Abstract
The discovery of host-encoded gene products that sense molecular patterns in infectious microbes, and the demonstration of their role in triggering innate and adaptive immune responses, has been a key milestone in our understanding of immunology. Twenty-three years after Janeway first outlined the fundamental concepts of the ‘pattern recognition’ model, and 15 years since the identification of Toll-like receptors (TLRs) as pattern recognition receptors (PRRs), new insights continue to be revealed, and questions remain. For example, innate immune responses to microbes that are mediated by PRRs have historically been viewed as the domain of innate immune cell populations such as dendritic cells and macrophages. New evidence, however, has pointed to the role of B-cell-intrinsic TLR activation in shaping antibody responses. These studies have revealed that TLRs regulate a complex transcriptional network that controls multiple steps in the development of antigen-specific antibodies. This review covers these recent developments regarding the role of TLRs in B-cell gene expression and function in vitro and in vivo, and highlights the remaining challenges in the field, with particular emphasis on the role of TLRs in antibody responses to viral infection. A more complete understanding of how TLRs regulate antibody responses will lead to improved vaccine design.
Keywords: antibody responses, innate immunity, B cells, Toll receptors/Toll-like receptors, viruses/viral immunity
Introduction
Toll-like receptors (TLRs) are an ancient family of receptors that have been conserved throughout millions of years of evolution and are found in both vertebrate and invertebrate species. The TLRs all share basic structural features of extracellular leucine-rich repeats, a transmembrane domain, and a cytosolic Toll/Interleukin-1 receptor (TIR) domain. Initially, TLRs were identified in genetic screens for genes that regulate embryonic patterning of Drosophila melanogaster.1 A vertebrate TLR was subsequently identified as an important mediator of inflammatory responses to bacterial lipopolysaccharide (LPS),2,3 sparking intense study of the role of TLRs in immunity. Thirteen vertebrate members of the TLR family have been identified. Humans express TLR1–10, while mice encode 13 (TLR1–13), although the murine TLR8 and TLR10 genes are not thought to express functional proteins. Each member of this family has evolved to respond to a different pathogen-associated molecular pattern. The TLRs that respond to bacterial products, such as triacyl lipoproteins (TLR1/2), diacyl lipoproteins (TLR2/6), LPS (TLR4) and flagellin (TLR5), are localized at the plasma membrane and sense extracellular microbes.4–6 By contrast, nucleic-acid-sensing TLRs such as TLR3, TLR7 and TLR9, which respond to dsRNA, ssRNA and CpG DNA respectively,7–9 are localized to endosomal compartments by an interaction with the membrane protein UNC93B.10,11 This sequestering of the nucleic-acid-sensing TLRs is essential to prevent inappropriate stimulation by self nucleic acid present in the extracellular space.12 Murine TLR11 has been shown to detect profilin from Toxoplasma gondii, and to prevent uropathogenic bacterial infections,13,14 but the human TLR11 gene does not express a functional protein.
The identification of these receptors lead to the characterization of their roles in initiating rapid inflammatory responses during microbial infection. Importantly, innate immune pathways, including TLRs, are now appreciated as being key regulators of adaptive immune responses by B and T lymphocytes.15
Expression of TLRs in B cells
B cells play an essential role in the development of antibody responses to infection and vaccination, and the molecular mechanisms that regulate these responses are of great interest. Mature naive B cells are subdivided into several distinct classes with specialized functional roles. B1 cells are found primarily in body cavities, whereas B2 cells are found in secondary lymphoid organs, and are further subdivided into marginal zone B cells or follicular B cells. Follicular B cells are responsible for T-cell-dependent antibody responses that develop into germinal centres (GCs), whereas marginal zone B cells express polyreactive B-cell receptors (BCRs) and are considered to have a more ‘innate’ role in host defence.16
Several studies have examined the specific expression of individual TLR members in different B-cell subsets in both mouse and human tissues. These studies have found that B cells express a distinct subset of the TLR family that determines their ability to respond to microbial patterns. The molecular basis and functional significance of restricted TLR expression in B cells is not yet clear. The expression of TLRs in B cells highlights that these cells have evolved to directly sense microbes.
Naive human B cells express only low levels of TLRs, whereas activated and memory B cells express significant levels of TLR1, TLR6, TLR7, TLR9 and TLR10, and low levels of TLR2.17–20 The TLRs expressed in human B cells are up-regulated following activation via BCR or CD40 stimulation, and this is especially prominent for TLR9 and TLR10.21 Interestingly, human CD138+ plasma cells express a broader range of TLRs, including TLR3 and TLR4, and stimulation of TLRs on plasma cells augments antibody secretion.22
Analyses of TLR expression in mouse B cells also found a distinct pattern of expression. TLR1, TLR2, TLR4, TLR6, TLR7 and TLR9 are expressed in most B-cell subsets, although levels vary between the individual subsets.23,24 For example, TLR9 is especially abundant in B1 cells, follicular and marginal zone B cells, but less so in Peyer’s patch B cells. In contrast with human B cells, murine B cells do not express TLR10 but do express TLR4, and murine B cells can be potently activated by LPS.25 Although, like human B cells, murine B cells express only low levels of TLR3, these cells can still respond to TLR3 ligands.26
Expression of TLRs in B cells is regulated by the action of cytokines as well as by signalling from the BCRs. Both TLR3 and TLR7 are strongly up-regulated in murine B cells by interferon-β (IFN-β),27 and by stimulation of BCRs.28 Expression of TLR7 in human B cells is also strongly up-regulated by type 1 interferons.29
The restricted TLR expression pattern in B cells raises a number of interesting questions. For example, how do humans, who lack expression of TLR3 and TLR4 in B cells, mount effective antibody responses to dsRNA viruses and Gram-negative bacteria respectively? TLR3 and TLR4 are expressed in CD11c+ dendritic cells (DCs),30,31 and it is possible that their engagement in DCs is sufficient to compensate for a lack of these receptors in B cells. It is also possible that alternative sensing pathways for these pathogens are expressed in B cells and are sufficient for the triggering of B-cell responses in the absence of TLR signalling.
