Abstract
Macrophages are the major source of the chemokines macrophage inflammatory protein-2 (MIP-2) and keratinocyte-derived chemokine (KC), which play a major role in neutrophil migration to sites of inflammation. Although extracellular ATP from inflammatory tissues induces several immune responses in macrophages, it is unclear whether ATP-stimulated macrophages affect neutrophil migration. Therefore, the aim of the present study was to investigate the role of ATP-induced MIP-2 production by macrophages. When ATP was injected intraperitoneally into mice, the number of neutrophils within the peritoneal cavity markedly increased, along with the levels of MIP-2 and KC in the peritoneal lavage fluid. Consistent with this, ATP induced MIP-2 production, but not that of KC, by peritoneal exudate macrophages (PEMs) in vitro. This occurred via interactions with the P2X7 receptor and P2Y2 receptor. Furthermore, treatment of PEMs with ATP led to the production of reactive oxygen species. The ATP-induced MIP-2 production was inhibited by treatment with the antioxidant N-acetyl-l-cysteine. Also, MIP-2 production was inhibited by pre-incubating PEMs with inhibitors of extracellular signal-regulated kinase 1/2 or p38 mitogen-activated protein kinase. The MIP-2 neutralization reduced the increase in neutrophil numbers observed in ATP-treated mice. Taken together, these results suggest that increased production of reactive oxygen species by ATP-stimulated macrophages activates the signalling pathways that promote MIP-2 production which, in turn, induces neutrophil migration.
Keywords: ATP, macrophages, macrophage inflammatory protein-2, neutrophils, P2 receptors
Introduction
Extracellular ATP, along with other extracellular nucleotides such as UTP, ADP and NAD, induce physiological and immune responses in a wide spectrum of cell types and tissues.1–3 Extracellular nucleotides bind to two major cell surface receptors: the ligand-gated ion channel P2X (P2X1–7) receptors and the G protein-coupled P2Y (P2Y1,2,4,6,11–14) receptors.1,2,4,5 P2X receptors are activated by ATP, whereas P2Y receptors are selective for ADP (P2Y1, P2Y12 and P2Y13), ATP and UTP (P2Y2 and rodent P2Y4), UTP (human P2Y4), UDP (P2Y6) and UDP-glucose (P2Y14).5 In general, intracellular ATP concentrations in the cytoplasm are maintained within the millimolar range (3–10 mm), but within the nanomolar range in plasma (400–700 nm).1 Release of ATP is caused by cytolysis resulting from, for example, tissue injury and cell death.1,6
The P2X7 receptor (P2X7R) is primarily expressed on macrophages, lymphocytes and other immune cells.1,7,8 P2X7R plays an important role in the regulation of both innate and adaptive immunity. Treatment with ATP induces different cellular responses, such as pore formation within the plasma membrane, induction of apoptosis and/or necrosis, cytokine production, up-regulation of MHC class II expression and the killing of intracellular mycobacteria.9–17 Extracellular ATP also stimulates the phosphorylation of cell signalling molecules, including extracellular signal-regulated kinase (ERK) 1/2 and p38 mitogen-activated protein kinase (MAPK).12,18,19 In addition, ATP-mediated caspase-1 activation induces the release of mature interleukin-1β (IL-1β) and IL-18 by macrophages primed with lipopolysaccharide (LPS) before ATP stimulation.9,11
Chemokines play key roles in leucocyte migration during an immune response.20 In humans, CXC ligand (CXCL) 8/IL-8 plays a major role in neutrophil migration.21–23 In mice, CXCL1/keratinocyte-derived chemokine (KC) and CXCL2/macrophage inflammatory protein-2 (MIP-2), both murine functional homologues of IL-8, recruit neutrophils to sites of injury and infection.24–29 Also, anti-KC neutralizing monoclonal antibody (mAb) and/or anti-MIP-2 neutralizing mAb suppress neutrophil migration and inflammation when injected in vivo into murine models of inflammation.27,30,31 Interestingly, extracellular ATP has an important role in inflammatory neutrophil migration in both humans and mice, chemotaxis via P2Y2R, and neutrophil function.32–35 P2Y2R is involved in the migration of neutrophils into the lungs in murine models of sepsis.36 However, the mechanism by which extracellular ATP induces neutrophil migration has not been clarified.
Recent studies show that reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), are the physiological mediators of cell signalling pathways involved in differentiation, proliferation, migration and cytokine secretion.37,38 In addition, stimulation of primary macrophages with ATP alone results in the production of high levels of ROS.12,18 The results of the present study show that ATP-mediated ROS generation by macrophages induces MIP-2 production, but not KC, thereby leading to neutrophil migration.
Materials and methods
Mice
C57BL/6 (B6) mice and B6.129P2-P2rx7tm1Gab/J mice (P2X7R-deficient: P2X7R−/−, derived from Pfizer Inc., New York, NY) (8–10 weeks old) were used for the experiments. Both strains of mouse were obtained from Jackson Laboratory, Inc. (Bar Harbor, ME) and maintained in the animal facility of Niigata University under specific pathogen-free conditions. All animal experiments were approved by the Committee on Animal Research of Niigata University.
Reagents, ATP-induced neutrophil migration within the peritoneal cavity and MIP-2 neutralization
ATP, 2′-O-(4-benzoyl-benzoyl) ATP and 3′-O-(4-benzoyl-benzoyl) ATP (BzATP), UTP, LPS, N-acetyl-l-cysteine (NAC) and SB202190 were purchased from Sigma (St Louis, MO). PD98059 was purchased from Calbiochem (La Jolla, CA) and PBS was purchased from Wako Pure Industries (Osaka, Japan). ATP was injected at several different doses (in 300 μl PBS) to induce neutrophil migration in the peritoneal cavity. PBS was injected as a control. The MIP-2 was functionally inhibited by intraperitoneal injection of 50 μg anti-MIP-2 (40605) mAb (R&D Systems Inc., Minneapolis, MN),27,30 or control rat IgG2b (BD Biosciences, San Diego, CA) 15 min before the injection of ATP.
Preparation of peritoneal exudate macrophages and peritoneal lavage fluid
Peritoneal exudate cells (PECs) were obtained from the mice by an intraperitoneal injection of 0·5 ml of 3·0% thioglycollate medium (Eiken Chemical Co., Ltd, Tokyo, Japan). Four days after injection, PECs were collected by lavage with cold PBS and seeded in RPMI-1640 medium (Nissui Pharmaceutical Co. Ltd., Tokyo, Japan) supplemented with 10% fetal calf serum (FCS), 2 mm l-glutamine and 5 × 10−5 m 2-mercaptoethanol at a density of 1 × 106 cells/ml in 48-well plates. After 2 hr of adherence at 37° in 5% CO2, PECs were washed twice with warm PBS to remove non-adherent cells. Adherent cells were regarded as peritoneal exudate macrophages (PEMs), and used in the experiments after incubation in complete medium. The peritoneal cavity was washed with 2 ml cold PBS to collect peritoneal lavage fluid. Samples contaminated with blood were not used in the experiment.
