections performed. Digital images of EpSPs and Non-SPs were used for morphometric analysis. EpSPs and Non-SPs exhibited similar morphological characteristics, including similar mitochondrial morphology (Fig. 5a–d). However, cellular area of EpSPs was significantly reduced compared to Non-SPs (EpSPs: 23.35 arb. units (AU) ± 1.6, n = 61; Non-SPs: 30.3 AU ± 1.2, n = 59; P <0.001). This was expected, as our initial flow gate comprises the smallest 25th percent of the total keratinocytes, and a small cellular size is one of the characteristics of undifferentiated keratinocytes (Barrandon and Green 1985). By tracing the total area of the mitochondria in the EpSPs and Non-SPs, we found that the percentage of mitochondrial area per cytoplasmic area was significantly decreased in EpSPs compared to Non-SPs (Fig. 5g). These data indicate that mitochondria occupy less cytoplasmic area in undifferentiated epidermal cells compared to their more differentiated progeny.
Fig. 5.
Decreased mitochondrial area and peroxisome number in EpSPs. Transmission electron microscopy of Non-SP (a, c, e) and EpSP (b, d, f) cellular pellet. a and b are representative images used to evaluate the mitochondrial area. c and d are higher magnifications of mitochondria. g Percentage of mitochondrial area per cytoplasmic area, data ± SEM. ***P <0.001 by unpaired t test. e and f are representative images showing the peroxisomes stained in black. h Percentage of cells containing 0, 1 or 2 and more peroxisomes per cells. Data are averages ± SEM. *P <0.05 by t test
EpSPs have fewer peroxisomes than Non-SPs
To determine if the decreased catalase activity and lower antioxidant protein levels exhibited by EpSPs (Figs. 3, 4) were associated with ultrastructural differences, we determined the number of peroxisomes per cell in EpSPs and Non-SP cells. Peroxisomes can be easily identified by transmission electron microscopy as they become black after diaminobenzidine incubation (Fig. 5e, f). As shown in Fig. 5h, we did not find difference between EpSPs and Non-SPs for low number of peroxisomes. However, almost no EpSPs had more than 2 peroxisomes (P <0.05) compared to Non-SPs. The mean number of peroxisomes per cell was lower in EpSPs (0.22) compared to Non-SPs (0.33). However, these data were not statistically significant. Together, these data suggest that differences between the two groups were only significant for numerical density >2 (>2 peroxisomes per cell).
EpSPs produce less ROS than other epidermal subpopulations
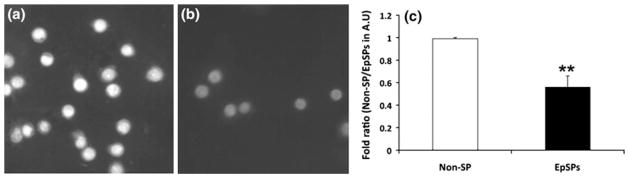
In order to determine the intracellular ROS levels in the different epidermal subpopulations under normal homeostatic conditions, we measured the fluorescent signal of oxidized dihydroethidium immediately after cells were flow-sorted. As the fluorescence is directly proportional to the amount of ROS present in the cell, we took pictures under identical conditions to insure accuracy of the analysis. Our results presented in Fig. 6 show lower levels of oxidized DHE in the EpSPs compared to the other epidermal subpopulations. Quantification of the intensity of the fluorescent signal shows a twofold reduction in the oxidation intensity of DHE in EpSPs compared to Non-SPs. Therefore, our data suggest a decreased level of ROS in undifferentiated keratinocytes.
Fig. 6.