TLR signalling and gene regulation
TLR signalling pathways have been extensively studied in cell types such as macrophages and DCs, but relatively few studies have specifically examined TLR signalling in B cells. Recognition by TLR of microbial ligands activates a signalling cascade through a variety of TIR domain containing adapter molecules such as Myeloid differentiation primary response gene 88 (Myd88), TIR-domain-containing adapter-inducing interferon-β (TRIF), Toll-interleukin 1 receptor domain containing adaptor protein (TIRAP), Trif-related adapter molecule (TRAM) and Sterile-alpha and Armadillo motif containing protein (SARM).32 Myd88 mediates signalling from all TLRs except TLR3, which signals through TRIF. TLR4 activates both Myd88-dependent and TRIF-dependent signalling. TIRAP, TRAM and SARM play accessory or regulatory roles in signalling through the canonical Myd88-dependent or TRIF-dependent pathways.33,34 Activation of the TLR signalling cascade results in the activation of pro-inflammatory and antimicrobial gene expression through transcription factors such as nuclear factor-κB, interferon regulatory factor 3 and activating protein 1.35 A key feature of the ability of TLRs to regulate adaptive immunity is the up-regulation of MHC antigen presentation and co-stimulatory molecules such as CD80 and CD86, that can help trigger antigen-specific T-cell responses.2
A comprehensive analysis of the molecular architecture of TLR signalling has been performed in murine DCs using an short hairpin RNA approach.36,37 These studies identified dozens of transcriptional regulators that coordinate the host response to TLR activation, highlighting the complexity of TLR signalling pathways. Different TLRs regulate overlapping transcriptional pathways but can also initiate gene expression specific to a particular TLR. It is also highly likely that the molecular architecture of TLR-controlled transcription networks differs between cell lineages – so it will be important to apply systematic approaches to understanding TLR signalling specifically in B cells. Interestingly, studies comparing the wiring of innate immune networks in different mouse strains have found striking strain-specific differences, indicating the evolutionary plasticity of innate immune signalling networks.38
In vitro responses of B cells to TLR stimulation
In vitro exposure of human or mouse B cells to TLR ligands alone is, in many cases, sufficient to promote a combination of responses, including expression of activation markers such as CD69, CD80 and CD86, antigen presentation, proliferation, class switch recombination and antibody secretion.39–43 The specific response of B cells to TLR stimulation differs depending on the B-cell subset and the TLR.44,45 For example, murine follicular B cells are less sensitive to LPS-induced proliferation than marginal zone B cells because of lower induction of c-myc expression.46 Also, TLR ligation is sufficient to promote development of murine B1 and marginal zone B cells into antibody-secreting cells, but is less potent at triggering antibody secretion from follicular B cells.24
Some evidence suggests that, in addition to promoting class switch recombination through up-regulation of activation-induced deaminase, TLRs can bias switching to selected immunoglobulin isotypes. For example, LPS induces switching to IgG3, whereas LPS plus interleukin-4 promotes IgG1 and IgG3.47 By contrast CpG oligodeoxynucleotides promote IgG2a, IgG2b and IgG3 and suppress IgG1 and IgE.48
Cytokine secretion is also a feature of TLR activation in B cells. Human B cells respond to TLR stimulation by expression and secretion of a wide range of cytokines, including macrophage inflammatory proteins 1α and 1β; interleukins 1α, 1β, 6, 8 and 10; interferon-inducible protein 10; and granulocyte and granulocyte–macrophage colony-stimulating factors.19,49,50 This response is more pronounced for CD27+ memory cells than for naive B cells.19 Studies in mice have shown that proliferation of B cells in response to TLR stimulation depends on an autocrine IFN-β loop.51 Different B-cell subsets have specialized cytokine secretion profiles in response to TLR stimulation – interleukin-10 is predominantly secreted by marginal zone and B1 B cells, IFN-γ is secreted by follicular B cells, and both subsets secrete interleukin-6.52–54
How B cells integrate information from TLRs with antigen-specific activation through BCRs, and T-cell help through CD40, is a key area that is not fully understood. In vitro studies have shown that TLR signalling can interact and synergize with stimulation of BCRs by antigen or stimulation of CD40 by CD40 ligand .55,56In vitro data have also suggested significant interspecies differences in the relationship between individual TLRs and BCRs or CD40 in B-cell activation. The TLR9 ligand CpG DNA alone is highly immunostimulatory towards murine B cells, but is less so to human B cells because of a requirement for additional signals such as BCRs, CD40 or cytokines.20 By cooperating with antigen-specific signals, TLRs can provide an extra level of regulation to ensure that B cells are only activated in the context of infection. A breakdown in this regulation can lead to autoimmunity, and the role of TLRs in autoimmunity has been reviewed elsewhere.57,58 Individual TLRs may have specialized roles with respect to the functional outcome of co-stimulation with BCRs or CD40 in B cells. Specifically, it has been reported that BCR or CD40 stimulation in combination with some TLRs (TLR3, TLR4 or TLR9) promotes proliferation and activation, whereas others (TLR1/2, TLR2/6, TLR4 and TLR7) promote development into antibody-secreting cells.56 The molecular basis of these differential responses, as well as their role in the context of an in vivo immune response, are not yet clear. It will be important to fully examine the nature and function of the transcriptional interaction of TLRs with CD40 and BCRs.
Although in vitro studies of B cells exposed to TLR agonists have provided important clues as to how these receptors regulate B-cell responses, the high doses and synthetic nature of the TLR agonists used in some studies may not accurately represent the behaviour of B cells in the presence of actual microbes. Hence, it will be important to re-evaluate these experiments and observations using more physiological settings before their true relevance can be fully gauged.
The role of TLRs in B-cell responses in vivo
B-cell responses in vivo are regulated by a complicated network of cellular and molecular interactions (Fig. 1). As many of the cell types that regulate this response express TLRs, there are numerous stages at which TLRs could influence the B-cell response. Dendritic cells in lymph nodes respond to TLR stimulation by presenting microbial peptides on MHCI and MHCII to cytotoxic CD8 T cells and helper CD4 T cells (Th), respectively. Up-regulation of co-stimulatory molecules such as CD80, CD86 on DCs also promotes these interactions and TLR-regulated cytokines secreted by DCs can influence the subsequent development and polarization of Th cells to Th1 or Th2 lineages.57,59 The DCs can also interact directly with B cells via the presentation of whole antigen,60 although it is unknown if TLRs regulate this process. Follicular B cells internalize microbial antigen and present peptides on MHCII to Th cells, which in turn up-regulate expression of CD40 ligand, and promote activation, proliferation and development of the B cells into GC B cells. The GC B cells up-regulate expression of TLRs, and undergo several rounds of proliferation, class switch recombination and somatic hypermutation to develop high-affinity antibody chains.61 The TLRs expressed in non-haematopoietic lineages can also regulate B-cell activation – up-regulation of the B-cell-activating factor in salivary gland epithelial cells during virus infection is TLR dependent.62
Figure 1.
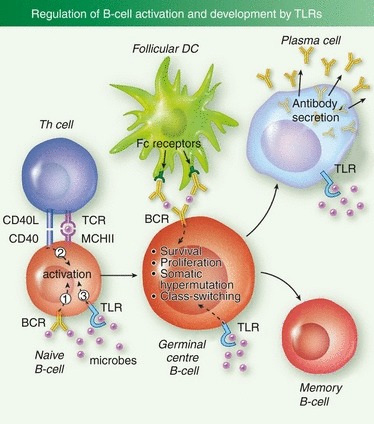
B-cell intrinsic regulation of antibody responses by Toll-like receptors (TLRs). TLR ligation by microbes contributes to the initial activation of antigen-specific follicular B cells, in combination with B-cell receptor (BCR) stimulation by antigen, and CD40 stimulation by follicular helper T cells (Tfh). Activated B cells then develop into germinal centre (GC) B cells and undergo multiple rounds of proliferation, somatic hypermutation and class switch recombination. TLR ligation enhances GC reactions. GC B cells can then undergo apoptosis or further develop into long-lived B-cell populations such as antibody-secreting plasma cells, or memory B cells. Plasma cells abundantly express TLRs, and TLR ligation enhances antibody secretion.