PEM culture
The PEMs were primed in complete RPMI-1640 medium containing 10% FCS in the presence of 1 μg/ml LPS for 4 hr, and pre-treated for 60 min with PD98059, for 30 min with SB202190, or for 60 min with NAC before ATP stimulation. Finally, the cells were stimulated with ATP, BzATP, UTP, LPS or control medium for 24 hr. The levels of cytokines and chemokines in the culture supernatant were measured by ELISA.
Measurement of cytokine and chemokine levels
Cytokine and chemokine levels were measured by ELISA according to the manufacturer’s instructions [IL-1β and tumour necrosis factor-α (TNF-α); BD Biosciences, and KC and MIP-2; R&D Systems].
Flow cytometric analysis
The PECs were pre-incubated with anti-mouse CD16/CD32 (2.4G2) mAb (BD Biosciences) to block Fcγ receptors, and then incubated with various mAbs for 30 min at 4° as previously described.3,15 Directly labelled anti-mouse anti-Mac-1 (M1/70) and anti-Gr-1 (RB6-8C5) mAbs (BD Biosciences) were used for phenotypic analysis. The PECs were analysed using a FACSCalibur (BD Biosciences) flow cytometer. Dead cells were removed using propidium iodide+ gating.
Cell sorting and Cytospin preparation
The Mac-1+ Gr-1high populations were isolated from peripheral blood leucocytes or PECs by sorting on a FACSAria II (BD Biosciences). Cytospin preparations were made in a cytocentrifuge (Thermo Fisher Scientific Inc., Runcorn, UK) at 80 g for 3 min. Smears were stained with May–Giemsa.
Measurement of intracellular ROS levels
The oxidation-sensitive fluorescent probe, 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA, 5 μm) (Molecular Probes, Inc., Eugene, OR), was used to detect intracellular ROS as described previously.39,40 Briefly, PEMs were grown on coverslips in complete RPMI-1640 medium containing 10% FCS at a density of 1 × 106 cells/ml in six-well plates and stimulated with ATP to investigate the time–course of ROS production. The PEMs were washed twice with PBS and CM-H2DCFDA in PBS was added and incubated for 10 min at 37°. After a gentle rinse with PBS, the cells were fixed with 4% paraformaldehyde for 10 min, and then stained with Hoechst for 30 min. The cells were subsequently transferred to glass slides and mounted in Fluoromount/Plus (Diagonostic Bio Systems, Pleasanton, CA). Fluorescence was detected using a confocal laser microscope (Biozero BZ-8100; Keyence, Osaka, Japan). More than 400 cells (or for 3 mm ATP-stimulated PEMs: > 200 cells) in six random fields were analysed, and the data are presented as the mean fluorescence intensity. Fluorescence intensity was quantified using the fluorescence analysis software (BZ-H1C; Keyence).
Measurement of ERK1/2 and p38 MAPK phosphorylation levels
The PECs were incubated with 300 μm ATP for different lengths of time in complete RPMI-1640 medium containing 10% FCS. Cells were fixed by addition of pre-warmed PhosFlow Lyse/Fix buffer (BD Biosciences) for 10 min at 37°, and then cells were washed twice with cold PBS and centrifugation at 600 g for 8 min. After washing, the cells were permeabilized in Perm Buffer III (BD Biosciences) for 30 min on ice. The cells were then washed in stain buffer (1 × PBS, 2% newborn calf serum, 0·09% sodium azide) and resuspended in staining buffer at a concentration of 1 × 106 cells/ml. To each tube were added the following antibodies: anti-mouse CD16/CD32, anti-mouse-Mac-1, isotype control (mouse IgG1), anti-phospho-ERK1/2 (pT202/pY204, clone: 20A), and anti-phospho-p38MAPK mAb (pT180/pY182, clone: 36/p38) (BD Biosciences). Cells were incubated at room temperature for 60 min in the dark, washed twice in stain buffer, and analysed using a FACSCalibur. The fluorescence intensity of ERK1/2 and p38 MAPK phosphorylation levels was represented as the Gmean of each sample.
Statistical analysis
All data were expressed as the mean ± SEM. The Student’s unpaired t-test was used to compare the means between two experimental groups. A P-value < 0·05 was considered significant.
Results
Extracellular ATP increases the number of neutrophils and macrophages in the peritoneal cavity of ATP-treated mice
We first examined whether the population of leucocytes within the peritoneal cavity changed after the injection of ATP. To address this, mice were injected intraperitoneally with ATP or PBS. The leucocyte subsets within the PEC populations were determined using two-colour flow cytometry. We used commercial PBS, which had passed the bacterial endotoxins test, mycoplasma test and sterility test. Figure 1(a) shows that the peritoneal granulocyte population (Mac-1+ Gr-1high cells) within the peritoneal cavity of mice injected with 100 μg ATP markedly increased (from 16·7% to 55·4%) compared with that in PBS-injected mice. Similarly, the peritoneal macrophage population (Mac-1+ Gr-1low cells) increased from 0·3% to 13·7%. Consistent with this, ATP-injected mice showed a significant increase in the absolute number of granulocytes and macrophages within the peritoneal cavity compared with PBS-injected mice (Fig. 1b). To further characterize the granulocyte subsets, the morphology of the Mac-1+ Gr-1high cells was observed. Figure 1(c) shows that Mac-1+ Gr-1high cells from the peritoneal cavity of ATP-injected mice were neutrophils, as were those in the blood. These results show that the numbers of neutrophils and macrophages increased simultaneously in the peritoneal cavity of ATP-injected mice.
Figure 1.
