Abstract
The canonical role of major histocompatibility complex class I (MHCI) molecules in antigen presentation involves the recognition of a short peptide of intracellular origin, bound to the upper surface of the class I molecule, by CD8+ T lymphocytes. Assembly and loading of the MHCI is a highly regulated, chaperone-mediated process and only when the fully folded MHCI molecule is correctly loaded with peptide is it released from the endoplasmic reticulum for trafficking to the cell surface. Current models of the interactions of MHCI molecules with their cognate receptors visualize them functioning as monomeric entities. However, in recent years, new data have revealed MHCI molecules with the ability to form disulphide-linked dimeric structures, with several distinct dimer entities being elucidated. We describe here three types of MHCI dimers; HLA-B27 dimers formed predominantly through the possession of an unpaired cysteine within the peptide-binding groove; HLA-G dimers, which form through a cysteine on its external surface; and a novel population we term redox-induced dimers, which can form between cysteine residues in the cytoplasmic tail domains. The characteristics of these dimeric MHCI molecules and their role in both normal immune responses and in disease pathogenesis are reviewed in this article.
Keywords: dimers, HLA-B27, HLA-G, MHCI, redox
Introduction to MHCI assembly, structure and function
Major histocompatibility complex class I (MHCI) molecules occupy a unique niche in the biology of the immune system. They allow T lymphocytes to ‘see’ inside other cells, by displaying representative fragments of, essentially, a cell’s entire protein content at the cell surface for screening by T cells. This allows the detection of proteins that would not normally be present, such as virus-derived peptides, and permits the precise removal of such infected cells by targeting with cytotoxic T cells, while leaving neighbouring uninfected cells unscathed. This unique system is an incredibly powerful and sensitive defence against intracellular pathogens.
The normal classical MHCI monomer molecule comprises a highly polymorphic heavy chain non-covalently linked to the β2-microglobulin (β2m) light chain (Fig. 1, first image). Before peptide loading the partially folded structure is stabilized by a multi-component assembly, the peptide loading complex (PLC), which includes the chaperone molecule calreticulin, tapasin and the oxidoreductases ERp57 and protein disulphide isomerase. The components of the PLC act cooperatively in the process of MHCI assembly and any disruption of the PLC leads to a significant reduction in the efficiency of antigen presentation to T cells.1–4 The PLC is tethered to the transporter associated with antigen presentation (TAP) via the class I-specific accessory molecule tapasin, so as to optimize peptide loading. The peptides themselves are derived from cytosolic or nuclear proteins which, when degraded by the proteasome, are transported from the cytosol into the endoplasmic reticulum (ER) by TAP. Once in the ER, trimming to a length of eight or nine amino acids by aminopeptidases occurs, followed by loading into the peptide-binding groove of newly synthesized MHCI molecules. As a result of the stringent nature of MHCI assembly and peptide loading any misfolded molecules are retained in the ER until correct folding or degradation can occur.5
Figure 1.
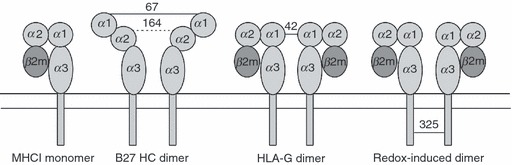
Depiction of MHC monomer and various dimeric forms of MHCI molecules. From left to right; typical monomer MHCI molecule; partially unfolded HLA-B27 dimers, formed at the cell surface by disulphide linkage through residue cysteine 67 in the peptide-binding groove, but also contributed to in the endoplasmic reticulum by cysteine at position 164; HLA-G dimer formed through disulphide linkage at cysteine at position 42; redox-induced dimers formed on cells undergoing apoptosis and on exosome vesicles as the result of altered intravesicle or cytoplasmic redox conditions, formed through cysteines in the cytoplasmic tail domains (typically positions 325 in HLA-B alleles, and 339 in HLA-A alleles).