EpSPs produce less ROS than Non-SPs. Freshly isolated Non-SPs (a) and EpSPs (b) were incubated with DHE and pictures acquired digitally. Intensity of the signal was recorded in arbitrary units (AU) and normalized to the signal of Non-SPs. Data are average of three experiments ± SEM. **P <0.005 by t test
EpSPs are more resistant to UV radiation than Non-SPs
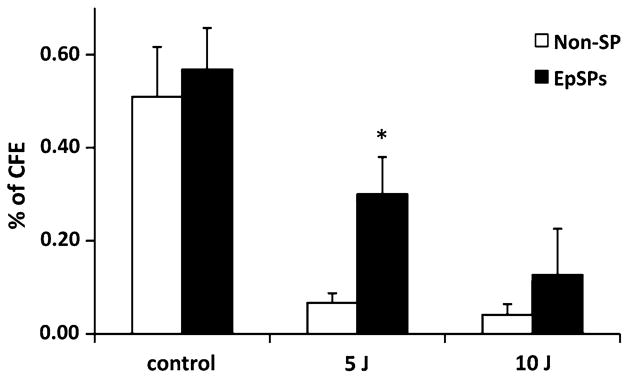
Ultraviolet radiation is known to generate free radicals leading to the formation of ROS in the epidermis that can cause DNA damage with oncogenic potential. Because catalase, SOD2 and GPx protein levels were low and catalase and SOD activities were low in freshly isolated (non-cultured) EpSPs, we asked whether EpSPs would be more susceptible to UV radiation (Fig. 7). We cultured keratinocytes and used their ability to form colonies [colony forming efficiency (CFE) assay] as a read out for the experiment. We observed that EpSPs were more resistant to radiation (5 J) compared to Non-SP cells (4.5-fold increase in EpSPs over Non-SPs, P <0.05). Although a similar trend was observed at 10 J, the data was not significant (P = 0.38).
Fig. 7.
EpSPs are more resistant to UV radiation than Non-SPs. Colony forming efficiency of EpSPs and Non-SPs was calculated after 5 and 10 J. Data are average ± SEM. *P <0.05 by t test between EpSPs and Non-SPs
Discussion
Reactive oxygen species and antioxidants levels determine the redox state within tissues and cells. Furthermore, numerous critical cellular functions are sensitive to the redox environment. Particularly, intracellular ROS regulate key cellular functions such as proliferation, differentiation and apoptosis, and therefore are critical for cellular signaling. Redox biology is essential to many cell types, including stem/progenitor cells, as it plays a role for their self-renewal and has implications for their differentiation and fate (Owusu-Ansah and Banerjee 2009). In our current study, we focused on murine epidermal keratinocytes, particularly an undifferentiated population (EpSPs) that we previously characterized as having stem cell-like properties (Dunnwald et al. 2001; Jiao et al. 2004; Oberley et al. 2008). We took a global approach and asked, in the context of the epidermis, what was the redox microenvironment of freshly isolated subpopulations. We focused on cells that were never placed in culture, to mimic as much as possible the homeostatic physiological state and avoid in vitro culture artifacts. Our data show that EpSPs have lower antioxidant protein levels (catalase, SOD2, and gluthatione peroxidase-1), decreased SOD and catalase activity, and produce less ROS, compared to the more differentiated Non-SPs. These data were complemented by ultrastructural analyses to determine mitochondrial area and peroxisome number.
We first investigated where antioxidant enzymes were present in the skin, as to our knowledge no previous report exists for murine skin. We were unable to detect SOD1, which could be related to technical difficulties with the antibody. However, SOD2 was found in the epidermis, and in some dermal cells. We found strong expression in the basal and granular layer. These results are slightly different than the one reported for adult human skin (Hornig-Do et al. 2007) and could be due to a species difference or an age difference, as our studies were done with neonatal murine back skin. GPx was expressed throughout the epidermis and dermis as previously described in human cutaneous sections, with an apical localization in the basal layer. Catalase was strongly expressed in the granular layer, one of the outermost layers of the epidermis. Although this enzyme plays a role in defending the organism against stress, it may not be essential to epidermal defense and development, as mice lacking catalase developed normally and responded to oxidant injury similar to wildtype animals (Ho et al. 2004). Nevertheless, its expression in the granular layer fits with a protective function against outside ultraviolet radiation, as reported with human keratinocytes in culture and in bioengineered skin (Rezvani et al. 2006, 2007), and also in bioengineered skin made with keratinocytes from patients with xeroderma pigmentosum (Rezvani et al. 2008). It is paradoxical that our EpSPs, which have very low levels and low activity of SOD and catalase, are more resistant to UV radiation than their progeny. This apparent discrepancy could be due to in vitro culture artifacts, as our SOD and catalase data were obtained with freshly isolated cells. Alternatively, EpSPs could upregulate compensatory mechanisms, like the Nuclear-EF2 related factor 2 pathway, to resist UV radiation better than their progeny. The Nuclear-EF2 related factor 2 pathway is a major UV cytoprotector (Schafer et al. 2010b) and activation of Nrf2 target genes strongly reduced UVB cytotoxicity through enhancement of ROS detoxification (Schafer et al. 2010a).