Both synthetic TLR ligands and traditional vaccine adjuvants that contain TLR ligands can promote antibody responses during vaccination, suggesting the potential of using this pathway to promote antibody responses.63 Mice that are deficient in members of the TLR family and TLR adapters have also been constructed and extensively analysed for their ability to mount B-cell responses to different immunogens and pathogens. The role of TLRs in B-cell responses in vivo has been a subject of some controversy, with early reports examining antibody responses to model antigens plus classical adjuvants producing seemingly contradictory results.64–66 However, more recent evidence confirms that both B-cell intrinsic and extrinsic TLRs can indeed significantly regulate B-cell responses in vivo, although the extent varies from one model system to another.
Most studies examining the role of TLRs in antibody responses have used germline TLR or Myd88-deficient mice, making it difficult to discern the contribution of B-cell intrinsic TLR signalling versus B-cell extrinsic TLR signalling. The first in vivo evidence for B-cell intrinsic TLR signalling regulating antibody responses came from Pasare and Medzhitov.67 These investigators demonstrated by transferring wild-type, TLR4-deficient, or Myd88-deficient B cells to mice that lack endogenous B cells, that TLR signalling was required in B cells to promote an antibody response to human serum albumin. Interestingly, this effect was specific to certain immunoglobulin isotypes – IgE was not affected nor was homing or survival. Mice with conditional alleles of Myd88 are now available, which has permitted a more detailed analysis of cell-lineage-specific requirements for TLRs. Mice with Myd88 conditionally deleted in DCs exhibit 10-fold reduced levels of antigen-specific IgG in response to immunization with ovalbumin and CpG oligodeoxynucleotides.68 More recently, conditional deletion of Myd88 in DCs and B cells was used to determine that the antibody response to virus-like particles required B-cell-intrinsic Myd88 but the response to purified antigen with adjuvant required DC-intrinsic Myd88.69 This result highlights the in vivo importance of B-cell-intrinsic TLRs and also demonstrates how antigen/adjuvant combinations commonly used to model immune responses can behave very differently from actual viral pathogens.
As TLR signalling can synergize with BCR activation to promote B-cell activation and proliferation in vitro, it is likely that co-engagement of BCRs and TLRs promotes initial microbe-specific B-cell activation in vivo. It has been proposed that B cells require three different signals for initial activation in vivo: (i) antigen (through BCRs), (ii) T-cell help (through CD40) and (iii) an innate immune signal, such as TLR ligation.70 By requiring B cells to receive a signal from BCR, CD40 and an innate pathogen sensor, B cells can be carefully regulated to only respond in the context of infection, and avoid activation in response to self antigens.
In addition to regulating initial B-cell activation, TLRs probably regulate later steps in the B-cell response. Several recent reports have indicated an in vivo role for TLRs in promoting class switch recombination, somatic hypermutation and the development or maintenance of GCs.69,71–75 Consistent with this hypothesis, GC B cells have elevated sensitivity to TLR ligands and this correlates with increased expression of Myd88, Myd88 adapter like protein (Mal) and Interleukin 1 receptor associated kinase M (IRAK-M).65 Furthermore, delivery of TLR agonists with synthetic nanoparticles significantly enhances the number and size of GC reactions in the context of immunization.72 Hence, it is likely that GC B cells perform continuous surveillance of microbial levels to regulate the duration and intensity of GC reactions.
In addition to the role of TLRs in primary responses to infection, it will be important to determine the role of TLRs in the maintenance and activation of memory B cells. So far, it has been difficult to separate the role of TLRs in the recall response of memory lymphocytes from the role of TLRs in the generation of memory cells during the primary response. Recently, an inducible knockout for Myd88 has been used to determine that the initial CD8 T-cell response to the arenavirus lymphocytic choriomeningitis virus is regulated by Myd88, but that secondary CD8 T-cell responses are Myd88 independent.76 A similar approach could be used to determine the role of TLRs in the reactivation of memory B cells and secondary antibody responses.
Pathways of TLR stimulation in B cells during viral infection
Although some reports have identified cell surface TLRs such as TLR2 or TLR4 as mediating recognition of specific viral proteins,77–80 the majority of studies have focused on the role of nucleic-acid-sensing TLRs (TLR3, TLR7, TLR8, TLR9) in antiviral immunity. As these TLRs are located in endosomal compartments, viral particles containing the genomic RNA or DNA must be delivered to the endosome to promote TLR-dependent immunity. There are a number of different pathways by which viral nucleic acid could potentially be delivered to endosomal TLRs in B cells (Fig. 2). In the subset of B cells with BCRs that are specific for virus surface antigens, viruses can be internalized and trafficked to endosomes after BCR binding. Inside endosomes, pH and degradative enzymes then disrupt viral particles to release the nucleic acid TLR ligands within. This BCR-dependent pathway thereby provides an important coupling between pathogen sensing and antigen-specific activation of B cells. A similar pathway has been shown to play an important role in the context of autoreactive B-cell activation81,82 and for B-cell responses to whole bacteria.83
Figure 2.
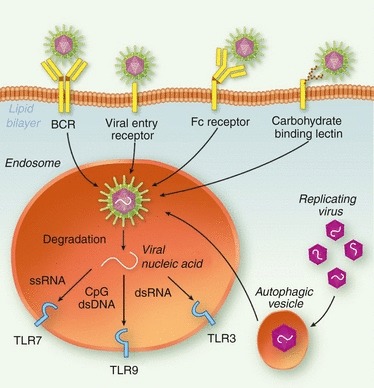
Potential routes of Toll-like receptor (TLR) stimulation in B cells by viruses. Viruses can bind to and enter B cells through several different pathways. B-cell receptors (BCRs) specific for viral antigens can bind and internalize virus, or viral particles can enter through binding either their natural entry receptor or a carbohydrate-binding lectin such as DC-SIGN. Antibody-coated viruses could also potentially enter B cells through Fc receptor-mediated internalization. Internalized virus is then degraded in endosomes to release viral genomic nucleic acid, which can stimulate the endosomal TLRs such as TLR7, TLR8, TLR9 or TLR3. Replicating virus in the B-cell cytoplasm could also potentially be delivered to endosomal TLRs by autophagy.
Some viruses can directly bind and enter B cells through receptor-mediated endocytosis, thereby accessing TLR-containing endosomes as part of their natural infectious cycle. This raises a problem for murine B cells, for which TLR7 and TLR9 stimulation are sufficient to promote activation. How do mice avoid polyclonal activation of B cells through TLR7 and TLR9 during infection with viruses that can directly infect B cells? Other potential BCR-independent modes of viral TLR stimulation in B cells include the internalization of antibody-coated virus particles through Fc recptors,84 or binding of glycosylated viral proteins by scavenging C-type lectins such as DC-SIGN.85 Autophagy, a natural homeostatic process by which long-lived cellular organelles and proteins can be enveloped in membranes and recycled, has also been shown to be capable of enveloping cytoplasmic viral components and directing them to TLRs.86 For non-BCR-dependent pathways of TLR stimulation, it is less clear how antigen specificity of B-cell activation would be achieved or whether these pathways are sufficient for B-cell activation in vivo. It will be important to determine the role that each of these pathways plays in vivo in the context of B-cell activation. The precise pathway by which microbial TLR ligands are delivered to TLRs in B cells could have an impact on the outcome in the context of antigen-specific B-cell responses. Are all these pathways equivalent or do they lead to qualitatively different outcomes?
