Abstract
The newly proposed criteria for posttraumatic stress disorder (PTSD) in the Diagnostic and Statistical Manual (DSM-V) include dysregulation of a variety of emotional states including fear, anger, guilt, and shame, in addition to dissociation and numbing. Consistent with these revisions, we postulate two models of emotion dysregulation in PTSD in which fear is not the prevailing emotion but is only one of several components implicated in a dysregulated emotional system that also mediates problems regulating anger, guilt, shame, dissociation, and numbing.
We discuss whether there is a relationship between fear and other emotion regulation systems that may help further our understanding of PTSD and its underlying neurocircuitry. Two pathways describing the relationship between fear and other emotion regulation systems in PTSD are proposed. The first pathway describes emotion dysregulation as an outcome of fear conditioning through stress sensitization and kindling. The second pathway views emotion dysregulation as a distal vulnerability factor and hypothesizes a further exacerbation of fear and other emotion regulatory problems, including the development of PTSD after exposure to one or several traumatic event(s) later in life. Future research and treatment implications are discussed.
Keywords: Anterior cingulate cortex, medial prefrontal cortex, amygdale, DSM-V, emotion, attachment, HPA-axis, infant, development
Since the conceptualization of posttraumatic stress disorder (PTSD) in the Diagnostic and Statistical Manual (DSM)-III, several models of this disorder have been entertained. Originally a stress model was felt to be most applicable to the disorder. However, over time, such a model appeared less pertinent since in a classic stress model removal of the stressor removes the stress response (Selye, 1956), and hypothalamic pituitary adrenal (HPA)-axis alterations repeatedly failed to show increased cortisol levels in PTSD (Bremner et al., 2003a; Glover & Poland, 2002; Heim, Ehlert, Hanker & Hellhammer, 1998; Yehuda et al., 1990, 1995; Yehuda, Boisoneau, Mason & Giller, 1993; Yehuda, Teicher, Trestman, Levengood & Siever, 1996; but also see Baker et al., 1999; Lemieux & Coe, 1995; Maes et al., 1998; Pitman & Orr, 1990; Rasmusson et al., 2001). Therefore, fear conditioning (Charney, Deutch, Krystal, Southwick & Davis, 1993; Pitman, 1989) and other forms of learning, including learned helplessness (van der Kolk, Greenberg, Boyd & Krystal, 1985) were suggested as viable models for PTSD. Currently, the dominant paradigm in PTSD informing research and treatment is the fear conditioning model (reviewed in Shin & Handwerger, 2009; Shin & Liberzon, 2010).
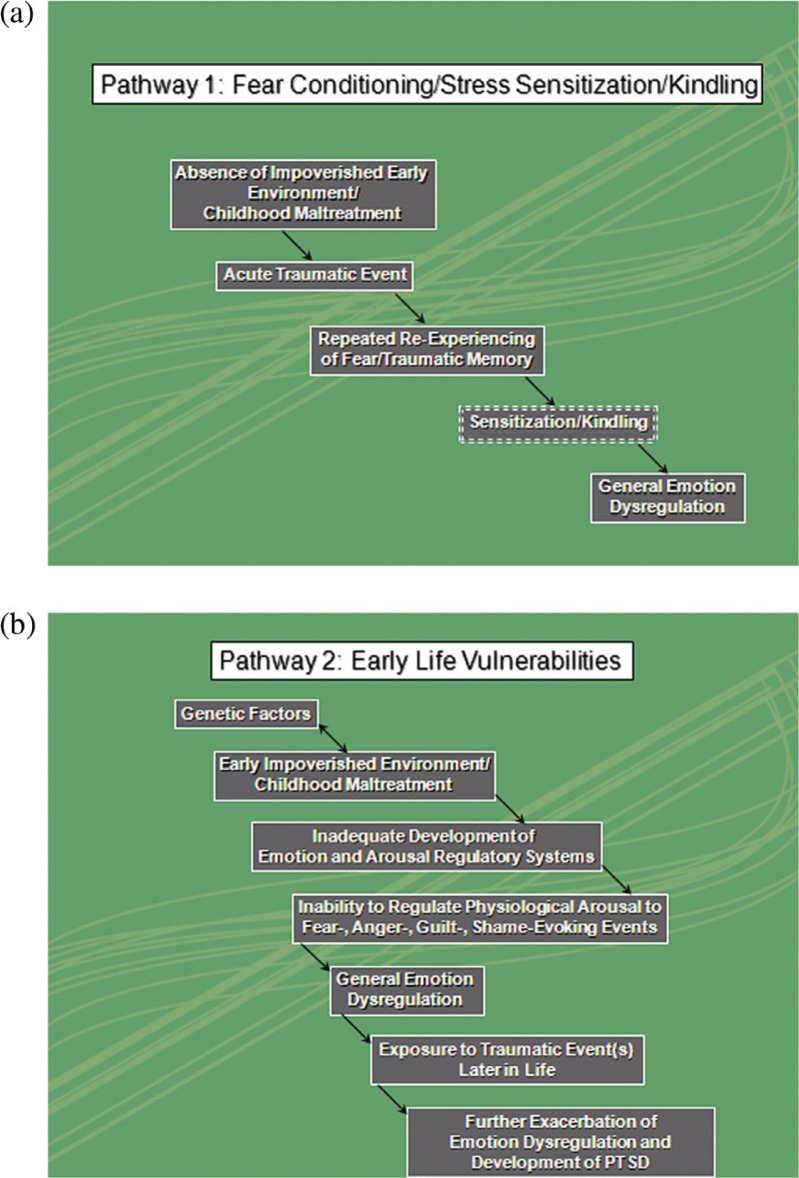
The newly proposed criteria for PTSD in DSM5, however, have moved beyond the conceptualization of PTSD as predominantly a fear response and include dysregulation of a variety of emotional states, including fear, anger, guilt, and shame in addition to dissociation and numbing (see also Resick & Miller, 2009). The term “emotion dysregulation” will be used in this paper to collectively refer to disturbances in a variety of emotional responses. A model that describes the relationship between fear and the other emotional symptoms in PTSD is presently lacking. In this review, we postulate a model of PTSD in which fear is not the prevailing emotion but is only one of several components that are implicated in a dysregulated emotional system. Two pathways to emotion dysregulation in PTSD describing the relationship between fear and other emotion regulation systems in PTSD are proposed (see Fig. 1). The first pathway describes emotion dysregulation as an outcome of fear conditioning through stress sensitization and kindling. The second pathway views