HLA-B27 dimers
The human HLA-B27 MHCI allele was first identified as being strongly linked to the arthritic condition ankylosing spondylitis, which predominantly affects the spine, in 1973.6,7 Between 90 and 95% of patients with ankylosing spondylitis express this allele; however, the role that HLA-B27 plays in ankylosing spondylitis, along with other recently identified genes such as IL23R and ERAP1,8,9 remains an enigma. In 1999, Allen et al.10 noted that recombinant HLA-B27 molecules folded in vitro often formed disulphide-linked heavy-chain dimers. The ability to form these dimers was controlled by the very uncommon possession of a cysteine at position 67 in the peptide-binding groove. Mutation of this residue abrogated dimer formation (Fig. 1, second image). This observation has spurred on a decade of research into how these structures may contribute to ankylosing spondylitis. HLA-B27 appears to have the ability to form at least two distinct forms of these dimeric MHCI structures, one on the cell surface, and one that remains resident in the ER. The exact relationship between these two dimeric forms remains to be fully elucidated, but each may play a role in the disease pathogenesis of ankylosing spondylitis, as discussed below.
Cell surface HLA-B27 dimers
Partial unfolding of cell surface HLA-B27 has been proposed to generate a molecule in which the normally hidden cysteine at position 67 in the peptide groove becomes more solvent when exposed and permits dimerization with another similarly unfolded HLA-B27 molecule. These molecules have been shown to be present on the cell surface of human lymphoid cell lines, on stimulated lymphoid cells from B27+ transgenic rats and on synovial and peripheral blood mononuclear cells of B27+ patients with ankylosing spondylitis,11–13 indicating a possible role in the aetiology of ankylosing spondylitis.
How the above molecules form is of some interest. Those fully folded MHCI molecules expressed at the cell surface are normally relatively stable but under certain conditions, for instance the low-affinity binding of a sub-optimal peptide, the class I heterotrimer (class I heavy chain, β2m light chain and peptide) can dissociate and heavy-chain dimers can form. This process is primarily thought to be dependent on the endocytic pathway, which is involved in the recycling of molecules from the cell surface rather than the trafficking of misfolded molecules from the ER.14 The acidic environment of the endosome presumably allows for the partial unfolding of the molecule and the aberrant formation of intra-molecular or inter-molecular disulphide bonds.
In addition to peptide recognition by the T-cell receptor of CD8+ cytotoxic T lymphocytes, MHCI molecules are also ligands for natural killer (NK) cells and other leucocyte subsets that express killer cell immunoglobulin-like receptors (KIR); C-type lectin receptors; or leucocyte immunoglobulin-like transcripts/leucocyte immunoglobulin-like receptors (LILR). The KIR molecules are highly polymorphic and therefore specific for different MHCI molecules, as they recognize the α1–α2 domains of MHCI, whereas LILR molecules have a much broader specificity arising from their ability to recognize β2m and the α3 domain of MHCI.15 The LILRB1 and LILRB2 receptors differ in their β2m dependency with LILRB1 requiring the presence of β2m as well as the α3 domain for activation. LILRB1 (also termed CD85j) are expressed on B cells, NK cells, myelomonocytic cells and T cells and recognize all MHCI; whereas LILRB2 (CD85d) are only expressed on cells of the myelomonocytic lineage and recognize most HLA-A and B molecules.
The HLA-B27 monomer molecule is recognized by the inhibitory receptors LILRB1, LILRB2 and KIR3DL1, while the cell surface β2m-dissociated heavy-chain dimer molecules are recognized by LILRB2 and KIR3DL2.11 Both monomer and dimer HLA-B27 molecules also appear to be recognized by the activating receptor LILRA1,16 although recent receptor binding studies show that LILRA1 has a stronger preference for the β2m-free heavy-chain form.17 The consequences of differential recognition and the interplay of inhibitory and activatory signals in response to the various conformations of HLA-B27 is not yet fully understood but it is possible that altering such a fine balance could significantly contribute to disease.