The role of TLRs in B-cell responses to viruses: lessons from murine infection models
Mice that are TLR deficient have been analysed for altered susceptibility and antibody responses to several viral pathogens. These results have, overall, confirmed that TLRs can contribute to B-cell responses in the context of viral infection. However, the details and extent of their role varies from one viral model to another, highlighting the difficulty of extrapolating between different virus families. The phenotype observed with respect to the role of TLRs in B-cell responses is probably determined by three parameters – the nature of the viral genome, the cellular tropism of the virus, and the expression pattern of both TLR and non-TLR sensors capable of detecting the virus. It is also likely that TLR signalling regulates multiple steps in the B-cell response, but whether TLRs are necessary for progression through each checkpoint will depend on the presence or absence of alternative virus-sensing pathways, either B-cell intrinsic or extrinsic, that can compensate. For some viruses, TLR-independent pathways may be sufficient for most or all steps in the B-cell response, leading to a relatively weak phenotype in TLR-deficient mice. Similarly, innate immune responses to the virus in cell types other than B cells could facilitate B-cell activation through the paracrine action of cytokines. For other viruses, alternative sensing pathways may not exist or may not be expressed in the appropriate cell types to permit development of B cells past a checkpoint, leading to a more stringent requirement for TLRs.
The following section summarizes available data from Myd88-deficient or TLR-deficient mice regarding antibody responses for several noteworthy virus families.
Orthomyxoviridae (negative-sense ssRNA genomes)
Influenza virus is capable of stimulating a potent innate immune response in B cells, and this response is regulated by TLR7 (Browne EP, unpublished observation). However, studies with murine models of influenza virus infection have revealed that Myd88 and TLR7 are not strictly required for an antibody response to infection,87 although mice deficient in these molecules exhibited increased virus-specific IgG1 and decreased IgG2a/c immunoglobulin class switching. Curiously, germline Myd88-deficient mice exhibit heightened susceptibility to primary influenza virus infection, but are as resistant to secondary infection as wild-type mice.88 Overall these results suggest a fine-tuning role for TLRs in the antibody response to primary influenza virus infection, rather than an explicit requirement. This may be a result of the presence of alternative innate sensors such as Retinoic acid inducible gene I (RIG-I) that can detect influenza virus.89 A clearer role for TLRs is found for responses to vaccination with inactivated influenza virus particles. Myd88-deficient or TLR7-deficient mice are not protected by inactivated influenza virus particles, and exhibit defects in inducing IgG2a/c recall responses, as well as plasma cell responses in bone marrow, to inactivated vaccine.90,91
Paramyxoviridae (negative-sense ssRNA genomes)
A murine model of respiratory syncytial virus (RSV) infection has found that Myd88-deficient mice are able to mount an effective clearing antibody response to RSV, albeit with a delay.92 Interestingly, these mice are also able to develop antibodies against an inactivated RSV vaccine, but these antibodies are qualitatively poor – they exhibit reduced avidity for RSV proteins and attenuated neutralizing power.93 This suggests that, during RSV vaccination, TLRs regulate a late step in the B-cell response such as affinity maturation. Consistent with this finding, Myd88-deficient mice have fewer GL7-expressing GC B cells after vaccination. This phenotype has not yet been linked to an individual TLR, although polymorphisms in TLR4 may correlate with susceptibility to clinical RSV infection.94 Remarkably, administration of LPS with the RSV vaccine significantly enhanced its protective efficacy and the neutralizing ability of the antibodies induced.93
Polyomaviridae (circular dsDNA genomes)
Myd88-deficient mice infected with murine polyomavirus initially develop strong humoral immunity to the virus, including both IgM and IgG isotypes.95 They also develop GC B cells and exhibit class switching and somatic hypermutation, but fail to develop virus-specific bone marrow plasma cells. Certain antibody isotypes such as IgG2a and IgG2b exhibit reduced levels among the virus-specific antibodies. Interestingly, Myd88-deficient mice fail to maintain a serum antibody response to polyomavirus, and titres of virus-specific antibodies declined over time relative to wild-type mice.95 This finding suggests a role for TLRs in the maintenance of long-term antibody responses to polyomavirus, possible at the level of formation or maintenance of plasma cells. An individual TLR has not yet been identified as being necessary or sufficient for detecting polyomavirus, but as polyomaviruses have dsDNA genomes it possible that TLR9 is involved.
Rhabdoviridae (negative-sense ssRNA genomes)
Although Myd88 is not required for an antibody response to the model rhabdovirus vesicular stomatitis virus (VSV), as measured by either total virus-specific IgG or by neutralizing antibody titre, a slight reduction in the representation of some istoypes was seen in Myd88-deficient mice.96 It is still unknown which TLR mediates this effect, although VSV can stimulate plasmacytoid DCs through TLR7,97 and VSV-G protein has been shown to stimulate TLR4.98
Herpesviridae (linear dsDNA genomes)
A number of TLRs have been implicated in the immune response to herpesviruses. TLR9 can detect the dsDNA herpesvirus genome, while TLR3 can detect dsRNAs that are generated abundantly during herpesvirus replication by overlapping transcription. TLR2 has also been proposed to directly detect some herpesvirus glycoproteins.99,100 TLR3- and TLR9-deficient mice, and to a lesser extent TLR2-deficient mice, have heightened sensitivity to murine cytomegalovirus, but this phenotype seems to be a result of defects in the innate response such as IFN-α and natural killer cells rather than through adaptive immunity.101 Both Myd88-deficient and TLR9-deficient mice have an apparently normal antibody response to murine cytomegalovirus infection, apart from a reduction in IgG1 specific for the virus.102 By contrast, Myd88-deficient mice have reduced B-cell responses to gamma herpesvirus 68, indicated by fewer activated B cells, fewer GC B cells and reduced antibody titres.103 Interestingly Epstein–Barr virus has been found to inhibit the sensitivity of B cells to TLR7/8 and TLR9 agonists,104 suggesting that these TLRs may regulate responses to this virus, but the lack of a murine model for Epstein–Barr virus makes an in vivo genetic analysis of this question difficult.