emotion dysregulation as a distal vulnerability factor and hypothesizes a further exacerbation of fear and other emotion regulatory problems, including the development of PTSD after exposure to one or several traumatic event(s) later in life. Investigations in PTSD to date have focused predominantly on cross sectional studies which are not able to directly address the causal relationships just described. However, the pathways described above pave a road map for subsequent longitudinal studies that examine this crucial causal relationship in order to elucidate the neuronal underpinnings of PTSD in a prospective manner.
Fig. 1.
(a) Pathway 1: fear conditioning/stress sensitization. Pathway 1 describes emotion dysregulation as on outcome of fear conditioning through stress sensitization and kindling. (b) Pathway 2: early life vulnerabilities. Pathway 2 views emotion dysregulation as a distal vulnerability factor and hypothesizes a further exacerbation of fear and other emotion regulatory problems after exposure to (a) traumatic event(s) later in life.
Pathway 1 (Fear conditioning/stress sensitization/kindling)
Stress sensitization
The main support for the process of fear leading to general emotion dysregulation comes from the research investigating the biological mechanisms underlying sensitization. There is support for the notion that fear reactions experienced during the acute aftermath of a traumatic event followed by repeated re-experiencing of the traumatic memory can lead to a process of sensitization to subtle reminders of traumatic and related memories. Sensitization of the fear and stress response has been hypothesized to involve complex interactions between the individual's distress, psychophysiological reactivity and related neurotransmitter and neurohormonal responses (Charney et al., 1993; Friedman, 1994; Post, Weiss, Smith, Li & McCann, 1997; Yehuda, 2006; Yehuda & Antelman, 1993). Sensitization can result in a progressive augmentation and kindling of the reactivity of an individual and lead to an associated emergence of general emotion dysregulation, including anger, grief, numbing, and dissociation as well as a generalization of the fear response. Kindling is a term used to describe the emergence of generalized seizures in response to repeated, subthreshold electrophysiological stimulation, usually directed at the amygdala. In PTSD, similarly to the kindling of seizures, the progressive augmentation, and expansion of symptoms occurs over time and may be related to the neural circuitry associated with the emotional memory response becoming increasingly strengthened and expanding into neighboring neural circuits (McFarlane, 2010; McFarlane, Yehuda & Clark, 2002; Post et al., 1997).
A number of neurotransmitters, neurohormones, and neuropeptides and their effects on a distinct network of brain regions, including the ventromedial prefrontal cortex, amygdala, and hippocampus have been proposed to underlie this process of sensitization (Heim & Nemeroff, 2009). In terms of neurochemical responses, a reduced cortisol response posttrauma has been postulated to facilitate the release of corticotrophin-releasing factor (CRF) and norepinephrine (NE), thereby leading to prolonged stress responses and sensitization of the stress-regulatory system (Yehuda, 2006). In addition, several of the neurochemical systems, including the NE and cortisol systems, have a direct influence on learning and extinction, thus directly affecting conditioned fear responses as well as the consolidation and retrieval of traumatic memories. The findings of increased NE activity and hypocortisolism may therefore result in enhanced encoding of traumatic memories with associated amplification of PTSD symptoms (de Quervain, 2008; de Quervain & Margraf, 2008).