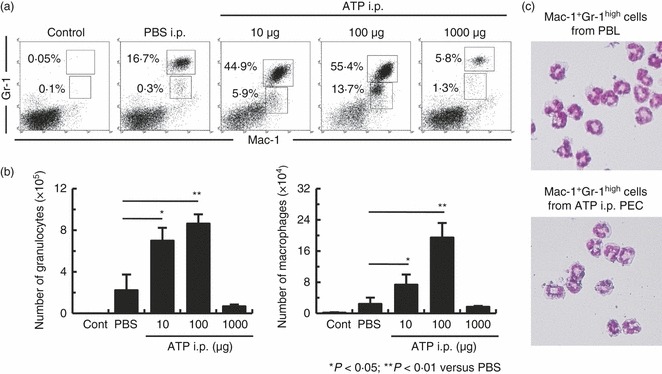
Extracellular ATP induces neutrophil migration in the peritoneal cavity of ATP-injected mice. B6 mice were injected intraperitoneally with ATP or PBS. Peritoneal exudate cells (PECs) were harvested 4 hr later and assayed by flow cytometry. Cells were stained with Gr-1 and Mac-1 to determine granulocyte and macrophage populations (a) and the absolute number of granulocytes and macrophages (b) in control mice and mice injected with ATP or PBS. (c) Granulocytes were isolated from ATP-injected mice and stained with May–Giemsa (objective lens × 60). The absolute numbers of granulocytes and macrophages were calculated from three repeated experiments. Data represent the mean ± SEM.
Injection of ATP into mice activates macrophages
Both KC and MIP-2 play an important role in neutrophil migration, and these chemokines are mainly produced by macrophages.24–29 The increase in number of neutrophils and macrophages in the peritoneal cavity of ATP-injected mice raises the question of whether macrophages are involved in neutrophil migration. To address this, the KC and MIP-2 concentrations in the peritoneal lavage fluid of ATP-injected mice were measured by ELISA. We also measured the levels of TNF-α and IL-1β in the peritoneal lavage fluid in these mice because these cytokines are mainly produced by macrophages.41 In addition, LPS-activated macrophages stimulated with ATP produce IL-1β.9,11,14Figure 2 shows that the IL-1β levels in peritoneal lavage fluid were similar in ATP-injected and PBS-injected mice. In contrast, ATP-injected mice showed significantly higher levels of TNF-α, KC and MIP-2 in peritoneal lavage fluid than mice injected with PBS.
Figure 2.
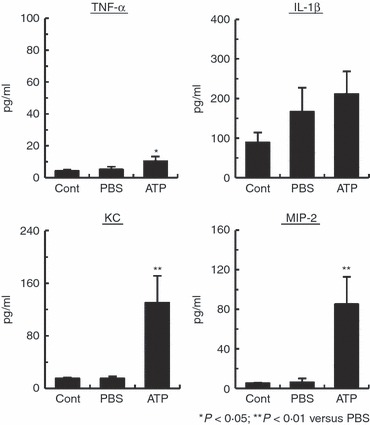
Induction of tumour necrosis factor-α (TNF-α), keratinocyte-derived chemokine (KC) and macrophage inflammatory protein-2 (MIP-2) production in peritoneal lavage fluid by ATP. B6 mice (n = 3 per group) were injected intraperitoneally with ATP. After 4 hr, the levels of interleukin-1β (IL-1β), TNF-α, KC and MIP-2 in the peritoneal lavage fluid were determined by ELISA. Data represent the mean ± SEM. Experiments were repeated three times.
To examine whether macrophages were responsible for this increase in TNF-α, KC and MIP-2 levels, PEMs were stimulated with ATP for 24 hr and the concentration of IL-1β, TNF-α, KC and MIP-2 in the culture supernatants was measured by ELISA. Figure 3 shows that ATP-stimulated PEMs produced significant levels of MIP-2 compared with non-stimulated PEMs. In contrast, IL-1β, TNF-α and KC secretion showed insignificant increases in ATP-stimulated PEMs. In addition, our preliminary experiments showed that the MIP-2 levels in the culture supernatant increased 4 hr after ATP stimulation, rising to maximal levels by 16–24 hr. These results show that the injection of ATP into mice stimulates PEMs and induces MIP-2 production.
Figure 3.
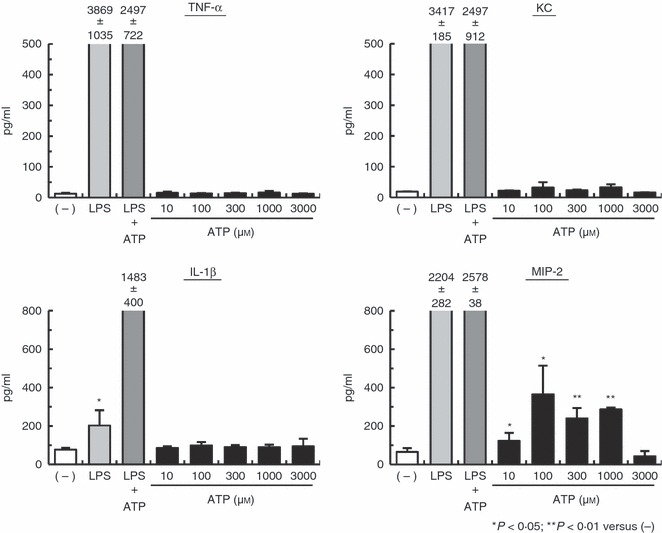
ATP stimulation of macrophages in vitro leads to macrophage inflammatory protein-2 (MIP-2) production. Peritoneal exudate macrophages (PEMs) were primed with 1 μg/ml lipopolysaccharide (LPS) for 4 hr and then stimulated with or without ATP for 24 hr. Culture supernatants were harvested and assayed for interleukin-1β (IL-1β), tumour necrosis factor-α (TNF-α), keratinocyte-derived chemokine (KC) and MIP-2 by ELISA. Data represent the mean ± SEM of triplicate samples. Experiments were repeated three times.
Extracellular ATP induces ROS production in PEMs
Stimulation of macrophages with ATP and a P2X7R agonist (BzATP) leads to ROS production.12,18 Therefore, we next examined whether ROS were detectable in ATP-stimulated PEMs. To determine this, PEMs were stimulated with ATP and, after treatment, were incubated with the ROS-sensitive dye CM-H2DCFDA. The production of ROS in PEMs was then quantified by confocal image analysis. Figure 4(a) shows that ATP-induced ROS production was confirmed by viewing ATP-stimulated PEMs using fluorescence microscopy. Furthermore, the ROS levels in the PEMs were transient. Figure 4(b) shows that ROS production was detectable within 5 min of ATP stimulation, rose to maximal levels within 15 min, and returned to basal levels by 30 min. In addition, MIP-2 production from ATP-stimulated PEMs was significantly inhibited by pre-treatment with the antioxidant NAC (Fig. 4c). Unexpectedly, pre-treatment of non-stimulated PEMs with NAC significantly inhibited MIP-2 production compared with non-stimulated PEMs (Fig. 4c). These results show that ATP-stimulated PEMs induce ROS production and, subsequently, MIP-2 production.
Figure 4.