Flaviviridae (positive-sense ssRNA genome)
Studies using TLR-deficient mice indicate that TLR7 is not required for control of West Nile virus,105 while Myd88 and TLR3 regulate inflammation and viral loads in the periphery.106,107 The role of these molecules in controlling antibody responses to the virus was not reported. However, a study looking at Dengue-virus-infected macaques found that subcutaneous doses of TLR3 and TLR7/8 agonists after infection led to enhanced humoral responses and an increased IgG2 to IgG1 ratio.108
Retroviridae (polyadenylated positive-sense ssRNA genomes)
For reasons that are not clear, infection with human immunodeficiency virus (HIV) fails to induce a potent neutralizing antibody response.109 Similarly, efforts to induce a broad neutralizing antibody response with purified HIV gp120 envelope have been unsuccessful.110 Unfortunately, as yet, no small animal model of HIV exists that permits genetic analysis of the role of TLRs in antibody responses. However, studies with mouse retroviral models have clearly demonstrated a requirement for TLR7 and Myd88 for an antibody response to infection.71,111,112 Retroviruses have also been demonstrated to stimulate murine B cells via TLR4 through an interaction with commensal bacteria.113,114 By conditional deletion of Myd88 the requirement for TLRs in the antibody response to retroviral infection was found to be primarily B-cell intrinsic, while DC-intrinsic TLRs were less important.71 Hence TLR-deficient mice exhibit a more pronounced phenotype with retroviral models than that observed with other viruses such as influenza, RSV or polyomavirus. Why would the B-cell response to retroviruses exhibit a more stringent requirement for TLRs than other viruses? It is interesting to note that retroviruses exhibit a low cytopathic effect on infected cells compared with most other viruses. For highly cytopathic viruses, immune stimulation could occur by dead or dying cells releasing molecules such as uric acid or ATP, or by expressing signals of cellular distress.115 In the absence of TLR signalling, these pathways could be sufficient to promote B-cell responses. So for less cytopathic viruses such as retroviruses, the immune response may depend more on pattern recognition and TLRs to stimulate a response.
Evidence from human genetics
Compared with the data from mice, direct genetic evidence for the role of TLRs in antibody responses in humans are less abundant. Inactivating germline mutations in innate immune pathways in human populations are rare, probably because of selection pressure from infectious disease. However, some human populations with functionally significant mutations in components of the TLR pathway such as Myd88, IRAK4 and UNC93B have been identified.116,117 These mutations have so far revealed largely normal development of B cells and responses to immunization with some exceptions.118 Notably, humans with IRAK4 and Myd88 mutations exhibit increased susceptibility to pyogenic bacterial infections,116,117 but do not exhibit heightened susceptibility to viral pathogens. In contrast, TLR3 deficiency has been shown to result in heightened sensitivity to herpes simplex virus-1 encephalitis, although it is unclear if antibody responses are defective in these individuals.119,120 Interestingly, an analysis of the antibody repertoire has found evidence for a role for Myd88 and TLRs in appropriate negative selection of autoreactive B cells.121 Specifically, Myd88 and IRAK4 were found to be required for central and peripheral checkpoints to prevent antibody autoreactivity. UNC93B, by contrast was found to be only required for a peripheral checkpoint.
Summary and future challenges
Despite the tremendous progress made in understanding the role of TLRs in B-cell responses, significant challenges remain. Although numerous studies have now shown that TLRs can modulate B-cell responses, both in vitro and in vivo, the diverse results obtained with different immunogens and pathogens have yet to be reconciled into a complete model for how TLRs regulate B cells. Findings from murine models of infection and immunity will need to be applied to the study of human infections and vaccines. Does the relative resistance of Myd88-deficient humans to infection compared with Myd88-deficient mice imply a fundamental interspecies difference in the role of TLRs? It is possible that in humans, the TLRs contribute to but are not necessary for B-cell responses because of compensation by TLR-independent innate pathways? Nevertheless, the conservation of these receptors through evolution and their expression in B cells implies a functional role in humans.
It will also be important to fully catalogue the extent of functionally significant polymorphisms and diversity within the TLR pathway present in the human population and to examine these polymorphisms for correlations with patterns of infectious disease and antibody responses to vaccination. Similarly, it will be interesting to expand our analysis of the role of TLRs in antibody responses beyond the C57BL/6 mouse strain to more diverse murine genetic backgrounds. This will probably shed light on the evolution of TLR signalling and function. Are the roles of innate pathways in stimulating adaptive immunity highly conserved and robust through speciation and evolution, or are they inherently plastic to permit adaptation to new infectious diseases? Another key area that needs to be addressed is defining and characterizing TLR-independent innate pathways that regulate B cells.
Does inadequate TLR signalling contribute to poorly neutralizing antibody responses to chronic viral infections such as HIV and hepatitis C virus? Recently, a number of broadly neutralizing antibodies to HIV have been identified, and their amino acid sequences exhibit unusually high levels of somatic hypermutation.122 As TLRs have been shown to regulate GC reactions where somatic hypermutation takes place, it is possible that targeting TLRs expressed in GC B cells could enhance somatic hypermutation and thereby the breadth of the antibody response to HIV?
There is also a critical need for novel TLR agonists that can potently promote antibody responses during vaccination, but that lack the toxicity issues that characterize existing agonists. By precisely defining the role of individual TLR-regulated genes in the B-cell response, we may be able to design molecules that can trigger TLRs to induce protective responses without causing unnecessary inflammation and toxicity. This might be achieved by developing methods to target TLRs expressed on only key cell lineages and avoid ‘bystander’ stimulation.
Acknowledgments
The author wishes to thank Philip Kong, Peter Bak, Mike Barnkob and Adam Drake for useful comments and suggestions.
Disclosures
The author has no conflicts of interest to disclose.