Different populations of keratinocytes have been identified within the epidermis, and their antioxidants and ROS levels are unknown. We were particularly interested in characterizing the levels of these molecules in the EpSPs and their more differentiated progeny, the Non-SP cells. These two populations of keratinocytes are isolated from the basal layer and separated based on their Hoechst dye exclusion properties. EpSPs have characteristics of undifferentiated keratinocytes: they exclude the Hoeschst dye, they have a long lifespan and recapitulate an epidermis in a tissue engineered model, and they are slow cycling (Dunnwald et al. 2001, 2003). We already know from our previous work that the vast majority of EpSPs are in the G0/G1 phase of the cell cycle and have increased levels of p27kip1 (Dunnwald et al. 2003). Increased levels of p27kip1 have also been reported after murine embryonic fibroblasts were treated with a thiol-antioxidant, shifting the intracellular redox state toward a more reducing environment (Menon et al. 2003). Additional data demonstrated that the cell cycle checkpoint between G0/G1 and S-phase is redox-sensitive and a more oxidizing environment is necessary for cells to initiate proliferation (Sarsour et al. 2005). Our current studies indicate that EpSPs produce low levels of ROS, as detected by dihydroethidium, and that at least two of the antioxidant enzymes, SOD and catalase, are functionally down-regulated. Although one would expect that low levels of antioxidants lead to higher levels of ROS, one could also argue that the levels of antioxidant enzyme are low because of low levels of ROS production, which is required for maintaining genomic stability (Li and Marban 2010). Also, we cannot exclude the possibility that enzymatic activity itself may be modulated by signaling or metabolic molecules, regardless of the protein level. This overall low redox environment might allow sensitivity to ROS as a differentiating signal in the context of injury and wound healing. This model would be consistent with previously reported studies, where an increase in hydrogen peroxide in hematopoietic stem cells caused stem cells to exit from their normal quiescent state (Ito et al. 2006) and more oxidized oligodendrocytes were more readily induced to differentiate (Noble et al. 2005).
Many aspects of cellular respiration and energy production take place in mitochondria and mitochondria are a major source of intracellular ROS. Antioxidant proteins are critical to the maintenance of redox homeostasis in mitochondria. We observed significant differences in SOD2, catalase and GPx protein levels. Because enzymatic activity assay requires substantial amount of cells, we focused on SOD2 and catalase based on the difference in their levels between EpSPs and Non-SPs. Both enzymes showed significant differences between the two groups. Consistent with the notion that undifferentiated cells have fewer organelles (He et al. 2004b; Larouche et al. 2008), including mitochondria and peroxisomes, and that differentiation is sensitive to mitochondrial content (von Wangenheim and Peterson 1998), we investigated the proportion of mitochondrial area in each subpopulations. We found a decreased cytoplasmic mitochondrial area in EpSPs compared to Non-SPs. Additionally, we could not find EpSPs with two or more peroxisome per cell, whereas Non-SPs with at least two peroxisomes were clearly visible. Of note, catalase is mainly expressed in the peroxisome, and the increased number of peroxisome per Non-SPs correlate with the increased level of catalase observed in western blot. Together, these data indicate that the notion that undifferentiated cells have fewer organelles also applies to keratinocyte progenitors.