Fear conditioning and failure of extinction of conditioned fear
Some authors have suggested that the progressive augmentation of symptoms and increased fear reactivity are related to a failure of the extinction of conditioned fear and may therefore be a mechanism underlying stress sensitization (Milad et al., 2007, 2008, 2009; Wessa & Flor, 2007). This mechanism may also underlie the generalization of fear responses to reminders of the traumatic event over the course of the disorder. Following the important work by LeDoux (1997), Davis (1992), and Quirk (1998) on fear conditioning in animals, the neural circuitry underlying fear conditioning has become a powerful model to examine the neural underpinnings of PTSD. Studies showed that the medial prefrontal cortex (MPFC)/anterior cingulate cortex (ACC) could modulate the amygdala response to fearful stimuli, including fearful faces and traumatic memory recall, and that decreased responsiveness of the MPFC/ACC led to a disinhibition of the amygdala (reviewed in Shin & Handwerger, 2009; Shin & Liberzon, 2010). Rauch, Shin and Phelps (2006) therefore suggested that an increased amygdala response with a concomitant decrease in ventromedial prefrontal cortex response plays a central role in the process of sensitization through excessive acquisition of fear associations, thereby leading to progressive amplification of PTSD symptoms and associated arousal (Rauch et al., 2006). This model is further supported by studies reporting an association between extinction recall and decreased activation of the ventromedial prefrontal cortex and the hippocampus (Milad et al., 2007, 2009). The hippocampus is implicated in the mediation of stress responses, declarative memory, and contextual aspects of fear conditioning and therefore provides a further mechanism underlying stress sensitization leading to augmentation of PTSD and fear symptoms over time. In summary, significant advances have been made in delineating the fear circuitry in PTSD, and it is now well established that the fear networks are central to the etiology and maintenance of the disorder (Jovanovic & Ressler, 2010; Shin & Liberzon, 2010).
Nevertheless, it is important to note that even though increased amygdala response has been observed in PTSD (e.g., Liberzon et al. 1999; Shin et al., 1997, 1999, 2004, 2005; reviewed in Etkin & Wager, 2007), others have not demonstrated this effect (e.g., Bremner et al., 1999a, 1999b; Lanius et al. 2001, 2002, 2003). In addition, decreased amygdala response to thermal pain stimuli in individuals with PTSD has been described and linked to symptoms of analgesia (Geuze et al., 2007; Kraus et al., 2009). These and other clinical studies have therefore suggested PTSD to be a disorder involving both emotional under-modulation (lack of control over or disinhibition of emotional responding) such as occurs during re-experiencing/hyperarousal reactivity, and states of emotional over-modulation (overcontrol of emotional states) in an attempt to restrict unwanted emotional experiences, as occurs during states of dissociation, numbing, and analgesia (Briere & Spinazzola 2005; Browne & Finkelhor, 1986; Ehlers, Mayou, & Bryant, 1998; Ford, Courtois, Steele, Hart & Nijenhuis, 2005; Frewen & Lanius, 2006; Lanius et al., in press; Litz, Orsillo, Kaloupek & Weathers, 2000; McCauley et al., 1997; Orth & Wieland 2006; Rizvi, Kaysen, Gutner, Griffin & Resick, 2008; Stovall-McClough & Cloitre, 2006; van der Hart, Nijenhuis & Steele, 2005; van der Kolk, Roth, Pelcovitz, Sunday & Spinazzola, 2005; Zlotnick et al.,1996, 2003). Emotional undermodulation has been proposed to be mediated by failure of prefrontal inhibition of limbic regions, whereas emotional overmodulation may be mediated by prefrontal inhibition of the same limbic regions (Lanius et al., in press). It is intriguing that these different responses somewhat recapitulate the neuroendocrine findings in PTSD that have reported low cortisol and high norepinephrine (NE), but at other times have not found significant differences or even showed opposite results (Geracioti et al., 2001; Hawk, Dougall, Ursano & Baum, 2000; Liberzon et al., 1999; Marshall et al., 2002; O'Donnell et al., 2004; Pitman & Orr, 1990; Southwick et al., 1999; Yehuda et al., 1998: Young & Breslau, 2004). Such alterations of response may reflect the fact that PTSD is a dynamic disorder and patients often alternate between states of physiological hyperarousal and hypoarousal (emotional numbing, avoidance). The concepts of emotional under- and over-modulation provide the basis for expanding the fear circuitry model to potentially accommodate abnormalities in the regulation of multiple emotional states that are often observed in PTSD (Frewen & Lanius, 2006; Lanius et al., 2010; Liberzon & Martis 2006).
Relationship between fear and other emotional systems
Having established the biological mechanisms of sensitization of the fear response, it now becomes important to examine how fear sensitization can be applied to the regulation of other emotions. Panksepp's (1998) seminal work on the neural circuitry underlying different emotional systems provides a model in which sensitization of the fear system can lead to a destabilization of other emotional systems and thus lead to general emotion dysregulation. Panksepp has outlined four highly interactive emotional systems that are defined by genetically coded neural circuits that, when activated, lead to specific behaviors. The four major emotional systems that have been proposed include (1) a fear system that is designed to minimize the probability of bodily destruction and aids in escaping from dangerous situations; (2) a rage system that mediates anger and aggression; (3) a seeking system which underlies goal-directed behaviors and may generate curiosity and intellectual pursuits, thus facilitating learning, and (4) a panic system that plays a key role in the maintenance of social contact by mediating social emotional processes related to attachment.
Research has shown significant overlap in the neural circuitry underlying these emotional systems, and substantial neural interaction at both the higher (e.g., medial prefrontal cortex, anterior cingulate cortex, and amygdala) and lower levels of the neuroaxis (e.g., periaqueductal grey) has therefore been proposed to occur among these systems (Panksepp, 1998; Panksepp & Northoff, 2009). For example, Panksepp has postulated that the fear system has excitatory influences on the rage, seeking, and panic systems, whereas the rage and seeking systems are thought to have inhibitory influences on the fear system. As a result of these complex interactions, the process of sensitization of the fear system in the individual with PTSD, through repeated recollection of traumatic and associated memories, would have effects on the other emotional systems, thus resulting in generalized emotional dysregulation (i.e., symptoms not only of fear but also anger, anhedonia-numbing, and socially related panic).