References
- 1.Hashimoto C, Hudson KL, Anderson KV. The Toll gene of Drosophila, required for dorsal–ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988;52:269–79. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 3.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi F, Smith KD, Ozinsky A, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi O, Kawai T, Muhlradt PF, Morr M, Radolf JD, Zychlinsky A, Takeda K, Akira S. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol. 2001;13:933–40. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- 6.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–9. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 7.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–31. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 8.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 9.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 10.Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–8. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 11.Brinkmann MM, Spooner E, Hoebe K, Beutler B, Ploegh HL, Kim YM. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J Cell Biol. 2007;177:265–75. doi: 10.1083/jcb.200612056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukui R, Saitoh S, Kanno A, et al. Unc93B1 restricts systemic lethal inflammation by orchestrating Toll-like receptor 7 and 9 trafficking. Immunity. 2011;35:69–81. doi: 10.1016/j.immuni.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–6. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 14.Yarovinsky F, Zhang D, Andersen JF, et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–9. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–5. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer-Bahlburg A, Rawlings DJ. Differential impact of Toll-like receptor signaling on distinct B cell subpopulations. Front Biosci. 2011;17:1499–516. doi: 10.2741/4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–7. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 18.Mansson A, Adner M, Hockerfelt U, Cardell LO. A distinct Toll-like receptor repertoire in human tonsillar B cells, directly activated by PamCSK, R-837 and CpG-2006 stimulation. Immunology. 2006;118:539–48. doi: 10.1111/j.1365-2567.2006.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal S, Gupta S. TLR1/2, TLR7, and TLR9 signals directly activate human peripheral blood naive and memory B cell subsets to produce cytokines, chemokines, and hematopoietic growth factors. J Clin Immunol. 2010;31:89–98. doi: 10.1007/s10875-010-9456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 21.Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A. The toll-like receptor repertoire of human B lymphocytes: inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood. 2003;102:956–63. doi: 10.1182/blood-2002-11-3355. [DOI] [PubMed] [Google Scholar]
- 22.Dorner M, Brandt S, Tinguely M, et al. Plasma cell toll-like receptor (TLR) expression differs from that of B cells, and plasma cell TLR triggering enhances immunoglobulin production. Immunology. 2009;128:573–9. doi: 10.1111/j.1365-2567.2009.03143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gururajan M, Jacob J, Pulendran B. Toll-like receptor expression and responsiveness of distinct murine splenic and mucosal B-cell subsets. PLoS ONE. 2007;2:e863. doi: 10.1371/journal.pone.0000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genestier L, Taillardet M, Mondiere P, Gheit H, Bella C, Defrance T. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J Immunol. 2007;178:7779–86. doi: 10.4049/jimmunol.178.12.7779. [DOI] [PubMed] [Google Scholar]
- 25.Andersson LC, Nordling S, Hayry P. Proliferation of B and T cells in mixed lymphocyte cultures. J Exp Med. 1973;138:324–9. doi: 10.1084/jem.138.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall-Clarke S, Downes JE, Haga IR, Bowie AG, Borrow P, Pennock JL, Grencis RK, Rothwell P. Polyinosinic acid is a ligand for toll-like receptor 3. J Biol Chem. 2007;282:24759–66. doi: 10.1074/jbc.M700188200. [DOI] [PubMed] [Google Scholar]
- 27.Chang WL, Coro ES, Rau FC, Xiao Y, Erle DJ, Baumgarth N. Influenza virus infection causes global respiratory tract B cell response modulation via innate immune signals. J Immunol. 2007;178:1457–67. doi: 10.4049/jimmunol.178.3.1457. [DOI] [PubMed] [Google Scholar]
- 28.Sato S, Sanjo H, Takeda K, et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–95. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 29.Bekeredjian-Ding IB, Wagner M, Hornung V, Giese T, Schnurr M, Endres S, Hartmann G. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J Immunol. 2005;174:4043–50. doi: 10.4049/jimmunol.174.7.4043. [DOI] [PubMed] [Google Scholar]
- 30.Muzio M, Bosisio D, Polentarutti N, et al. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 31.Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, Segal DM. Regulation of Toll-like receptors in human monocytes and dendritic cells. J Immunol. 2001;166:249–55. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- 32.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–5. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 33.Carty M, Goodbody R, Schroder M, Stack J, Moynagh PN, Bowie AG. The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nat Immunol. 2006;7:1074–81. doi: 10.1038/ni1382. [DOI] [PubMed] [Google Scholar]
- 34.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–64. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 35.Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9:692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- 36.Chevrier N, Mertins P, Artyomov MN, et al. Systematic discovery of TLR signaling components delineates viral-sensing circuits. Cell. 2011;147:853–67. doi: 10.1016/j.cell.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amit I, Garber M, Chevrier N, et al. Unbiased reconstruction of a mammalian transcriptional network mediating pathogen responses. Science. 2009;326:257–63. doi: 10.1126/science.1179050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stephan K, Smirnova I, Jacque B, Poltorak A. Genetic analysis of the innate immune responses in wild-derived inbred strains of mice. Eur J Immunol. 2007;37:212–23. doi: 10.1002/eji.200636156. [DOI] [PubMed] [Google Scholar]
- 39.Jiang W, Lederman MM, Harding CV, Rodriguez B, Mohner RJ, Sieg SF. TLR9 stimulation drives naive B cells to proliferate and to attain enhanced antigen presenting function. Eur J Immunol. 2007;37:2205–13. doi: 10.1002/eji.200636984. [DOI] [PubMed] [Google Scholar]
- 40.Capolunghi F, Cascioli S, Giorda E, et al. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J Immunol. 2008;180:800–8. doi: 10.4049/jimmunol.180.2.800. [DOI] [PubMed] [Google Scholar]
- 41.Lutzker S, Rothman P, Pollock R, Coffman R, Alt FW. Mitogen- and IL-4-regulated expression of germ-line Igγ 2b transcripts: evidence for directed heavy chain class switching. Cell. 1988;53:177–84. doi: 10.1016/0092-8674(88)90379-0. [DOI] [PubMed] [Google Scholar]
- 42.He B, Qiao X, Cerutti A. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J Immunol. 2004;173:4479–91. doi: 10.4049/jimmunol.173.7.4479. [DOI] [PubMed] [Google Scholar]
- 43.Xu W, Santini PA, Matthews AJ, Chiu A, Plebani A, He B, Chen K, Cerutti A. Viral double-stranded RNA triggers Ig class switching by activating upper respiratory mucosa B cells through an innate TLR3 pathway involving BAFF. J Immunol. 2008;181:276–87. doi: 10.4049/jimmunol.181.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanten JA, Vasilakos JP, Riter CL, Neys L, Lipson KE, Alkan SS, Birmachu W. Comparison of human B cell activation by TLR7 and TLR9 agonists. BMC Immunol. 2008;9:39. doi: 10.1186/1471-2172-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cognasse F, Hamzeh-Cognasse H, Lafarge S, Chavarin P, Pozzetto B, Richard Y, Garraud O. Identification of two subpopulations of purified human blood B cells, CD27− CD23+ and CD27high CD80+, that strongly express cell surface Toll-like receptor 9 and secrete high levels of interleukin-6. Immunology. 2008;125:430–7. doi: 10.1111/j.1365-2567.2008.02844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer-Bahlburg A, Bandaranayake AD, Andrews SF, Rawlings DJ. Reduced c-myc expression levels limit follicular mature B cell cycling in response to TLR signals. J Immunol. 2009;182:4065–75. doi: 10.4049/jimmunol.0802961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol. 2008;26:261–92. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin L, Gerth AJ, Peng SL. CpG DNA redirects class-switching towards “Th1-like” Ig isotype production via TLR9 and MyD88. Eur J Immunol. 2004;34:1483–7. doi: 10.1002/eji.200324736. [DOI] [PubMed] [Google Scholar]