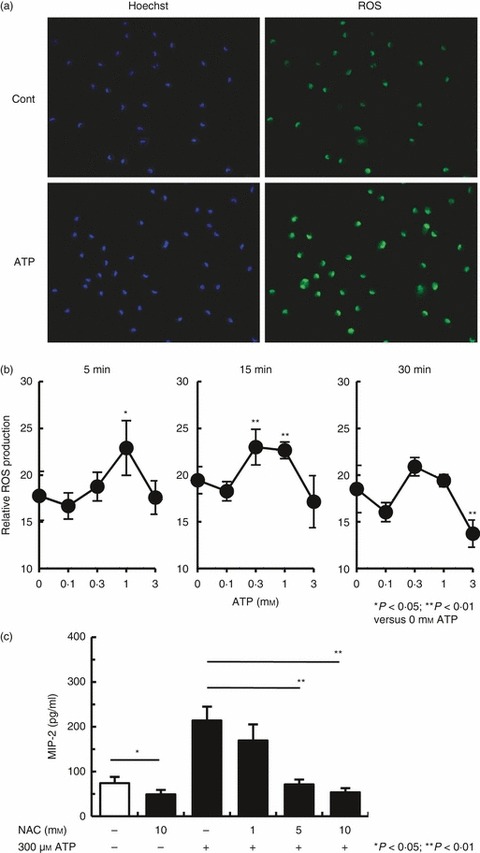
ATP induces reactive oxygen species (ROS) production in macrophages in vitro. (a) Peritoneal exudate macrophages (PEMs) were stimulated with 300 μm ATP for 15 min at 37° and then treated with 5 μm 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) for 10 min at 37°. The PEMs were visualized immediately with a fluorescence microscope (objective lens × 20). (b) PEMs were stimulated with several concentrations of ATP for 5, 15 and 30 min and ROS production was measured using the fluorescent probe, CM-H2DCFDA. Data represent the mean ± SEM of > 400 cells (or for 3 mm ATP-stimulated PEMs: > 200 cells). One result from two representative experiments is shown. (c) PEMs were pre-incubated for 60 min with N-acetyl-l-cysteine (NAC) before the addition of 300 μm ATP. Stimulation in the presence of NAC continued for 24 hr. Data represent the mean ± SEM of triplicate samples. The experiments were repeated three times.
MIP-2 production by ATP-stimulated PEMs depends on P2X7R and P2Y2R
The ATP-activated P2X7R mediate IL-1β and IL-18 production by LPS-primed macrophages.9,11,14 Therefore, to examine whether ATP stimulation induces MIP-2 production via P2X7R in macrophages, PEMs were incubated with ATP or the P2X7R agonist, BzATP.14,18 Furthermore, P2X7R−/− mouse-derived PEMs were incubated with ATP. Figure 5(a) shows that BzATP-stimulated PEMs expressed significant levels of MIP-2 compared with the background controls. However, unexpectedly, ATP-stimulated P2X7R−/− PEMs expressed significant levels of MIP-2 compared with non-stimulated PEMs (Fig. 5b). ATP and UTP activate both mouse P2Y2R and P2Y4R,5 and peritoneal macrophages express P2X1,4,7R and P2Y1,2,6R but not P2Y4R.42 Therefore, we examined whether ATP stimulation induces MIP-2 production via P2Y2R on macrophages. Figure 5(c) shows that PEMs stimulated with both ATP and UTP produced MIP-2. Next we investigated whether P2X7R was required for neutrophil migration to the peritoneal cavity of ATP-injected mice. Figure 5(d) shows that the proportion of peritoneal neutrophils in P2X7R−/− mice decreased compared with that in B6 mice (25·7% and 45·2%, respectively). In agreement, ATP-injected P2X7R−/− mice showed a significant decrease in absolute neutrophil numbers within the peritoneal cavity compared with ATP-injected B6 mice (Fig. 5e). These results show that stimulation of PEMs with ATP induces MIP-2 production, pointing to a mechanism involving PEM activation via stimulation of P2X7R and P2Y2R by ATP-derived ligands.
Figure 5.
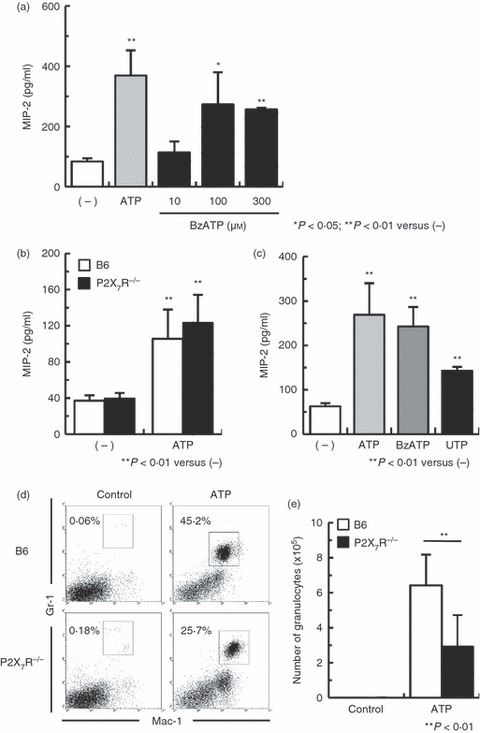
Induction of macrophage inflammatory protein-2 (MIP-2) in peritoneal exudate macrophages (PEMs) incubated with ATP requires functional P2 receptors. (a) The PEMs were stimulated with 300 μm ATP or different concentrations of 2′-O-(4-benzoyl-benzoyl) ATP and 3′-O-(4-benzoyl-benzoyl) ATP (BzATP; a P2X7R agonist) for 24 hr. (b) B6 and P2X7R−/− PEMs were stimulated with 300 μm ATP for 24 hr. (c) PEMs were stimulated with 300 μm ATP, BzATP or UTP for 24 hr. Data represent the mean ± SEM of triplicate samples. The experiments were repeated three times. (d) B6 mice and P2X7R−/− mice were injected intraperitoneally with 100 μg ATP. Peritoneal exudate cells were harvested 4 hr later and assayed by flow cytometry. The granulocyte population was gated on Mac-1+ Gr-1high cells. One result from five representative experiments is shown. (e) The absolute numbers of granulocytes were calculated from five repeated experiments. Data represent the mean ± SEM.