We next wanted to determine if the low level of ROS production and low level of antioxidants in EpSPs affect their resistance to oxidative stress. Our results using UV radiation as the stress inducer show that EpSPs were capable to form keratinocyte colonies at a dose when Non-SPs cells could not, suggesting they are more resistant to UV-induced oxidative stress than Non-SPs. These results complement our previous work using the same isolated side population to promote ischemia repair and angiogenesis and survive necrotic and ischemic environment (Jiao et al. 2004). The overall resistance to oxidative stress by undifferentiated cells may appear to be a general characteristic of these cells, as endothelial progenitor, neuronal progenitor and other stem cells have shown increased resistance to multiple types of oxidative stress (Ivanova et al. 2002; He et al. 2004a; Ingram et al. 2007; Madhavan et al. 2006).
In summary, our data provide new evidence that keratinocytes are not homogenous in their redox machinery and environment. The suggested low levels of antioxidants and ROS production by EpSPs may be critical to stem maintenance, insure their proliferative capacity, and prime them to rapidly respond to tissue damage. Further, resistance of EpSPs to oxidative stress may allow and enhance their efficacy in novel cell-based tissue repair therapies, particularly in the context of diabetes or chronic leg ulcers.
Acknowledgments
This manuscript is dedicated to Dr. Larry Oberley, who was the inspiration for these studies. The authors would like to acknowledge Dr. N. Akhins-Burns for helpful discussion. Also, the authors would like to thank Justin Fishbaugh, Gene Hess and George Rasmussen from Flow Cytometry Core Facility at the University of Iowa for technical assistance, and Jian Shao for electron microscopy expertise. This work was supported in part by the Bioscience Funding Program from the University of Iowa to M.D., NIH R03AR055313 and R01DK059965 to M.D., and NIH R01CA115438 and P01CA066081 to L.W.O. W.J.C was a fellow of the Iowa Bioscience Advantage program (R25 GM58939).
Contributor Information
Wanakee J. Carr, Department of Pediatrics, 206 MRC, The University of Iowa, Iowa City, IA 52242, USA
Rebecca E. Oberley-Deegan, Department of Medicine, National Jewish Health, Denver, CO 80206, USA
Yuping Zhang, Department of Radiation Oncology, The University of Iowa, Iowa City, IA 52242, USA. Department of Free Radical Biology Program, The University of Iowa, Iowa City, IA 52242, USA.
Christopher C. Oberley, Department of Pediatrics, 206 MRC, The University of Iowa, Iowa City, IA 52242, USA
Larry W. Oberley, Department of Radiation Oncology, The University of Iowa, Iowa City, IA 52242, USA. Department of Free Radical Biology Program, The University of Iowa, Iowa City, IA 52242, USA
Martine Dunnwald, Department of Pediatrics, 206 MRC, The University of Iowa, Iowa City, IA 52242, USA, martine-dunnwald@uiowa. Interdisciplinary Program in Molecular and Cellular Biology, The University of Iowa, Iowa City, IA 52242, USA. Interdisciplinary Program in Genetics, The University of Iowa, Iowa City, IA 52242, USA.