Intracellular HLA-B27 dimers
Within the ER, HLA-B27 has a tendency to misfold. This is primarily because of the specific residues in the B-pocket of the peptide-binding groove, which have been implicated in the affinity of the heavy chain to bind β2m or peptide.18–20 The assembly kinetics are slower for most of the disease-associated forms of this allele (B*2705, B*2704 and B*2702 as compared with B*2706, and B*2709 which are not disease associated) and also as compared with most other MHCI molecules.18,21 This increase in the time taken for the molecule to assemble appears to lead to the accumulation of misfolded molecules in the ER, a proportion of which are in the form of MHCI dimers. These misfolded HLA-B27 molecules appear to result in the cellular response of ER stress induction and initiation of the Unfolded Protein Response,22,23 in an attempt to either rescue or dispose of the burden of misfolded proteins. Animal models also implicate the presence of these HLA-B27 dimers with disease. In the HLA-B27 transgenic rat model, inflammatory disease similar to human spondyloarthropathy occurs in strains expressing high levels of HLA-B27.24 The cysteine at position 67 is involved in intracellular dimer formation, but we have also noted a role for the structurally conserved cysteines at positions 101 and especially164 in the formation of a pool of intracellular dimers.25 As a consequence of the current limitations of reagents specific for HLA-B27 dimers compared with monomers it remains unclear whether cells handle misfolded monomer and dimeric HLA-B27 molecules differently, and therefore whether dimers specifically induce the Unfolded Protein Response; however, we have recently identified components of the degradation pathway that appear to preferentially bind HLA-B27 dimers, suggesting that cells do indeed see these structures as a significant risk to cellular homeostasis (Guiliano DB, Fussell H, Lenart I, Powis SJ and Antoniou AN, unpublished data).
HLA-G homodimers
The non-classical HLA-G MHCI allele exhibits less sequence variability than classical MHCI molecules, which are highly polymorphic in the peptide-binding region, and as such HLA-G presents a more restricted peptide repertoire, with a single peptide accounting for approximately 15% of all recovered peptides in the placenta.26 The basic structure of the molecule is the same as classical MHCI with the heavy chain being non-covalently linked to β2m; however, it has a truncated cytoplasmic tail domain that prolongs retention of the molecule in the ER.27,28 The molecule also displays a much longer half-life at the cell surface because of loss of the cytoplasmic tail domain endocytosis motif, which promotes recycling via the endocytic pathway.29,30 The crystal structure of the peptide-binding groove of HLA-G reveals a peptide that is more deeply buried within the molecule and with many more binding contacts than most MHCI molecules.31 HLA-G is primarily expressed in fetal placental trophoblast cells, which invade the maternal uterine tissues during placentation, although it has also been reported to be expressed on some T cells and in other tissues and tumour cells.32 There are seven isoforms with HLA-G1 being membrane bound and the most predominant while HLA-G5 to HLA-G7 are truncated and soluble in nature.33 The function of these different isoforms is still little understood but it has been proposed that they may be involved in the modulation of immune activity at the maternal–fetal interface.34
HLA-G possesses an unpaired cysteine residue at position 42 on an external loop of the peptide-binding groove and it is through this cysteine that disulphide bond formation occurs, as mutation of this cysteine to a serine abrogates dimer formation (Fig. 1, third image).15,35 Dimerization does not appear to occur until after the molecule has passed through the Golgi apparatus, as the dimeric form of HLA-G is completely endoglycosidase H resistant.36 Because of the positioning of cysteine 42, it is possible for dimer molecules to exist in a fully folded, β2m-associated form, in contrast to the cysteine 67-dependent HLA-B27 dimers, and with no significant conformational changes as compared with the HLA-G monomer molecule.
Fully folded, β2m-associated HLA-G dimers have been implicated in the production of tolerizing signals in pregnancy,37 and also in graft rejection.38 HLA-G monomers are recognized by the inhibitory receptors LILRB1 and LILRB2 and by KIR2DL4.37 The LILR receptors, although they bind to most MHCI molecules, have a greater affinity for HLA-G39 and this affinity is substantially increased with the molecule in dimeric form. As a result of this increase in avidity there is a concordant increase in signalling potential via LILRB1.15,40 So far, direct evidence of downstream NK cell inhibition has only been shown in assays using peripheral blood NK cells or NK cell lines rather than decidual NK cells ex vivo,36 indicating that perhaps other, more sensitive functional assays are required to assess decidual NK cell responses or that cell types other than decidual NK cells are involved in establishing a state of maternal immune tolerance in the tissues surrounding the implanted fetus. Free heavy chains, lacking peptide and β2m, have also been identified at the cell surface but these are not recognized by the receptors known to bind fully folded HLA-G dimers.41
MHCI redox-induced dimers
Recently, a third population of dimer MHCI molecules has been characterized, detected on exosomes.42 Exosomes are secreted microvesicles, 50–150 nm in size, that are involved in cell–cell communication and are formed from the inward budding of the endosomal membrane. A subset of the resulting multivesicular bodies, fuse with the plasma membrane of the cell and release their cargo of vesicles into the extracellular space.43 A wide variety of cell types release exosomes and the discovery of high levels of MHCI dimers on the surface of these vesicles raises the possibility that these structures may act as novel ligands for immune cells. Mutation of the various unpaired cysteine residues along the length of HLA-B27 (at positions 67, 308 and 325) indicate that this dimer population is not dependent on cysteine 67 as seen with the cell surface dimers described by Bowness and colleagues;11 and only marginally by cysteine 308 on the edge of the transmembrane and cytoplasmic domains; but rather, the terminal cysteine at position 325 in the cytoplasmic tail domain region appears to be crucial, as mutation of this residue leads to loss of dimers (Fig. 1, fourth image).42 Hence, the wide range of HLA-B alleles that possess cysteine at 325 may form this type of dimer. Of some significance it was also noted that mixed-allele dimers could form, between HLA-A and HLA-B alleles, because all HLA-A alleles contain a cysteine residue close to the end of the cytoplasmic tail domain. This would further imply that mixed dimers of HLA-B alleles could also form, though we have not yet investigated this.