Extracellular ATP leads to MIP-2 production through ERK1/2 and p38 MAPK pathways
The finding that PEMs stimulated with ATP produce MIP-2 via P2X7R and P2Y2R pathways suggests that ATP-activated cell signalling is related to MIP-2 production in macrophages. Recent studies show that extracellular ATP activates ERK1/2 and p38 MAPK.12,18,19 To examine this, we stimulated PEMs with ATP, and measured ERK1/2 and p38 MAPK phosphorylation by flow cytometry. Figure 6(a) shows that ATP-stimulated PEMs increased ERK1/2 and p38 MAPK phosphorylation compared with non-stimulated PEMs as measured by fluorescence intensity. In addition, non-stimulated PEMs showed an increase in the fluorescence intensity of the MAPK phosphorylation compared with the isotype controls (Fig. 6a). Next we investigated whether ERK1/2 and p38 MAPK phosphorylation was required for MIP-2 production from ATP-stimulated PEMs. The PEMs were incubated with the ERK1/2 inhibitor, PD98059, or the p38 MAPK inhibitor, SB202190, before the addition of ATP. Figure 6(b) shows that MIP-2 production was inhibited by pre-incubation with ERK1/2 or p38 MAPK inhibitors. Therefore, ATP-induced MIP-2 production by PEMs mediates activation of the ERK1/2 and p38 MAPK signalling pathways.
Figure 6.
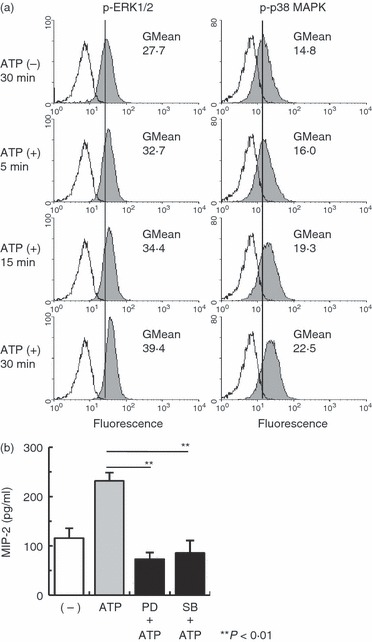
Induction of macrophage inflammatory protein-2 (MIP-2) in peritoneal exudate macrophages (PEMs) incubated with ATP requires activation of the extracellular signal-regulated kinase 1/2 (ERK1/2) and p38 mitogen-activated protein kinase (MAPK) signalling pathways. (a) Peritoneal exudate cells (PECs) were incubated with 300 μm ATP at different times. ERK1/2 and p38 MAPK phosphorylation were measured by flow cytometry. ATP-stimulated PECs were stained with anti-phospho-ERK1/2 (pT202/pY204) or p38 MAPK monoclonal antibody (pT180/pY182) (shaded histograms). Unstimulated PECs, which were incubated for 30 min, were stained with isotype control (white histograms). ERK1/2 and p38 MAPK phosphorylation levels were gated on the macrophage population (Mac-1+ cells). One result from three representative experiments is shown. (b) PEMs were pre-incubated for 60 min with an ERK1/2 inhibitor (PD98059) or for 30 min with a p38 MAPK inhibitor (SB202190) before the addition of 300 μm ATP. Stimulation in the presence of these inhibitors continued for 24 hr. Data represent the mean ± SEM of triplicate samples. The experiments were repeated three times.
Extracellular ATP-mediated MIP-2 increases the number of neutrophils in mice
The finding that ATP-stimulated PEMs produce MIP-2 suggests that ATP-stimulated MIP-2 secretion by PEMs plays an important role in neutrophil migration in the peritoneal cavity of ATP-injected mice. To further examine this, mice were treated with an anti-MIP2 neutralizing mAb before ATP injection. Figure 7(a) shows that the proportion of peritoneal neutrophils in anti-MIP-2 mAb/ATP-administered mice decreased compared with that in rat IgG/ATP-treated mice (from 60·3% to 41·6%, respectively). In agreement with this, mice treated with anti-MIP-2 mAb/ATP showed significantly lower absolute neutrophil numbers in the peritoneal cavity compared with mice treated with rat IgG/ATP (Fig. 7b). These results show that the enhanced neutrophil migration in ATP-treated mice is suppressed by anti-MIP-2 neutralizing mAbs.
Figure 7.
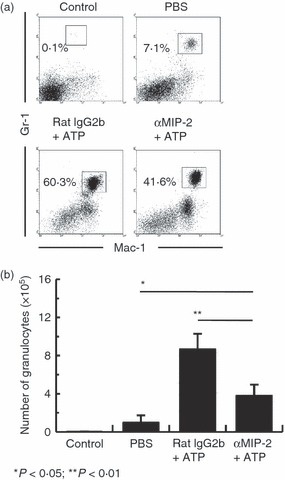
Extracellular ATP-mediated macrophage inflammatory protein-2 (MIP-2) increases neutrophil migration in the peritoneal cavity. B6 mice (n = 3 per group) were injected intraperitoneally with 50 μg anti-MIP-2 monoclonal antibody (mAb) or control rat IgG2b, and injected intraperitoneally with 100 μg ATP 15 min later. Peritoneal exudate cells was harvested 4 hr later and assayed by flow cytometry. Cells were stained with Gr-1 and Mac-1 to determine granulocyte population (a) and the absolute number of granulocytes (b) in control mice and mice injected with PBS, rat IgG2b/ATP or anti-MIP-2 mAb/ATP. The absolute numbers of granulocytes were calculated from three repeated experiments. Data represent the mean ± SEM.
Discussion
In this study, we first showed that the number of neutrophils and macrophages in the PEC population increases in ATP-injected mice. Four hours after treatment with low concentrations of ATP, the absolute number of both these cell types increased, whereas high concentrations of ATP did not increase the absolute number of cells (Fig. 1). The activation of P2X7R with high concentrations (millimolar range) of extracellular ATP can induce cell death in macrophages.1,6,12 Chemokines (e.g. MIP-2, and KC) produced by macrophages play key roles in neutrophil migration to sites of injury and infection.24 Interestingly, nucleotides such as ATP can act as chemotactic signals (i.e. ‘find me’ signal) released by apoptotic cells to promote the P2Y2R-dependent recruitment of macrophages, and provide efficient apoptotic cell clearance in vivo.43 Hence, the majority of neutrophil migration may be controlled by low-dose ATP-induced macrophage migration, but not high-dose ATP.