As described above, it is important to note that even though brain structures like the medial prefrontal cortex, anterior cingulate cortex, and amygdala have been shown to play a crucial role in the fear circuitry, their functions go far beyond the mediation of fear, being involved in guilt, grief, anger, dissociation, and analgesia (e.g., Denson, Pedersen, Ronquillo & Nandy, 2009; Freed, Yanagihara, Hirsch & Mann, 2009; Krajbich, Adolphs, Tranel, Denburg & Camerer, 2009; Ludascher et al., 2010). The amygdala, anterior cingulate cortex, and dorso- and ventromedial prefrontal cortex are also known to mediate important aspects of general emotion regulation such as emotion perception, affect generation, reappraisal of negative stimuli, suppression of emotional stimuli, and regulation of arousal (Anderson et al., 2004; Bernat, Cadwallader, Ward & Patrick, 2004; Eippert et al., 2007; Goldin, McRae, Ramel & Gross, 2008; Jackson, Malmstadt, Larson & Davidson, 2000; Johnstone, van Reekum, Urry, Kalin & Davidson, 2007; Ochsner, Bunge, Gross & Gabrieli, 2002; Ochsner et al., 2004; Orr et al., 2000; Phan et al., 2005; van Reekum et al., 2007; Urry et al., 2006). These findings strongly suggest a role for these brain structures in the executive top-down regulatory control of a variety of negative affects and autonomic arousal states rather than solely in the mediation of fear responses. We therefore propose that sensitization of the fear circuitry will ultimately lead to general emotion dysregulation.
Testable hypotheses
Based on the literature just described, the pathway proposed above would predict that in the aftermath of traumatic events, individuals who go on to develop acute PTSD will predominantly display symptoms of fear and anxiety, whereas in more chronic cases one will observe the emergence of more generalized emotion dysregulation; this can be tested in time-series analyses. We also hypothesize that strong associations between symptoms of fear and other emotional sequelae should be evident, such as indicated by factor analyses. We further hypothesize that individuals following this pathway will be most commonly adults suffering from adult-onset traumas who do not have a significant history of childhood maltreatment, as this would have already resulted in disturbed neurodevelopment of the emotion, and arousal regulatory systems and symptoms of general emotion dysregulation would be already apparent (see below). We also expect that successful early intervention of excessive fear responding could prevent the emergence of emotion dysregulation in this pathway, and thus treatments specific to emotion dysregulation may not be required for participants following this clinical course.
Pathway 2 (Early life vulnerabilities)
In contrast to the first pathway where fear is central to the origin of PTSD, pathway 2 expands its focus to include something that occurred before the traumatic event (Yehuda et al., in press). It thus constitutes a classical stress-diathesis model. Specifically, this pathway proposes that an early childhood environment, which is generally impoverished due to the unavailability of a responsive attachment figure, or specifically involves instances of childhood maltreatment and abuse, leads to inadequate neurodevelopment of the emotional and arousal regulatory systems and to associated emotion dysregulation. The latter in turn results in an inability to regulate physiological arousal to threatening events, including traumatic experiences, thereby leading to an exacerbation of emotion dysregulation, including the development of PTSD after exposure to traumatic event(s) later in life.
Caregiver–infant attachment relationship
Evidence suggests that the caregiver–infant attachment relationship during infancy plays a crucial role in the development of the emotion and arousal regulatory systems. Infants are known to rely on attachment relationship for protection from external threats, for emotion regulation and for stabilizing physiological reactions following stressors (Schore, 2003a, 2003b; Schuder & Lyons-Ruth, 2004). Studies have shown a strong relationship between responsive parenting behaviors and the offspring's ability to regulate the physiological arousal to stressors (Fish et al., 2004; Gunnar, 2005; Spangler & Grossman, 1999). Moreover, there is now increasing evidence that the quality of care in infancy can moderate the expression of genetic characteristics and thus influence the development of stress-related psychopathology, emotion regulation, and disorganized attachment behavior (Bakermans-Kranenburg & van Ijzendorn, 2004; Barry, Kochanska & Philibert, 2008; Caspi et al., 2002; Gervai et al., 2005, 2007; Kaufman et al., 2004; Kochanska, Philibert & Barry, 2009; Pauli-Pott, Friedl, Hinney & Hebebrand, 2009; Suomi, 2005). There is also increasing evidence from the animal literature that early life adverse experiences have significant and long-lasting effects on the development of neurobiological systems and may lead to “programming” of later stress reactivity and susceptibility to stress-related disorders (Coplan et al., 1996; Meaney & Szyf, 2005; Plotsky & Meaney, 1993; Seckl & Meany, 2006).