We have termed these exosomal dimers ‘redox-induced’ because we believe that they form because of the relative absence of the reducing agent glutathione inside exosomes, in comparison to the low millimolar levels normally found in the cell cytoplasm. This observation further led us to consider whether whole cells undergoing other stresses that would affect their redox status might also form such redox MHCI dimers. We have recently reported that such conditions do indeed occur. Cells undergoing apoptosis, by chemical treatments such as hydrogen peroxide and thimerosal, or by triggering of the Fas receptor pathway, also form MHCI dimers controlled by the cysteine residues in their cytoplasmic tail domains.44 This observation may also account for the population of non-HLA-B27 dimers that we recently reported in a study of MHCI dimer formation in monocyte-derived dendritic cells.45
KIR/LILR recognition studies of the fully folded β2m-associated dimers present on the surface of exosomes and apoptotic cells have yet to be carried out although they would be of particular interest because of the nature of exosome release into the extracellular milieu. It is intriguing to draw a parallel between this population of dimers and the fully folded β2m-associated HLA-G dimers implicated in pregnancy and to ask whether there is any differential recognition/binding of these molecules. Our studies have implicated cysteine 325 in dimer formation in the exosome population and whether this conformation would affect recognition by the LILR receptors remains to be determined. Mutation of the cysteine residues in the cytoplasmic tail of HLA-C,29 and of cysteine 308 and 325 in HLA-B7, has been shown to lead to a loss of LILR recognition.27 Hence, the elucidation of the signalling potential of these novel conformations and the downstream interplay of immune responses in health and disease is eagerly awaited.
Conclusions
Far from acting alone, as single entities, there is accumulating evidence that HLA class I molecules can, under certain circumstances, form a range of dimeric structures, some of which have potentially novel and unique immune activities. The current evidence for immune function is strongest for the cell surface HLA-G dimer, where immunomodulation at the maternal–fetal interface is of crucial importance. Somewhat similar to this proposed structure, with close proximity of two fully folded peptide-binding grooves, are the redox-induced dimers we describe in this review. However, the evidence supporting immunostimulatory or immunomodulatory functions for these dimers on either exosomes or apoptotic cells remains to be determined. This leaves the fascinating case of HLA-B27, where cysteine 67-linked cell surface dimers are known to be recognized by various immune receptors, and where similar intracellular dimers are associated with the induction of cellular stress. Determining the contribution of one or both subsets of these HLA-B27 dimers to pathogenesis is likely to reveal further extraordinary features about the roles of MHCI molecules in the regulation of the immune response.
Acknowledgments
ECC is funded by the Chief Scientist Office of the Scottish Government. The authors confirm no financial conflicts of interest.
Disclosures
The authors disclose no conflicting financial interests.