We also showed that macrophages in ATP-injected mice were functionally enhanced. Administration of ATP increased the levels of TNF-α, KC and MIP-2 in the peritoneal lavage fluid (Fig. 2); this suggests macrophage activation because TNF-α, KC and MIP-2 are known to be produced by macrophages.30,41 Consistent with this (Fig. 3), in vitro stimulation of PEMs with ATP induced production of MIP-2, but not TNF-α or KC. In addition, P2X7R and P2Y2R had an important role in this MIP-2 production pathway (Fig. 5a–c). Interestingly, a similar mechanism was recently reported suggesting that high levels of extracellular ATP (1 mm) induced MIP-2 (CXCL2) production from microglia through P2X7R.44 However, little is known about the involvement of P2Y2R in MIP-2 production, because primary microglia expresses P2X4,7R and P2Y6,12R but not P2Y2R.45 Hence, taken together, these results provide evidence for a mechanism involving MIP-2 production, in which ATP activates macrophages by signalling through P2X7R and P2Y2R.
The generation of ROS in response to ATP activates cell signalling mediators, such as ERK1/2 and p38 MAPK, in macrophages.12,18,19 Recent reports show that H2O2 activates the ERK1/2 and p38 MAPK pathways in macrophages, which then induce MIP-2 production.40,46 Furthermore, ATP induced activation of the ERK1/2, p38 MAPK and JNK pathway in microglia, and ATP-induced mRNA expression of MIP-2 was inhibited by these signalling inhibitors.44 These reports support the possibility that ATP-mediated ROS production triggers ERK1/2 and p38 MAPK activation, which induces MIP-2 production. In the present study, we demonstrated that ATP-stimulated PEMs induce ROS production and that ATP-induced MIP-2 production is inhibited by NAC (Fig. 4). Moreover, ATP-induced MIP-2 production was inhibited by ERK1/2 and p38 MAPK inhibitors (Fig. 6). Therefore, our data extend the results of previous studies by showing that activation of the ERK1/2 and p38 MAPK pathways by ROS, via ATP ligands, is required for ATP-induced MIP-2 production by PEMs. In addition, H2O2 is capable of stimulating neutrophil chemotaxis.47 Hence, ATP-induced ROS may be involved in the direct neutrophil migration activity of H2O2.
P2X7R is involved in mediating high concentration (millimolar range) ATP-induced cell death within the first hours of cell contact.12,48 High concentrations of ATP (1 mm) induced MIP-2 release in microglia, which peaked at 6 hr following ATP treatment and persisted for at least 24 hr.44 Our results showed that 1 mm ATP-stimulated PEMs secreted significantly higher levels of MIP-2 in the culture supernatants than non-stimulated PEMs after 24 hr of culture (Fig. 3). Simultaneously, the cell death of PEMs induced by 1 and 3 mm ATP was observed by microscopy (data not shown). These results suggest that high concentrations of ATP (1 mm) induce MIP-2 production from PEMs within the first hours before cell death. We also demonstrated that stimulation of PEMs with BzATP (P2X7R agonist) and UTP (P2Y2R agonist) induce the production of MIP-2 (Fig. 5a,c). However, these data do not exclude the possibility that BzATP-induced MIP-2 production could also be the result of contaminating ATP having effects on P2Y2R. In addition, BzATP can induce cell death in macrophages and dendritic cells.12,48–50 Hence, these results suggest that BzATP-induced MIP-2 production from PEMs may be mediated via P2Y2R by ATP that is released by BzATP-induced cell death.
Because P2X7R ligands, such as ATP, alone do not induce IL-1β production by macrophages, macrophages must first be stimulated with LPS before being stimulated with ATP.9,11 In contrast, our data show that stimulation of PEM with ATP alone induced MIP-2 production (Fig. 3). A previous study showed that microglia stimulated with high concentrations of ATP (1 mm) induced the release of MIP-2.44 Therefore, macrophages stimulated with ATP alone can induce MIP-2 production. We also demonstrated that non-stimulated PEMs show ROS-mediated MIP-2 production (Fig. 4c) and ERK1/2 and p38 MAPK phosphorylation (Fig. 6a), suggesting that PEMs may be activated macrophages. Hence, high concentrations of ATP might not be necessary for MIP-2 production by activated macrophages induced with thioglycollate medium.
Neutrophils are the first immune cells to migrate to sites of tissue injury or infection.51,52 Murine MIP-2 is one of the CXC chemokine family members thought to be functionally analogous to human IL-8 and to rat neutrophil chemoattractants.41 Recently, Nakamura et al.27 demonstrated that pre-treatment with an anti-MIP-2 neutralizing mAb reduced both hepatic neutrophil accumulation and liver injury in a murine model of concanavalin A-induced hepatitis. However, Chen et al.33 provided strong evidence that ATP, acting via P2Y2R, is directly involved in the control of neutrophil chemotaxis and chemokinesis. In the present study, the increase in the number of peritoneal neutrophils in ATP-injected mice was observed concurrently with an increase in peritoneal lavage fluid MIP-2 levels (Figs 1 and 2). Moreover, the absolute number of peritoneal neutrophils decreased in anti-MIP-2 neutralizing mAb-primed mice injected with ATP (Fig. 7). Our data extend the results of previous studies by showing that ATP-mediated MIP-2 production plays a key role in neutrophil migration. Furthermore, considering that this neutrophil migration was not completely inhibited by an anti-MIP-2 neutralizing mAb, other mediators (e.g. KC) and/or neutrophil chemotaxis via P2Y2R may also be involved. Further studies are required to elucidate the mechanism of neutrophil migration mediated by KC and P2Y2R in ATP-injected mice.
Based on these results, we suggest one mechanism by which neutrophils migrate to inflammatory tissues. This mechanism involves a two-step model for extracellular ATP-induced neutrophil migration. The first step involves the leakage of intracellular ATP into the extracellular environment through cytolysis resulting from inflammation (e.g. cell damage by hypoxia and ischaemia, bacterial infection, tissue injury, or cell death).6,53,54 ATP is also released by bacteria.9 ATP release triggers the second step. Our results show that binding of extracellular ATP activates P2X7R and P2Y2R on macrophages and induces MIP-2 production which, subsequently, mediates neutrophil migration. Therefore, ATP-induced neutrophil migration may effectively exclude the pathogen, but may aggravate the inflammatory response.
In summary, the present study suggests that stimulation of P2X7R and P2Y2R by extracellular ATP induces MIP-2 production by macrophages, which induces neutrophil migration. This process requires ROS-mediated activation of the ERK1/2 and p38 MAPK pathways. Taken together, these findings indicate that ATP-induced neutrophil migration (via P2X7R and P2Y2R) may be involved in the progression of inflammatory diseases.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research of Japan and Grant for Promotion of Niigata University Research Projects. The authors thank Dr Yoshitaka Maeda and Ms Kanako Oda for their skilful assistance with the transplantation of fertilized eggs.