- 49.Sindhava V, Woodman ME, Stevenson B, Bondada S. Interleukin-10 mediated autoregulation of murine B-1 B-cells and its role in Borrelia hermsii infection. PLoS ONE. 2010;5:e11445. doi: 10.1371/journal.pone.0011445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jagannathan M, Hasturk H, Liang Y, et al. TLR cross-talk specifically regulates cytokine production by B cells from chronic inflammatory disease patients. J Immunol. 2009;183:7461–70. doi: 10.4049/jimmunol.0901517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Green NM, Laws A, Kiefer K, et al. Murine B cell response to TLR7 ligands depends on an IFN-β feedback loop. J Immunol. 2009;183:1569–76. doi: 10.4049/jimmunol.0803899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barr TA, Brown S, Ryan G, Zhao J, Gray D. TLR-mediated stimulation of APC: distinct cytokine responses of B cells and dendritic cells. Eur J Immunol. 2007;37:3040–53. doi: 10.1002/eji.200636483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lampropoulou V, Hoehlig K, Roch T, et al. TLR-activated B cells suppress T cell-mediated autoimmunity. J Immunol. 2008;180:4763–73. doi: 10.4049/jimmunol.180.7.4763. [DOI] [PubMed] [Google Scholar]
- 54.Lenert P, Brummel R, Field EH, Ashman RF. TLR-9 activation of marginal zone B cells in lupus mice regulates immunity through increased IL-10 production. J Clin Immunol. 2005;25:29–40. doi: 10.1007/s10875-005-0355-6. [DOI] [PubMed] [Google Scholar]
- 55.Jain S, Chodisetti SB, Agrewala JN. CD40 signaling synergizes with TLR-2 in the BCR independent activation of resting B cells. PLoS ONE. 2011;6:e20651. doi: 10.1371/journal.pone.0020651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boeglin E, Smulski CR, Brun S, Milosevic S, Schneider P, Fournel S. Toll-like receptor agonists synergize with CD40L to induce either proliferation or plasma cell differentiation of mouse B cells. PLoS ONE. 2011;6:e25542. doi: 10.1371/journal.pone.0025542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Green NM, Marshak-Rothstein A. Toll-like receptor driven B cell activation in the induction of systemic autoimmunity. Semin Immunol. 2011;23:106–12. doi: 10.1016/j.smim.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meyer-Bahlburg A, Rawlings DJ. B cell autonomous TLR signaling and autoimmunity. Autoimmun Rev. 2008;7:313–6. doi: 10.1016/j.autrev.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boonstra A, Asselin-Paturel C, Gilliet M, Crain C, Trinchieri G, Liu YJ, O’Garra A. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation. J Exp Med. 2003;197:101–9. doi: 10.1084/jem.20021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–14. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 61.Gatto D, Brink R. The germinal center reaction. J Allergy Clin Immunol. 2010;126:898–907. doi: 10.1016/j.jaci.2010.09.007. quiz 8–9. [DOI] [PubMed] [Google Scholar]
- 62.Ittah M, Miceli-Richard C, Gottenberg JE, et al. Viruses induce high expression of BAFF by salivary gland epithelial cells through TLR- and type-I IFN-dependent and -independent pathways. Eur J Immunol. 2008;38:1058–64. doi: 10.1002/eji.200738013. [DOI] [PubMed] [Google Scholar]
- 63.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–63. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 64.Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–8. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meyer-Bahlburg A, Khim S, Rawlings DJ. B cell intrinsic TLR signals amplify but are not required for humoral immunity. J Exp Med. 2007;204:3095–101. doi: 10.1084/jem.20071250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–50. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 67.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–8. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 68.Hou B, Reizis B, DeFranco AL. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 2008;29:272–82. doi: 10.1016/j.immuni.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hou B, Saudan P, Ott G, et al. Selective utilization of Toll-like receptor and MyD88 signaling in B cells for enhancement of the antiviral germinal center response. Immunity. 2011;34:375–84. doi: 10.1016/j.immuni.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruprecht CR, Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur J Immunol. 2006;36:810–6. doi: 10.1002/eji.200535744. [DOI] [PubMed] [Google Scholar]
- 71.Browne EP. TLR7 controls the germinal center response to retroviral infection. PLoS Pathog. 2011;7:e1002293. doi: 10.1371/journal.ppat.1002293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kasturi SP, Skountzou I, Albrecht RA, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–7. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aranburu A, Ceccarelli S, Giorda E, Lasorella R, Ballatore G, Carsetti R. TLR ligation triggers somatic hypermutation in transitional B cells inducing the generation of IgM memory B cells. J Immunol. 2010;185:7293–301. doi: 10.4049/jimmunol.1002722. [DOI] [PubMed] [Google Scholar]
- 74.Garin A, Meyer-Hermann M, Contie M, et al. Toll-like receptor 4 signaling by follicular dendritic cells is pivotal for germinal center onset and affinity maturation. Immunity. 2010;33:84–95. doi: 10.1016/j.immuni.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 75.Bessa J, Kopf M, Bachmann MF. Cutting edge: IL-21 and TLR signaling regulate germinal center responses in a B cell-intrinsic manner. J Immunol. 2010;184:4615–9. doi: 10.4049/jimmunol.0903949. [DOI] [PubMed] [Google Scholar]
- 76.Rahman AH, Zhang R, Blosser CD, Hou B, Defranco AL, Maltzman JS, Wherry EJ, Turka LA. Antiviral memory CD8 T-cell differentiation, maintenance, and secondary expansion occur independently of MyD88. Blood. 2011;117:3123–30. doi: 10.1182/blood-2010-11-318485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boehme KW, Guerrero M, Compton T. Human cytomegalovirus envelope glycoproteins B and H are necessary for TLR2 activation in permissive cells. J Immunol. 2006;177:7094–102. doi: 10.4049/jimmunol.177.10.7094. [DOI] [PubMed] [Google Scholar]
- 78.Bieback K, Lien E, Klagge IM, et al. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J Virol. 2002;76:8729–36. doi: 10.1128/JVI.76.17.8729-8736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duesberg U, von dem Bussche A, Kirschning C, Miyake K, Sauerbruch T, Spengler U. Cell activation by synthetic lipopeptides of the hepatitis C virus (HCV) – core protein is mediated by toll like receptors (TLRs) 2 and 4. Immunol Lett. 2002;84:89–95. doi: 10.1016/s0165-2478(02)00178-5. [DOI] [PubMed] [Google Scholar]
- 80.Cooper A, Tal G, Lider O, Shaul Y. Cytokine induction by the hepatitis B virus capsid in macrophages is facilitated by membrane heparan sulfate and involves TLR2. J Immunol. 2005;175:3165–76. doi: 10.4049/jimmunol.175.5.3165. [DOI] [PubMed] [Google Scholar]
- 81.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–7. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 82.Viglianti GA, Lau CM, Hanley TM, Miko BA, Shlomchik MJ, Marshak-Rothstein A. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–47. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 83.Jendholm J, Morgelin M, Perez Vidakovics ML, Carlsson M, Leffler H, Cardell LO, Riesbeck K. Superantigen- and TLR-dependent activation of tonsillar B cells after receptor-mediated endocytosis. J Immunol. 2009;182:4713–20. doi: 10.4049/jimmunol.0803032. [DOI] [PubMed] [Google Scholar]