References
- Baillet A, Chanteperdrix V, Trocme C, Casez P, Garrel C, Besson G. The role of oxidative stress in amyotrophic lateral sclerosis and Parkinson’s disease. Neurochem Res. 2010;35(10):1530–1537. doi: 10.1007/s11064-010-0212-5. [DOI] [PubMed] [Google Scholar]
- Barrandon Y, Green H. Cell size as a determinant of the clone-forming ability of human keratinocytes. Proc Natl Acad Sci USA. 1985;82:5390–5394. doi: 10.1073/pnas.82.16.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers RF, Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195(1):133–140. [PubMed] [Google Scholar]
- Bickenbach JR. Identification and behavior of label retaining cells in oral mucosa and skin. J Dent Res. 1981;60C:1611–1620. doi: 10.1177/002203458106000311011. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun T-T, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Das DK, George A, Liu XK, Rao PS. Detection of hydroxyl radical in the mitochondria of ischemic-reperfused myocardium by trapping with salicylate. Biochem Biophys Res Commun. 1989;165(3):1004–1009. doi: 10.1016/0006-291x(89)92702-2. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Du J, Daniels DH, Asbury C, Venkataraman S, Liu J, Spitz DR, Oberley LW, Cullen JJ. Mitochondrial production of reactive oxygen species mediate dicumarol-induced cytotoxicity in cancer cells. J Biol Chem. 2006;281(49):37416–37426. doi: 10.1074/jbc.M605063200. [DOI] [PubMed] [Google Scholar]
- Dunnwald M, Tomanek-Chalkey A, Alexandrunas D, Fishbaugh J, Bickenbach JR. Isolating a pure population of epidermal stem cells for use in tissue engineering. Exp Dermatol. 2001;10(1):45–54. doi: 10.1034/j.1600-0625.2001.100106.x. [DOI] [PubMed] [Google Scholar]
- Dunnwald M, Chinnathambi S, Alexandrunas D, Bickenbach JR. Mouse epidermal stem cells proceed through the cell cycle. J Cell Physiol. 2003;195:194–201. doi: 10.1002/jcp.10311. [DOI] [PubMed] [Google Scholar]
- Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- He T, Peterson TE, Holmuhamedov EL, Terzic A, Caplice NM, Oberley LW, Katusic ZS. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arterioscler Thromb Vasc Biol. 2004a;24:2021–2027. doi: 10.1161/01.ATV.0000142810.27849.8f. [DOI] [PubMed] [Google Scholar]
- He ZP, Tan WQ, Tang YF, Zhang HJ, Feng MF. Activation, isolation, identification and in vitro proliferation of oval cells from adult rat livers. Cell Prolif. 2004b;37(2):177–187. doi: 10.1111/j.1365-2184.2004.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho YS, Xiong Y, Ma W, Spector A, Ho DS. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J Biol Chem. 2004;279(31):32804–32812. doi: 10.1074/jbc.M404800200. [DOI] [PubMed] [Google Scholar]
- Hornig-Do HT, von Kleist-Retzow JC, Lanz K, Wickenhauser C, Kudin AP, Kunz WS, Wiesner RJ, Schauen M. Human epidermal keratinocytes accumulate superoxide due to low activity of Mn-SOD, leading to mitochondrial functional impairment. J Invest Dermatol. 2007;127(5):1084–1093. doi: 10.1038/sj.jid.5700666. [DOI] [PubMed] [Google Scholar]
- Ingram DA, Krier TR, Mead LE, McGuire C, Prater DN, Bhavsar J, Saadatzadeh MR, Bijangi-Vishehsaraei K, Li F, Yoder MC, Haneline LS. Clonogenic endothelial progenitor cells are sensitive to oxidative stress. Stem Cells. 2007;25(2):297–304. doi: 10.1634/stemcells.2006-0340. [DOI] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nature Med. 2006;12(4):446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- Ivanova NB, Dimos JT, Schaniel C, Hackney J, Moore KA, Lemischka IR. A stem cell molecular signature. Science. 2002;298:601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40(11):1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- Jiao C, Bronner SJ, Mercer KLN, Sheriff DD, Schatteman GC, Dunnwald M. Epidermal cells accelerate the restoration of the blood flow in diabetic ischemic limbs. J Cell Sci. 2004;117:1055–1063. doi: 10.1242/jcs.00926. [DOI] [PubMed] [Google Scholar]