Parental care and the HPA-axis
The HPA-axis in human infants has been shown to be particularly sensitive to parental care. Altered cortisol levels have been associated with poor parental care in infancy, particularly in the first year of life (Gunnar & Donzella, 2001). Toddlers raised in Romanian or Russian orphanages known for their extremely depriving and under-stimulating environments failed to show expected patterns of daily cortisol production compared to children reared at home with an adequate parental–infant attachment relationship (Carlson & Earls, 1997; Kroupina, Gunnar & Johnson, 1997). Similar dysfunction of the HPA-axis has been reported in infants who were raised in family environments with observed neglect (Gilles, Berntson, Zipf & Gunnar, 2000).
A relationship between the type of attachment relationship and HPA-axis responsivity as measured by cortisol secretion has also been documented. Specifically, infants displaying a secure attachment to their primary caregiver did not exhibit elevations in cortisol during separation from their caregiver. However, infants with a disorganized attachment status associated with childhood maltreatment demonstrated elevated cortisol levels during separation from their primary caregiver as well as a slow return to baseline after this separation as compared to securely attached infants (Hertsgaard, Gunnar, Erickson & Nachmias, 1995).
Studies focusing on actual childhood maltreatment rather than unavailability of an attachment figure have also proposed a key role for the HPA-axis in response to fear and stress reactions. Alterations in glucocorticoid levels as a result of prolonged maltreatment in childhood have been reported and may be one mechanism underlying sustained fear responses in these individuals (Bremner et al., 2003b; Heim, Ehlert & Hellhammer, 2000). In addition to the HPA-axis, neuropetides such as arginine, vasopressin, and oxytocin have been shown to be affected by prolonged childhood maltreatment (Heim et al., 2009; Wismer Fries, Zigler, Kurian, Jacoris & Pollack, 2005). Oxytocin is thought to play an important role in maternal attachment and social bonding. Moreover, oxytocin has been shown to reduce fear and HPA-axis responses to stress, thereby providing a mechanism underlying sustained fear reactions and generalized emotion dysregulation involving both social and nonsocial emotions, a well-documented phenomenon in individuals with histories of prolonged trauma (Frewen et al., in press-a, b).
Emotion dysregulation following childhood maltreatment: fear and beyond
Emerging research clearly shows that the affective disturbances experienced by many PTSD subjects, especially those with a history of prolonged childhood trauma, are not limited to fear. Lanius et al. (2001, 2003) observed that script-driven imagery of personalized trauma narratives in individuals with PTSD predominantly due to prolonged childhood maltreatment elicited not only elevated fear and anxiety responses but also other negative emotions, including anger, guilt, and shame. Subsequent affective imagery and perception studies utilizing standardized stimuli confirm that emotional disturbances associated with PTSD related to childhood abuse are rarely restricted to fear. Both McTeague et al. (2010) and Frewen et al. (in press-a) observed elevated self-reported negative affect in response to standardized scripts, the content of which focused on arousal of anger or safety concerns (McTeague et al., 2010) or rejection-shame (Frewen et al., in press-a). Furthermore, Kim and Cicchetti (2010), using structural equation modeling in a sample of 215 maltreated and 206 non-maltreated children (ages 6–12 years), demonstrated that a history of neglect, physical and/or sexual abuse, multiple maltreatment subtypes, and earlier onset of maltreatment was directly related to symptoms of emotion dysregulation. Symptoms of emotion dysregulation are also at the core of a construct called Developmental Trauma Disorder, a diagnosis intended to be used in children and adolescents reflecting the complex adaptations to prolonged psychological trauma in childhood (van der Kolk & d'Andrea, 2010). Further support for this notion stems from a recent study by Cloitre et al. (2010) that specifically examined the treatment of PTSD related to childhood abuse. Results from this randomized controlled trial showed that a phase-based emotion/interpersonal regulation-to-exposure treatment was associated with greater benefits and fewer side effects than treatment that excluded emotion and interpersonal training (Cloitre et al., 2010).
The neural correlates underlying nontraumatic emotional processing and emotion regulation in PTSD
Neuroimaging studies in PTSD that encompassed affective disturbances other than fear also suggested a key role for the prefrontal/amygdala circuitry. Responses to recall of non traumatic sad and anxious memories in PTSD was associated with decreased response in the rostral anterior cingulate cortex and thalamus similar to what was found during recall of traumatic memories (Lanius et al., 2003). A further study reported decreased ventromedial prefrontal cortex response and decreased amygdala response during viewing of negatively valenced/aversive pictures (Phan, Britton, Taylor, Fig & Liberzon, 2006). A more recent study examined effortful modification of emotional responses that included enhancing, diminishing or maintaining responses to negative pictures. Deliberate attempts to downregulate emotional responses to negative pictures were more successful in nontraumatized healthy controls compared to subjects with a history of sexual assault with and without PTSD, and success was associated with an increased response of prefrontal regions in the nontraumatized group (New et al., 2009). More recent studies have examined neural activation patterns to standardized social (rejection-shame) and nonsocial (fear-anxiety) emotional imagery (Frewen et al., in press-a). These studies not only provide support for the failure of cortical inhibition during the processing of non traumatic-specific stimuli, but also suggest affective disturbances and emotion dysregulation to non-fear-related stimuli. The latter effect likely results from inadequate neurodevelopment of the emotional and arousal regulatory systems and is exacerbated by additional exposure to traumatic stressors later in life.