- 84.Shibuya A, Sakamoto N, Shimizu Y, et al. Fc α/μ receptor mediates endocytosis of IgM-coated microbes. Nat Immunol. 2000;1:441–6. doi: 10.1038/80886. [DOI] [PubMed] [Google Scholar]
- 85.Rappocciolo G, Piazza P, Fuller CL, et al. DC-SIGN on B lymphocytes is required for transmission of HIV-1 to T lymphocytes. PLoS Pathog. 2006;2:e70. doi: 10.1371/journal.ppat.0020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 87.Heer AK, Shamshiev A, Donda A, Uematsu S, Akira S, Kopf M, Marsland BJ. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J Immunol. 2007;178:2182–91. doi: 10.4049/jimmunol.178.4.2182. [DOI] [PubMed] [Google Scholar]
- 88.Seo SU, Kwon HJ, Song JH, Byun YH, Seong BL, Kawai T, Akira S, Kweon MN. MyD88 signaling is indispensable for primary influenza A virus infection but dispensable for secondary infection. J Virol. 2010;84:12713–22. doi: 10.1128/JVI.01675-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kato H, Takeuchi O, Sato S, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–5. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 90.Koyama S, Ishii KJ, Kumar H, Tanimoto T, Coban C, Uematsu S, Kawai T, Akira S. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J Immunol. 2007;179:4711–20. doi: 10.4049/jimmunol.179.7.4711. [DOI] [PubMed] [Google Scholar]
- 91.Kang SM, Yoo DG, Kim MC, et al. MyD88 plays an essential role in inducing B cells capable of differentiating into antibody-secreting cells after vaccination. J Virol. 2011;85:11391–400. doi: 10.1128/JVI.00080-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bhoj VG, Sun Q, Bhoj EJ, et al. MAVS and MyD88 are essential for innate immunity but not cytotoxic T lymphocyte response against respiratory syncytial virus. Proc Natl Acad Sci U S A. 2008;105:14046–51. doi: 10.1073/pnas.0804717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Delgado MF, Coviello S, Monsalvo AC, et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Puthothu B, Forster J, Heinzmann A, Krueger M. TLR-4 and CD14 polymorphisms in respiratory syncytial virus associated disease. Dis Markers. 2006;22:303–8. doi: 10.1155/2006/865890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guay HM, Andreyeva TA, Garcea RL, Welsh RM, Szomolanyi-Tsuda E. MyD88 is required for the formation of long-term humoral immunity to virus infection. J Immunol. 2007;178:5124–31. doi: 10.4049/jimmunol.178.8.5124. [DOI] [PubMed] [Google Scholar]
- 96.Lang KS, Navarini AA, Recher M, et al. MyD88 protects from lethal encephalitis during infection with vesicular stomatitis virus. Eur J Immunol. 2007;37:2434–40. doi: 10.1002/eji.200737310. [DOI] [PubMed] [Google Scholar]
- 97.Ahmed M, Mitchell LM, Puckett S, Brzoza-Lewis KL, Lyles DS, Hiltbold EM. Vesicular stomatitis virus M protein mutant stimulates maturation of Toll-like receptor 7 (TLR7)-positive dendritic cells through TLR-dependent and -independent mechanisms. J Virol. 2009;83:2962–75. doi: 10.1128/JVI.02030-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Georgel P, Jiang Z, Kunz S, et al. Vesicular stomatitis virus glycoprotein G activates a specific antiviral Toll-like receptor 4-dependent pathway. Virology. 2007;362:304–13. doi: 10.1016/j.virol.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 99.Gaudreault E, Fiola S, Olivier M, Gosselin J. Epstein–Barr virus induces MCP-1 secretion by human monocytes via TLR2. J Virol. 2007;81:8016–24. doi: 10.1128/JVI.00403-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sato A, Linehan MM, Iwasaki A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc Natl Acad Sci U S A. 2006;103:17343–8. doi: 10.1073/pnas.0605102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tabeta K, Georgel P, Janssen E, et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A. 2004;101:3516–21. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Delale T, Paquin A, Asselin-Paturel C, et al. MyD88-dependent and -independent murine cytomegalovirus sensing for IFN-α release and initiation of immune responses in vivo. J Immunol. 2005;175:6723–32. doi: 10.4049/jimmunol.175.10.6723. [DOI] [PubMed] [Google Scholar]
- 103.Gargano LM, Moser JM, Speck SH. Role for MyD88 signaling in murine γ herpesvirus 68 latency. J Virol. 2008;82:3853–63. doi: 10.1128/JVI.02577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–7. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 105.Welte T, Reagan K, Fang H, et al. Toll-like receptor 7-induced immune response to cutaneous West Nile virus infection. J Gen Virol. 2009;90(Pt 11):2660–8. doi: 10.1099/vir.0.011783-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–73. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 107.Szretter KJ, Daffis S, Patel J, Suthar MS, Klein RS, Gale M, Jr, Diamond MS. The innate immune adaptor molecule MyD88 restricts West Nile virus replication and spread in neurons of the central nervous system. J Virol. 2010;84:12125–38. doi: 10.1128/JVI.01026-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sariol CA, Martinez MI, Rivera F, et al. Decreased dengue replication and an increased anti-viral humoral response with the use of combined Toll-like receptor 3 and 7/8 agonists in macaques. PLoS ONE. 2011;6:e19323. doi: 10.1371/journal.pone.0019323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kwong PD, Doyle ML, Casper DJ, et al. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420:678–82. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- 110.Kim JH, Rerks-Ngarm S, Excler JL, Michael NL. HIV vaccines: lessons learned and the way forward. Curr Opin HIV AIDS. 2010;5:428–34. doi: 10.1097/COH.0b013e32833d17ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kane M, Case LK, Wang C, Yurkovetskiy L, Dikiy S, Golovkina TV. Innate immune sensing of retroviral infection via Toll-like receptor 7 occurs upon viral entry. Immunity. 2011;35:135–45. doi: 10.1016/j.immuni.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Browne EP, Littman DR. Myd88 is required for an antibody response to retroviral infection. PLoS Pathog. 2009;5:e1000298. doi: 10.1371/journal.ppat.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kane M, Case LK, Kopaskie K, Kozlova A, MacDearmid C, Chervonsky AV, Golovkina TV. Successful transmission of a retrovirus depends on the commensal microbiota. Science. 2011;334:245–9. doi: 10.1126/science.1210718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rassa JC, Meyers JL, Zhang Y, Kudaravalli R, Ross SR. Murine retroviruses activate B cells via interaction with toll-like receptor 4. Proc Natl Acad Sci U S A. 2002;99:2281–6. doi: 10.1073/pnas.042355399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–21. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 116.Picard C, Puel A, Bonnet M, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299:2076–9. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 117.von BernuthH, Picard C, Jin Z, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–6. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ku CL, von Bernuth H, Picard C, et al. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J Exp Med. 2007;204:2407–22. doi: 10.1084/jem.20070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang SY, Jouanguy E, Ugolini S, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–7. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 120.Guo Y, Audry M, Ciancanelli M, et al. Herpes simplex virus encephalitis in a patient with complete TLR3 deficiency: TLR3 is otherwise redundant in protective immunity. J Exp Med. 2011;208:2083–98. doi: 10.1084/jem.20101568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Isnardi I, Ng YS, Srdanovic I, et al. IRAK-4- and MyD88-dependent pathways are essential for the removal of developing autoreactive B cells in humans. Immunity. 2008;29:746–57. doi: 10.1016/j.immuni.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Walker LM, Huber M, Doores KJ, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–70. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]