Abbreviations
- BzATP
2′-O-(4-benzoyl-benzoyl) ATP and 3′-O-(4-benzoyl-benzoyl) ATP
- CM-H2DCFDA
5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester
- CXCL
CXC ligand
- ERK
extracellular signal-regulated kinase
- FCS
fetal calf serum
- H2O2
hydrogen peroxide
- IL
interleukin
- KC
keratinocyte-derived chemokine
- LPS
lipopolysaccharide
- mAb
monoclonal antibody
- MAPK
mitogen-activated protein kinase
- MIP-2
macrophage inflammatory protein-2
- NAC
N-acetyl-l-cysteine
- P2X7R
P2X7 receptor
- PECs
peritoneal exudate cells
- PEMs
peritoneal exudate macrophages
- ROS
reactive oxygen species
- TNF
tumour necrosis factor
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Di Virgilio F, Chiozzi P, Ferrari D, et al. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- 3.Kawamura H, Aswad F, Minagawa M, et al. P2X7 receptor-dependent and -independent T cell death is induced by nicotinamide adenine dinucleotide. J Immunol. 2005;174:1971–9. doi: 10.4049/jimmunol.174.4.1971. [DOI] [PubMed] [Google Scholar]
- 4.North RA, Barnard EA. Nucleotide receptors. Curr Opin Neurobiol. 1997;7:346–57. doi: 10.1016/s0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- 5.Abbracchio MP, Burnstock G, Boeynaems JM, et al. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Virgilio F. The P2Z purinoceptor: an intriguing role in immunity, inflammation and cell death. Immunol Today. 1995;16:524–8. doi: 10.1016/0167-5699(95)80045-X. [DOI] [PubMed] [Google Scholar]
- 7.Hogquist KA, Nett MA, Unanue ER, Chaplin DD. Interleukin 1 is processed and released during apoptosis. Proc Natl Acad Sci U S A. 1991;88:8485–9. doi: 10.1073/pnas.88.19.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanovello P, Bronte V, Rosato A, Pizzo P, Di Virgilio F. Responses of mouse lymphocytes to extracellular ATP. II. Extracellular ATP causes cell type-dependent lysis and DNA fragmentation. J Immunol. 1990;145:1545–50. [PubMed] [Google Scholar]
- 9.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–83. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 10.North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–80. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- 11.Mehta VB, Hart J, Wewers MD. ATP-stimulated release of interleukin (IL)-1β and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J Biol Chem. 2001;276:3820–6. doi: 10.1074/jbc.M006814200. [DOI] [PubMed] [Google Scholar]
- 12.Noguchi T, Ishii K, Fukutomi H, Naguro I, Matsuzawa A, Takeda K, Ichijo H. Requirement of reactive oxygen species-dependent activation of ASK1-p38 MAPK pathway for extracellular ATP-induced apoptosis in macrophage. J Biol Chem. 2008;283:7657–65. doi: 10.1074/jbc.M708402200. [DOI] [PubMed] [Google Scholar]
- 13.Aswad F, Kawamura H, Dennert G. High sensitivity of CD4+ CD25+ regulatory T cells to extracellular metabolites nicotinamide adenine dinucleotide and ATP: a role for P2X7 receptors. J Immunol. 2005;175:3075–83. doi: 10.4049/jimmunol.175.5.3075. [DOI] [PubMed] [Google Scholar]
- 14.Labasi JM, Petrushova N, Donovan C, et al. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–45. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- 15.Kawamura H, Aswad F, Minagawa M, Govindarajan S, Dennert G. P2X7 receptors regulate NKT cells in autoimmune hepatitis. J Immunol. 2006;176:2152–60. doi: 10.4049/jimmunol.176.4.2152. [DOI] [PubMed] [Google Scholar]
- 16.Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol. 2007;179:1913–25. doi: 10.4049/jimmunol.179.3.1913. [DOI] [PubMed] [Google Scholar]
- 17.Lammas DA, Stober C, Harvey CJ, Kendrick N, Panchalingam S, Kumararatne DS. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity. 1997;7:433–44. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- 18.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282:2871–9. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aga M, Johnson CJ, Hart AP, Guadarrama AG, Suresh M, Svaren J, Bertics PJ, Darien BJ. Modulation of monocyte signaling and pore formation in response to agonists of the nucleotide receptor P2X7. J Leukoc Biol. 2002;72:222–32. [PubMed] [Google Scholar]
- 20.Zlotnik A, Morales J, Hedrick JA. Recent advances in chemokines and chemokine receptors. Crit Rev Immunol. 1999;19:1–47. [PubMed] [Google Scholar]
- 21.Goodman RB, Strieter RM, Frevert CW, Cummings CJ, Tekamp-Olson P, Kunkel SL, Walz A, Martin TR. Quantitative comparison of C-X-C chemokines produced by endotoxin-stimulated human alveolar macrophages. Am J Physiol. 1998;275:L87–95. doi: 10.1152/ajplung.1998.275.1.L87. [DOI] [PubMed] [Google Scholar]
- 22.Wagner JG, Roth RA. Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol Rev. 2000;52:349–74. [PubMed] [Google Scholar]
- 23.Kraan MC, Patel DD, Haringman JJ, Smith MD, Weedon H, Ahern MJ, Breedveld FC, Tak PP. The development of clinical signs of rheumatoid synovial inflammation is associated with increased synthesis of the chemokine CXCL8 (interleukin-8) Arthritis Res. 2001;3:65–71. doi: 10.1186/ar141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tekamp-Olson P, Gallegos C, Bauer D, Mcclain J, Sherry B, Fabre M, Van deventer S, Cerami A. Cloning and characterization of cDNAs for murine macrophage inflammatory protein 2 and its human homologues. J Exp Med. 1990;172:911–9. doi: 10.1084/jem.172.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bozic CR, Kolakowski LF, Jr, Gerard NP, et al. Expression and biologic characterization of the murine chemokine KC. J Immunol. 1995;154:6048–57. [PubMed] [Google Scholar]
- 26.Kopydlowski KM, Salkowski CA, Cody MJ, van Rooijen N, Major J, Hamilton TA, Vogel SN. Regulation of macrophage chemokine expression by lipopolysaccharide in vitro and in vivo. J Immunol. 1999;163:1537–44. [PubMed] [Google Scholar]
- 27.Nakamura K, Okada M, Yoneda M, Takamoto S, Nakade Y, Tamori K, Aso K, Makino I. Macrophage inflammatory protein-2 induced by TNF-α plays a pivotal role in concanavalin A-induced liver injury in mice. J Hepatol. 2001;35:217–24. doi: 10.1016/s0168-8278(01)00109-x. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Klintman D, Liu Q, Sato T, Jeppsson B, Thorlacius H. Critical role of CXC chemokines in endotoxemic liver injury in mice. J Leukoc Biol. 2004;75:443–52. doi: 10.1189/jlb.0603297. [DOI] [PubMed] [Google Scholar]