Testable hypotheses
Based on the literature just described, we hypothesize that individuals with childhood-onset trauma will exhibit signs of disturbed neurodevelopment of the emotion and arousal regulatory systems. We therefore predict the early emergence of broad problems with emotion regulation during childhood and expect that these concerns are less obviously secondary to fear conditioning, but are further exacerbated by exposure to one or several traumatic event(s) later in life. We also hypothesize that the frequency and intensity of emotion regulatory problems will be significantly elevated in individuals with trauma originating in childhood due to the already chronic and indeed trait-like presentation of symptoms of emotion dysregulation in many of these individuals. We also expect that treatment including emotion regulation in addition to exposure-based strategies will be more effective for these individuals than a single treatment approach, a prediction which is supported by a recent report by Cloitre et al. (2010).
Conclusions
We have proposed that largely similar brain regions may be implicated in fear processing and emotion regulation of other emotions, and postulated that the relationship between fear and other emotion regulation systems may help us further our understanding of PTSD and its underlying neurocircuitry. The latter proposition is of particular relevance since PTSD has been shown to be a dynamic disorder that can involve different forms of emotion dysregulation. Both emotional undermodulation (such as presenting with expressed fear, anger, and re-experiencing/hyperarousal reactivity), and states of emotional overmodulation (e.g., attempts to restrict unwanted emotional experiences including in dissociation, numbing, and analgesia) are important aspects of the complex symptom picture often observed in PTSD. This conceptualization of PTSD is consistent with the significant revisions to the diagnostic formulation for PTSD that have been proposed for the fifth revision of the DSM (http://www.dsm5.org).
Two pathways that allow direct testing of the relationship between the fear-specific and broader emotion regulation systems in PTSD were proposed. The first fear conditioning/stress sensitization pathway suggests that the process of sensitization can result in a progressive augmentation and kindling of the reactivity of an individual, ultimately culminating in the emergence of dysregulation of a variety of emotional states. This pathway is compatible with the idea that PTSD is a response to a traumatic event that then alters stress/fear neural circuitry such that it becomes more highly responsive to triggers. A second pathway was proposed that views emotion dysregulation as a distal vulnerability factor and hypothesizes a further exacerbation of fear and other emotion regulatory problems, including the development of PTSD, after exposure to traumatic event(s) later in life. This pathway is particularly consistent with the idea of pre-traumatic risk and proposes that an early impoverished environment due to the unavailability of a responsive attachment figure or childhood maltreatment leads to inadequate neurodevelopment of the emotional and arousal regulatory systems and thus to the emergence of both fear specifically and general emotion dysregulation.
These pathways suggest that it will be crucial to focus on longitudinal studies to examine the underlying biology of PTSD in a prospective manner in order to capture the dynamic nature of the disorder. To date, most neurobiological studies of PTSD have been cross-sectional in nature. However, it will be important to also examine how stress, and psychophysiological and neural responses underlying PTSD can change with exposure to stressors, progression of the illness, the age of the individual as well as with different pre-trauma risk factors. Such studies will be essential not only to allow the identification of different phenotypes of the disorder that may be important in identifying potentially different subtypes of PTSD, but also to inform the nosology, phenomenology and treatment of this complex disorder.
Acknowledgement
This research was supported by Canadian Institutes of Health Research.
Conflict of interest and funding
There is no conflict of interest in the present study for any of the authors.
References
- Anderson M. C., Ochsner K. N., Kuhl B., Cooper J., Robertson E., Gabrieli S. W., et al. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–235. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- Baker D. G., West S. A., Nicholson W. E., Ekhator N. N., Kasckow J. W., Hill K. K., et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activitiy in combat veterans with posttraumatic stress disorder. The American Journal of Psychiatry. 1999;156(4):585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg M. J., van Ijzendoorn M. H. No association of dopamine D4 receptor (DRD4) and –521 C/T promoter polymorphisms with infant attachment disorganization. Attachment and Human Development. 2004;6:211–218. doi: 10.1080/14616730412331281584. [DOI] [PubMed] [Google Scholar]
- Barry R. A., Kochanska G., Philibert R. A. G x E interaction in the organization of attachment: Mother's responsiveness as a moderator of child's genotypes. The Journal of Child Psychology and Psychiatry. 2008;49(12):1313–1320. doi: 10.1111/j.1469-7610.2008.01935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat E., Cadwallader M., Ward M., Patrick C. Emotion regulation during picture viewing: Comparing responses to pleasant and unpleasant stimuli; Poster presented at 45th annual meeting of the Society for Psychophysiological Research; September 20–25, 2005; Lisbon, Portugal. 2004. [Google Scholar]