- Juntilla MM, Patil VD, Calamito M, Joshi RP, Birnbaum MJ, Koretzky GA. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood. 2010;115(20):4030–4038. doi: 10.1182/blood-2009-09-241000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larouche D, Tong X, Fradette J, Coulombe PA, Germain L. Vibrissa hair bulge houses two populations of skin epithelial stem cells distinct by their keratin profile. Faseb J. 2008;22(5):1404–1415. doi: 10.1096/fj.07-8109com. [DOI] [PubMed] [Google Scholar]
- Li TS, Marban E. Physiological levels of reactive oxygen species are required to maintain genomic stability in stem cells. Stem Cells. 2010;28(7):1178–1185. doi: 10.1002/stem.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- Li A, Simmons PJ, Kaur P. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci USA. 1998;95:3902–3907. doi: 10.1073/pnas.95.7.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lyle S, Yang Z, Cotsarelis G. Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. J Invest Dermatol. 2003;121:963–968. doi: 10.1046/j.1523-1747.2003.12600.x. [DOI] [PubMed] [Google Scholar]
- Madhavan L, Ourednik V, Ourednik J. Increased “vigilance” of antioxidant mechanisms in neural stem cells potentiates their capability to resist oxidative stress. Stem Cells. 2006;24(9):2110–2119. doi: 10.1634/stemcells.2006-0018. [DOI] [PubMed] [Google Scholar]
- Menon SG, Sarsour EH, Spitz DR, Higashikubo R, Sturm M, Zhang H, Goswami PC. Redox regulation of the G1 to S phase transition in the mouse embryo fibroblast cell cycle. Cancer Res. 2003;63(9):2109–2117. [PubMed] [Google Scholar]
- Michel M, Török N, Godbout M-J, Lussier M, Gaudreau P, Royal A, Germain L. Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. J Cell Sci. 1996;109:1017–1028. doi: 10.1242/jcs.109.5.1017. [DOI] [PubMed] [Google Scholar]
- Michel M, L’Heureux N, Pouliot R, Xu W, Auger FA, Germain L. Characterization of a new tissue-engineered human skin equivalent with hair. In Vitro Cell Dev Biol. 1999;35:318–326. doi: 10.1007/s11626-999-0081-x. [DOI] [PubMed] [Google Scholar]
- Moldovan L, Moldovan NI. Oxygen free radicals and redox biology of organelles. Histochem Cell Biol. 2004;122(4):395–412. doi: 10.1007/s00418-004-0676-y. [DOI] [PubMed] [Google Scholar]
- Monteiro HP, Stern A. Redox modulation of tyrosine phosphorylation-dependent signal transduction pathways. Free Radic Biol Med. 1996;21(3):323–333. doi: 10.1016/0891-5849(96)00051-2. [DOI] [PubMed] [Google Scholar]
- Moreno S, Mugnaini E, Ceru MP. Immunocytochemical localization of catalase in the central nervous system of the rat. J Histochem Cytochem. 1995;43(12):1253–1267. doi: 10.1177/43.12.8537642. [DOI] [PubMed] [Google Scholar]
- Noble M, Mayer-Proschel M, Proschel C. Redox regulation of precursor cell function: insights and paradoxes. Antioxid Redox Signal. 2005;7(11&12):1456–1467. doi: 10.1089/ars.2005.7.1456. [DOI] [PubMed] [Google Scholar]
- Oberley TD. Oxidative damage and cancer. Am J Pathol. 2002;160(2):403–408. doi: 10.1016/S0002-9440(10)64857-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberley CC, Gourronc F, Hakimi S, Riordan M, Bronner S, Jiao C, Dunnwald M. Murine epidermal side population possesses unique angiogenic properties. Exp Cell Res. 2008;314(4):720–728. doi: 10.1016/j.yexcr.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Oberley-Deegan RE, Rebits BW, Weaver MR, Tollefson AK, Bai X, McGibney M, Ovrutsky AR, Chan ED, Crapo JD. An oxidative environment promotes growth of Mycobacterium abscessus. Free Radic Biol Med. 2010;49:1666–1673. doi: 10.1016/j.freeradbiomed.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461(7263):537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M. P63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2001;98(6):3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervaiz S, Taneja R, Ghaffari S. Oxidative stress regulation of stem and progenitor cells. Antioxid Redox Signal. 2009;11(11):2777–2789. doi: 10.1089/ars.2009.2804. [DOI] [PubMed] [Google Scholar]