References
- 1.Gao B, Adhikari R, Howarth M, et al. Assembly and antigen-presenting function of MHC class I molecules in cells lacking the ER chaperone calreticulin. Immunity. 2002;16:99–109. doi: 10.1016/s1074-7613(01)00260-6. [DOI] [PubMed] [Google Scholar]
- 2.Garbi N, Tanaka S, Momburg F, Hammerling GJ. Impaired assembly of the major histocompatibility complex class I peptide-loading complex in mice deficient in the oxidoreductase ERp57. Nat Immunol. 2006;7:93–102. doi: 10.1038/ni1288. [DOI] [PubMed] [Google Scholar]
- 3.Grandea AG, IIII, Golovina TN, Hamilton SE, et al. Impaired assembly yet normal trafficking of MHC class I molecules in Tapasin mutant mice [In Process Citation] Immunity. 2000;13:213–22. doi: 10.1016/s1074-7613(00)00021-2. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Baig E, Williams DB. Functions of ERp57 in the folding and assembly of major histocompatibility complex class I molecules. J Biol Chem. 2006;281:14622–31. doi: 10.1074/jbc.M512073200. [DOI] [PubMed] [Google Scholar]
- 5.Otero JH, Lizak B, Hendershot LM. Life and death of a BiP substrate. Semin Cell Dev Biol. 2010;21:472–8. doi: 10.1016/j.semcdb.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brewerton DA, Hart FD, Nicholls A, Caffrey M, James DC, Sturrock RD. Ankylosing spondylitis and HL-A 27. Lancet. 1973;1:904–7. doi: 10.1016/s0140-6736(73)91360-3. [DOI] [PubMed] [Google Scholar]
- 7.Schlosstein L, Terasaki PI, Bluestone R, Pearson CM. High association of an HL-A antigen, W27, with ankylosing spondylitis. N Engl J Med. 1973;288:704–6. doi: 10.1056/NEJM197304052881403. [DOI] [PubMed] [Google Scholar]
- 8.Burton PR, Clayton DG, Cardon LR, et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet. 2007;39:1329–37. doi: 10.1038/ng.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans DM, Spencer CC, Pointon JJ, et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat Genet. 2011;43:761–7. doi: 10.1038/ng.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen RL, O’Callaghan CA, McMichael AJ, Bowness P. Cutting edge: HLA-B27 can form a novel β2-microglobulin-free heavy chain homodimer structure. J Immunol. 1999;162:5045–8. [PubMed] [Google Scholar]
- 11.Kollnberger S, Bird L, Sun MY, Retiere C, Braud VM, McMichael A, Bowness P. Cell-surface expression and immune receptor recognition of HLA-B27 homodimers. Arthritis Rheum. 2002;46:2972–82. doi: 10.1002/art.10605. [DOI] [PubMed] [Google Scholar]
- 12.Kollnberger S, Bird LA, Roddis M, et al. HLA-B27 heavy chain homodimers are expressed in HLA-B27 transgenic rodent models of spondyloarthritis and are ligands for paired Ig-like receptors. J Immunol. 2004;173:1699–710. doi: 10.4049/jimmunol.173.3.1699. [DOI] [PubMed] [Google Scholar]
- 13.Tsai WC, Chen CJ, Yen JH, Ou TT, Tsai JJ, Liu CS, Liu HW. Free HLA class I heavy chain-carrying monocytes – a potential role in the pathogenesis of spondyloarthropathies. J Rheumatol. 2002;29:966–72. [PubMed] [Google Scholar]
- 14.Bird LA, Peh CA, Kollnberger S, Elliott T, McMichael AJ, Bowness P. Lymphoblastoid cells express HLA-B27 homodimers both intracellularly and at the cell surface following endosomal recycling. Eur J Immunol. 2003;33:748–59. doi: 10.1002/eji.200323678. [DOI] [PubMed] [Google Scholar]
- 15.Shiroishi M, Kuroki K, Rasubala L, et al. Structural basis for recognition of the nonclassical MHC molecule HLA-G by the leukocyte Ig-like receptor B2 (LILRB2/LIR2/ILT4/CD85d) Proc Natl Acad Sci U S A. 2006;103:16412–7. doi: 10.1073/pnas.0605228103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen RL, Raine T, Haude A, Trowsdale J, Wilson MJ. Leukocyte receptor complex-encoded immunomodulatory receptors show differing specificity for alternative HLA-B27 structures. J Immunol. 2001;167:5543–7. doi: 10.4049/jimmunol.167.10.5543. [DOI] [PubMed] [Google Scholar]