- Bremner J. D., Narayan M., Staib L. H., Southwick S. M., McGlashan T., Charney D. S. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. The American Journal of Psychiatry. 1999a;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J. D., Narayan M., Staib L. H., Southwick S. M., McGlashan T., Charney D. S. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. The American Journal of Psychiatry. 1999b;156(11):1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J. D., Vythilingam M., Anderson G., Vermetten E., McGlashan T., Heninger G., et al. Assessment of the hypothalamic-pituitary-adrenal axis over a 24-hour diurnal period and in response to neuroendocrine challenges in women with and without childhood sexual abuse and posttraumatic stress disorder. Biological Psychiatry. 2003a;54(7):710–718. doi: 10.1016/s0006-3223(02)01912-1. [Retraction in: 2004 Biological Psychiatry, 55, 1202] [DOI] [PubMed] [Google Scholar]
- Bremner J. D., Vythilingam M., Vermetten E., Southwick S. M., Mcglashan T., Staib L.H., et al. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biological Psychiatry. 2003b;53(10):879–889. doi: 10.1016/s0006-3223(02)01891-7. [DOI] [PubMed] [Google Scholar]
- Briere J., Spinazzola J. Phenomenology and psychological assessment of complex posttraumatic states. Journal of Traumatic Stress. 2005;18:401–412. doi: 10.1002/jts.20048. [DOI] [PubMed] [Google Scholar]
- Browne A., Finkelhor D. Impact of child sexual abuse: A review of the research. Psychological Bulletin. 1986;99:66–77. [PubMed] [Google Scholar]
- Carlson M., Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Annals of the New York Academy of Sciences. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Caspi A., McClay J., Moffitt T. E., Mill J., Martin J., Craig I. W., et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Charney D. S., Deutch A. Y., Krystal J. H., Southwick S. M., Davis M. Psychobiologic mechanisms of posttraumatic stress disorder. Archives of General Psychiatry. 1993;50(4):295–305. doi: 10.1001/archpsyc.1993.01820160064008. [DOI] [PubMed] [Google Scholar]
- Cloitre M., Stovall-McClough K. C., Nooner K., Zorbas P., Cherry S., Jackson C. L., et al. Treatment for PTSD related to childhood abuse: A randomized controlled trial. The American Journal of Psychiatry. 2010;167(8):915–24. doi: 10.1176/appi.ajp.2010.09081247. [DOI] [PubMed] [Google Scholar]
- Coplan J. D., Andrews M. W., Owens M. J., Friedman S., Gorman J. M., Nemeroff C. B. Persistent elevations of cerebrospinal fluid concentrations of corticotropin – releasing factor in adult nonhuman primates exposed to early – life stressors: Implications for the pathophysiology of mood and anxiety disorders. Proceedings of the National Academy of Sciences. 1996;93:1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- de Quervain D.J. Glucocorticoid-induced reduction of traumatic memories: Implications for the treatment of PTSD. Progress in Brain Research. 2008;167:239–247. doi: 10.1016/S0079-6123(07)67017-4. [DOI] [PubMed] [Google Scholar]
- de Quervain D. J., Margraf J. Glucocorticoids for the treatment of post-traumatic stress disorder and phobias: A novel therapeutic approach. European Journal of Pharmacology. 2008;583(2–3):365–371. doi: 10.1016/j.ejphar.2007.11.068. [DOI] [PubMed] [Google Scholar]
- Denson T. F., Pedersen W. C., Ronquillo J., Nandy A. S. The angry brain: Neural correlates of anger, angry rumination, and aggressive personality. Journal of Cognitive Neuroscience. 2009;21(4):734–744. doi: 10.1162/jocn.2009.21051. [DOI] [PubMed] [Google Scholar]
- Ehlers A., Mayou R. A., Bryant B. Psychological predictors of chronic posttraumatic stress disorder after motor vehicle accidents. Journal of Abnormal Psychology. 1998;107:508–519. doi: 10.1037//0021-843x.107.3.508. [DOI] [PubMed] [Google Scholar]
- Eippert F., Veit R., Weiskopf N., Erb M., Birbaumer N., Anders S. Regulation of emotional responses elicited by threat-related stimuli. Human Brain Mapping. 2007;28:409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish E. W., Shahrock D., Bagot R., Caldji C., Bredy T., Szyf M., et al. Epigenetic programming of stress response through variations in maternal care. Annals of the New York Academy of Sciences. 2004;1036:167–180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- Ford J. D., Courtois C. A., Steele K., Hart O., Nijenhuis E. R. Treatment of complex posttraumatic self-dysregulation. Journal of Traumatic Stress. 2005;18:437–447. doi: 10.1002/jts.20051. [DOI] [PubMed] [Google Scholar]
- Freed P. J., Yanagihara T. K., Hirsch J., Mann J. J. Neural mechanisms of grief regulation. Biological Psychiatry. 2009;66(1):33–40. doi: 10.1016/j.biopsych.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen P. A., Dozois D. J. A., Neufeld R. W. J., Densmore M., Stevens T., Lanius R. A. Self-referential processing in women with PTSD related to childhood abuse: Affective and neural response. Psychological Trauma: Theory, Research, Practice, & Policy. Manuscript submitted for publication. in press-a. [Google Scholar]
- Frewen P. A., Dozois D. J. A., Neufeld R. W. J., Densmore M., Stevens T. K., Lanius R. A. Social emotions & emotional valence during imagery in women with PTSD: Affective and neural correlates. Psychological Trauma: Theory, Research, Practice & Policy. Manuscript submitted for publication. in press-b. [Google Scholar]