- Potten CS, Morris RJ. Epithelial stem cells in vivo. J Cell Sci. 1988;10(suppl):45–62. doi: 10.1242/jcs.1988.supplement_10.4. [DOI] [PubMed] [Google Scholar]
- Redvers RP, Li A, Kaur P. Side population in adult murine epidermis exhibits phenotypic and functional characteristics of keratinocyte stem cells. Proc Natl Acad Sci USA. 2006;103(35):13168–13173. doi: 10.1073/pnas.0602579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani HR, Mazurier F, Cario-Andre M, Pain C, Ged C, Taieb A, de Verneuil H. Protective effects of catalase overexpression on UVB-induced apoptosis in normal human keratinocytes. J Biol Chem. 2006;281(26):17999–18007. doi: 10.1074/jbc.M600536200. [DOI] [PubMed] [Google Scholar]
- Rezvani HR, Cario-Andre M, Pain C, Ged C, de Verneuil H, Taieb A. Protection of normal human reconstructed epidermis from UV by catalase overexpression. Cancer Gene Ther. 2007;14(2):174–186. doi: 10.1038/sj.cgt.7701000. [DOI] [PubMed] [Google Scholar]
- Rezvani HR, Ged C, Bouadjar B, de Verneuil H, Taieb A. Catalase overexpression reduces UVB-induced apoptosis in a human xeroderma pigmentosum reconstructed epidermis. Cancer Gene Ther. 2008;15(4):241–251. doi: 10.1038/sj.cgt.7701102. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Sarsour EH, Agarwal M, Pandita TK, Oberley LW, Goswami PC. Manganese superoxide dismutase protects the proliferative capacity of confluent normal human fibroblasts. J Biol Chem. 2005;280:18033–18041. doi: 10.1074/jbc.M501939200. [DOI] [PubMed] [Google Scholar]
- Schafer M, Dutsch S, auf dem Keller U, Navid F, Schwarz A, Johnson DA, Johnson JA, Werner S. Nrf2 establishes a glutathione-mediated gradient of UVB cytoprotection in the epidermis. Genes Dev. 2010a;24(10):1045–1058. doi: 10.1101/gad.568810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M, Dutsch S, auf dem Keller U, Werner S. Nrf2: a central regulator of UV protection in the epidermis. Cell Cycle. 2010b;9(15):2917–2918. doi: 10.4161/cc.9.15.12701. [DOI] [PubMed] [Google Scholar]
- Smith J, Ladi E, Mayer-Proschel M, Noble M. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci USA. 2000;97(18):10032–10037. doi: 10.1073/pnas.170209797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern MM, Bickenbach JR. Epidermal stem cells are resistant to cellular aging. Aging Cell. 2007;6(4):439–452. doi: 10.1111/j.1474-9726.2007.00318.x. [DOI] [PubMed] [Google Scholar]
- Tani H, Morris RJ, Kaur P. Enrichment for murine keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci USA. 2000;97(20):10960–10965. doi: 10.1073/pnas.97.20.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempus C, Morris RJ, Bortner CD, Cotsarelis G, Faircloth RS, Reece JM, Tennant RW. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol. 2003;120:501–511. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- Trouba KJ, Hamadeh HK, Amin RP, Germolec DR. Oxidative stress and its role in skin disease. Antioxid Redox Signal. 2002;4(4):665–673. doi: 10.1089/15230860260220175. [DOI] [PubMed] [Google Scholar]
- von Wangenheim KH, Peterson HP. Control of cell proliferation by progress in differentiation: clues to mechanisms of aging, cancer causation and therapy. J Theor Biol. 1998;193(4):663–678. doi: 10.1006/jtbi.1998.0731. [DOI] [PubMed] [Google Scholar]
- Weisiger RA, Fridovich I. Mitochondrial superoxide simutase site of synthesis and intramitochondrial localization. J Biol Chem. 1973;248(13):4793–4796. [PubMed] [Google Scholar]
- Weydert CJ, Cullen JJ. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc. 2010;5(1):51–66. doi: 10.1038/nprot.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290(5490):328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33(3):337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]