- 17.Jones DC, Kosmoliaptsis V, Apps R, et al. HLA class I allelic sequence and conformation regulate leukocyte Ig-like receptor binding. J Immunol. 2011;186:2990–7. doi: 10.4049/jimmunol.1003078. [DOI] [PubMed] [Google Scholar]
- 18.Antoniou AN, Ford S, Taurog JD, Butcher GW, Powis SJ. Formation of HLA-B27 homodimers and their relationship to assembly kinetics. J Biol Chem. 2004;279:8895–902. doi: 10.1074/jbc.M311757200. [DOI] [PubMed] [Google Scholar]
- 19.Dangoria NS, DeLay ML, Kingsbury DJ, Mear JP, Uchanska-Ziegler B, Ziegler A, Colbert RA. HLA-B27 misfolding is associated with aberrant intermolecular disulfide bond formation (dimerization) in the endoplasmic reticulum. J Biol Chem. 2002;277:23459–68. doi: 10.1074/jbc.M110336200. [DOI] [PubMed] [Google Scholar]
- 20.Mear JP, Schreiber KL, Munz C, Zhu X, Stevanovic S, Rammensee HG, Rowland-Jones SL, Colbert RA. Misfolding of HLA-B27 as a result of its B pocket suggests a novel mechanism for its role in susceptibility to spondyloarthropathies. J Immunol. 1999;163:6665–70. [PubMed] [Google Scholar]
- 21.Galocha B, de Castro JA. Folding of HLA-B27 subtypes is determined by the global effect of polymorphic residues and shows incomplete correspondence to ankylosing spondylitis. Arthritis Rheum. 2008;58:401–12. doi: 10.1002/art.23164. [DOI] [PubMed] [Google Scholar]
- 22.Colbert RA, DeLay ML, Layh-Schmitt G, Sowders DP. HLA-B27 misfolding and spondyloarthropathies. Adv Exp Med Biol. 2009;649:217–34. doi: 10.1007/978-1-4419-0298-6_16. [DOI] [PubMed] [Google Scholar]
- 23.Turner MJ, Sowders DP, DeLay ML, et al. HLA-B27 misfolding in transgenic rats is associated with activation of the unfolded protein response. J Immunol. 2005;175:2438–48. doi: 10.4049/jimmunol.175.4.2438. [DOI] [PubMed] [Google Scholar]
- 24.Hammer RE, Maika SD, Richardson JA, Tang JP, Taurog JD. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human β2m: an animal model of HLA-B27-associated human disorders. Cell. 1990;63:1099–112. doi: 10.1016/0092-8674(90)90512-d. [DOI] [PubMed] [Google Scholar]
- 25.Lenart I, Guiliano DB, Burn G, Campbell EC, Morley KD, Fussell H, Powis SJ, Antoniou AN. The MHC Class I heavy chain structurally conserved cysteines 101 and 164 participate in HLA-B27 dimer formation. Antioxid Redox Signal. 2012;16:33–43. doi: 10.1089/ars.2010.3693. [DOI] [PubMed] [Google Scholar]
- 26.Ishitani A, Sageshima N, Lee N, Dorofeeva N, Hatake K, Marquardt H, Geraghty DE. Protein expression and peptide binding suggest unique and interacting functional roles for HLA-E, F, and G in maternal–placental immune recognition. J Immunol. 2003;171:1376–84. doi: 10.4049/jimmunol.171.3.1376. [DOI] [PubMed] [Google Scholar]
- 27.Gruda R, Achdout H, Stern-Ginossar N, et al. Intracellular cysteine residues in the tail of MHC class I proteins are crucial for extracellular recognition by leukocyte Ig-like receptor 1. J Immunol. 2007;179:3655–61. doi: 10.4049/jimmunol.179.6.3655. [DOI] [PubMed] [Google Scholar]
- 28.Park B, Lee S, Kim E, Chang S, Jin M, Ahn K. The truncated cytoplasmic tail of HLA-G serves a quality-control function in post-ER compartments. Immunity. 2001;15:213–24. doi: 10.1016/s1074-7613(01)00179-0. [DOI] [PubMed] [Google Scholar]
- 29.Davis DM, Mandelboim O, Luque I, Baba E, Boyson J, Strominger JL. The transmembrane sequence of human histocompatibility leukocyte antigen (HLA)-C as a determinant in inhibition of a subset of natural killer cells. J Exp Med. 1999;189:1265–74. doi: 10.1084/jem.189.8.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis DM, Reyburn HT, Pazmany L, Chiu I, Mandelboim O, Strominger JL. Impaired spontaneous endocytosis of HLA-G. Eur J Immunol. 1997;27:2714–9. doi: 10.1002/eji.1830271035. [DOI] [PubMed] [Google Scholar]