- 29.Kukulski F, Ben Yebdri F, Bahrami F, Lévesque SA, Martín-Satué M, Sévigny J. The P2 receptor antagonist PPADS abrogates LPS-induced neutrophil migration in the murine air pouch via inhibition of MIP-2 and KC production. Mol Immunol. 2010;47:833–9. doi: 10.1016/j.molimm.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Filippo K, Henderson RB, Laschinger M, Hogg N. Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J Immunol. 2008;180:4308–15. doi: 10.4049/jimmunol.180.6.4308. [DOI] [PubMed] [Google Scholar]
- 31.Horvat JC, Starkey MR, Kim RY, Beagley KW, Preston JA, Gibson PG, Foster PS, Hansbro PM. Chlamydial respiratory infection during allergen sensitization drives neutrophilic allergic airways disease. J Immunol. 2010;184:4159–69. doi: 10.4049/jimmunol.0902287. [DOI] [PubMed] [Google Scholar]
- 32.Junger WG. Purinergic regulation of neutrophil chemotaxis. Cell Mol Life Sci. 2008;65:2528–40. doi: 10.1007/s00018-008-8095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Corriden R, Inoue Y, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–5. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 34.Kukulski F, Ben Yebdri F, Lecka J, Kauffenstein G, Lévesque SA, Martín-Satué M, Sévigny J. Extracellular ATP and P2 receptors are required for IL-8 to induce neutrophil migration. Cytokine. 2009;46:166–70. doi: 10.1016/j.cyto.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Shukla A, Namiki S, Insel PA, Junger WG. A putative osmoreceptor system that controls neutrophil function through the release of ATP, its conversion to adenosine, and activation of A2 adenosine and P2 receptors. J Leukoc Biol. 2004;76:245–53. doi: 10.1189/jlb.0204066. [DOI] [PubMed] [Google Scholar]
- 36.Inoue Y, Chen Y, Hirsh MI, Yip L, Junger WG. A3 and P2Y2 receptors control the recruitment of neutrophils to the lungs in a mouse model of sepsis. Shock. 2008;30:173–7. doi: 10.1097/shk.0b013e318160dad4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhee SG. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–3. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 38.Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med. 2002;166:S4–8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 39.Kawai Y, Nakao T, Kunimura N, Kohda Y, Gemba M. Relationship of intracellular calcium and oxygen radicals to Cisplatin-related renal cell injury. J Pharmacol Sci. 2006;100:65–72. doi: 10.1254/jphs.fp0050661. [DOI] [PubMed] [Google Scholar]
- 40.Kim D, Kim YJ, Seo JN, et al. 2,4-Dinitrofluorobenzene modifies cellular proteins and induces macrophage inflammatory protein-2 gene expression via reactive oxygen species production in RAW 264.7 cells. Immunol Invest. 2009;38:132–52. doi: 10.1080/08820130802667499. [DOI] [PubMed] [Google Scholar]
- 41.Nathan CF. Secretory products of macrophages. J Clin Invest. 1987;79:319–26. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.del Rey A, Renigunta V, Dalpke AH, et al. Knock-out mice reveal the contributions of P2Y and P2X receptors to nucleotide-induced Ca2+ signaling in macrophages. J Biol Chem. 2006;281:35147–55. doi: 10.1074/jbc.M607713200. [DOI] [PubMed] [Google Scholar]
- 43.Elliott MR, Chekeni FB, Trampont PC, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–6. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiratori M, Tozaki-Saitoh H, Yoshitake M, Tsuda M, Inoue K. P2X7 receptor activation induces CXCL2 production in microglia through NFAT and PKC/MAPK pathways. J Neurochem. 2010;114:810–9. doi: 10.1111/j.1471-4159.2010.06809.x. [DOI] [PubMed] [Google Scholar]
- 45.Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, et al. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446:1091–5. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaramillo M, Olivier M. Hydrogen peroxide induces murine macrophage chemokine gene transcription via extracellular signal-regulated kinase- and cyclic adenosine 5′-monophosphate (cAMP)-dependent pathways: involvement of NF-kappa B, activator protein 1, and cAMP response element binding protein. J Immunol. 2002;169:7026–38. doi: 10.4049/jimmunol.169.12.7026. [DOI] [PubMed] [Google Scholar]
- 47.Klyubin IV, Kirpichnikova KM, Gamaley IA. Hydrogen peroxide-induced chemotaxis of mouse peritoneal neutrophils. Eur J Cell Biol. 1996;70:347–51. [PubMed] [Google Scholar]
- 48.Coutinho-Silva R, Perfettini JL, Persechini PM, Dautry-Varsat A, Ojcius DM. Modulation of P2Z/P2X7 receptor activity in macrophages infected with Chlamydia psittaci. Am J Physiol Cell Physiol. 2001;280:C81–9. doi: 10.1152/ajpcell.2001.280.1.C81. [DOI] [PubMed] [Google Scholar]
- 49.Kawano A, Tsukimoto M, Noguchi T, Hotta N, Harada H, Takenouchi T, Kitani H, Kojima S. Involvement of P2X4 receptor in P2X7 receptor-dependent cell death of mouse macrophages. Biochem Biophys Res Commun. 2012;419:374–80. doi: 10.1016/j.bbrc.2012.01.156. [DOI] [PubMed] [Google Scholar]
- 50.Nihei OK, de Carvalho AC, Savino W, Alves LA. Pharmacologic properties of P2Z/P2X7 receptor characterized in murine dendritic cells: role on the induction of apoptosis. Blood. 2000;96:996–1005. [PubMed] [Google Scholar]
- 51.Ben-Baruch A, Michiel DF, Oppenheim JJ. Signals and receptors involved in recruitment of inflammatory cells. J Biol Chem. 1995;270:11703–6. doi: 10.1074/jbc.270.20.11703. [DOI] [PubMed] [Google Scholar]
- 52.Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003;24:25–9. doi: 10.1016/s1471-4906(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 53.Mo FM, Ballard HJ. The effect of systemic hypoxia on interstitial and blood adenosine, AMP, ADP and ATP in dog skeletal muscle. J Physiol. 2001;536:593–603. doi: 10.1111/j.1469-7793.2001.0593c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vassort G. Adenosine 5-triphosphate: a P2-purinergic agonist in the myocardium. Physiol Rev. 2001;81:767–806. doi: 10.1152/physrev.2001.81.2.767. [DOI] [PubMed] [Google Scholar]