- 31.Clements CS, Kjer-Nielsen L, Kostenko L, et al. Crystal structure of HLA-G: a nonclassical MHC class I molecule expressed at the fetal–maternal interface. Proc Natl Acad Sci U S A. 2005;102:3360–5. doi: 10.1073/pnas.0409676102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Apps R, Gardner L, Moffett A. A critical look at HLA-G. Trends Immunol. 2008;29:313–21. doi: 10.1016/j.it.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 33.LeMaoult J, Le Discorde M, Rouas-Freiss N, Moreau P, Menier C, McCluskey J, Carosella ED. Biology and functions of human leukocyte antigen-G in health and sickness. Tissue Antigens. 2003;62:273–84. doi: 10.1034/j.1399-0039.2003.00143.x. [DOI] [PubMed] [Google Scholar]
- 34.Hunt JS, Morales PJ, Pace JL, Fazleabas AT, Langat DK. A commentary on gestational programming and functions of HLA-G in pregnancy. Placenta. 2007;28(Suppl A):S57–63. doi: 10.1016/j.placenta.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyson JE, Erskine R, Whitman MC, et al. Disulfide bond-mediated dimerization of HLA-G on the cell surface. Proc Natl Acad Sci U S A. 2002;99:16180–5. doi: 10.1073/pnas.212643199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Apps R, Sharkey A, Gardner L, et al. Ex vivo functional responses to HLA-G differ between blood and decidual NK cells. Mol Hum Reprod. 2011;17:577–86. doi: 10.1093/molehr/gar022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apps R, Gardner L, Sharkey AM, Holmes N, Moffett A. A homodimeric complex of HLA-G on normal trophoblast cells modulates antigen-presenting cells via LILRB1. Eur J Immunol. 2007;37:1924–37. doi: 10.1002/eji.200737089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Favier B, HoWangYin KY, Wu J, Caumartin J, Daouya M, Horuzsko A, Carosella ED, LeMaoult J. Tolerogenic function of dimeric forms of HLA-G recombinant proteins: a comparative study in vivo. PLoS ONE. 2011;6:e21011. doi: 10.1371/journal.pone.0021011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shiroishi M, Tsumoto K, Amano K, et al. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci U S A. 2003;100:8856–61. doi: 10.1073/pnas.1431057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonen-Gross T, Achdout H, Gazit R, et al. Complexes of HLA-G protein on the cell surface are important for leukocyte Ig-like receptor-1 function. J Immunol. 2003;171:1343–51. doi: 10.4049/jimmunol.171.3.1343. [DOI] [PubMed] [Google Scholar]
- 41.Gonen-Gross T, Achdout H, Arnon TI, et al. The CD85J/leukocyte inhibitory receptor-1 distinguishes between conformed and β2-microglobulin-free HLA-G molecules. J Immunol. 2005;175:4866–74. doi: 10.4049/jimmunol.175.8.4866. [DOI] [PubMed] [Google Scholar]
- 42.Lynch S, Santos SG, Campbell EC, Nimmo AM, Botting C, Prescott A, Antoniou AN, Powis SJ. Novel MHC class I structures on exosomes. J Immunol. 2009;183:1884–91. doi: 10.4049/jimmunol.0900798. [DOI] [PubMed] [Google Scholar]
- 43.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 44.Makhadiyeva D, Lam L, Moatari M, Vallance J, Zheng Y, Campbell EC, Powis SJ. MHC class I dimer formation by alteration of the cellular redox environment and induction of apoptosis. Immunology. 2012;135:133–9. doi: 10.1111/j.1365-2567.2011.03518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campbell EC, Fettke F, Bhat S, Morley KD, Powis SJ. Expression of MHC class I dimers and ERAP1 in an ankylosing spondylitis patient cohort. Immunology. 2011;133:379–85. doi: 10.1111/j.1365-2567.2011.03453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