- Frewen P. A., Lanius R. A. Toward a psychobiology of posttraumatic self-dysregulation: Reexperiencing, hyperarousal, dissociation, and emotional numbing. Annals of the New York Academy of Sciences. 2006;1071:110–124. doi: 10.1196/annals.1364.010. [DOI] [PubMed] [Google Scholar]
- Friedman M. J. Neurobiological sensitization models of post-traumatic stress disorder: Their possible relevance to multiple chemical sensitivity syndrome. Toxicology and Industrial Health. 1994;10(4–5):449–462. [PubMed] [Google Scholar]
- Geracioti T. D., Jr, Baker D. G., Ekhator N. N., West S. A., Hill K. K., Bruce A. B., et al. CSF norepinephrine concentrations in posttraumatic stress disorder. The American Journal of Psychiatry. 2001;158(8):1227–1230. doi: 10.1176/appi.ajp.158.8.1227. [DOI] [PubMed] [Google Scholar]
- Gervai J., Nemoda Z., Lakatos K., Ronai Z., Toth I., Ney K., et al. Transmission disequilibrium tests confirm the link between DRD4 gene polymorphism and infant attachment. American Journal of Medical Genetics. Part B (Neuropsychiatric Genetics) 2005;132B:126–130. doi: 10.1002/ajmg.b.30102. [DOI] [PubMed] [Google Scholar]
- Gervai J., Novak A., Lakatos K., Toth I., Danis I., Ronai Z., et al. Infant genotype may moderate sensitivity to maternal affective communications: Attachment disorganization, quality of care, and the DRD4 polymorphism. Social Neuroscience. 2007;2:307–319. doi: 10.1080/17470910701391893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze E., Westenberg H. G., Jochims A., de Kloet C. S., Bohus M., Vermetten E., et al. Altered pain processing in veterans with posttraumatic stress disorder. Archives of General Psychiatry. 2007;64(1):76–85. doi: 10.1001/archpsyc.64.1.76. [DOI] [PubMed] [Google Scholar]
- Gilles E. E., Berntson G. G., Zipf W. B., Gunnar M. R. Neglect is associated with a blunting of behavioural and biological stress responses in human infants; Paper presented at the International Conference of Infant Studies; Brighton, England. 2000. [Google Scholar]
- Glover D. A., Poland R. E. Urinary cortisol and catecholamines in mothers of child cancer survivors with and without PTSD. Psychoneuroendocrinology. 2002;27(7):805–819. doi: 10.1016/s0306-4530(01)00081-6. [DOI] [PubMed] [Google Scholar]
- Goldin P. R., McRae K., Ramel W., Gross J. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M. Attachment and stress in early development. In: Carter C. S., editor. Attachment and bonding: A new synthesis. Cambridge, MA: MIT Press; 2005. pp. 245–255. [Google Scholar]
- Gunnar M. R., Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2001;27(1–2):199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Hawk L. W., Dougall A. L., Ursano R. J., Baum A. Urinary catecholamines and cortisol in recent-onset posttraumatic stress disorder after motor vehicle accidents. Psychosomatic Medicine. 2000;62(3):423–434. doi: 10.1097/00006842-200005000-00016. [DOI] [PubMed] [Google Scholar]
- Heim C., Ehlert U., Hellhammer D. H. The potential role of hypocortisolism in the pahtophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Heim C., Ehlert U., Hanker J. P., Hellhammer D. H. Abuse-related posttraumatic stress disorder and alterations of the hypothalamic-pituitary-adrenal axis in women with chronic pelvic pain. Psychosomatic Medicine. 1998;60(3):309–318. doi: 10.1097/00006842-199805000-00017. [DOI] [PubMed] [Google Scholar]
- Heim C., Nemeroff C. B. Neurobiology of posttraumatic stress disorder. CNS Spectrum. 2009;14(Suppl. 1):13–24. [PubMed] [Google Scholar]
- Heim C., Young L. J., Newport D. J., Mietzko T., Miller A. H., Nemeroff C. B. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Molecular Psychiatry. 2009;14(10):954–958. doi: 10.1038/mp.2008.112. [DOI] [PubMed] [Google Scholar]
- Hertsgaard L., Gunnar M., Erickson M. F., Nachmias M. Adrenocortical responses to the strange situation in infants with disorganized/disoriented attachment relationships. Child Development. 1995;66(4):1100–1106. [PubMed] [Google Scholar]
- Jackson D. C., Malmstadt J. R., Larson C. L., Davidson R. J. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37:515–522. [PubMed] [Google Scholar]
- Johnstone T., van Reekum C. M., Urry H. L., Kalin N. H., Davidson R. J. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. The Journal of Neuroscience. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T., Ressler K. J. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. The American Journal of Psychiatry. 2010 Jun;167(6):648–62. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J., Yang B., Douglas-Palumberi H., Houshyar S., Lipschitz D., Krystal J. H., et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proceedings of the National Academy of Sciences. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Cicchetti D. Longitudinal pathways linking child maltreatment, emotion regulation, peer relations, and psychopathology. The Journal of Child Psychology and Psychiatry. 2010;51(6):706–716. doi: 10.1111/j.1469-7610.2009.02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G., Philibert R. A., Barry R. A. Interplay of genes and early mother–child relationship in the development of self-regulation from toddler to preschool-age. The Journal of Child Psychology and Psychiatry. 2009;50(11):1331–1338. doi: 10.1111/j.1469-7610.2008.02050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajbich I., Adolphs R., Tranel D., Denburg N. L., Camerer C. F. Economic games quantify diminished sense of guilt in patients with damage to the prefrontal cortex. The Journal of Neuroscience. 2009;29(7):2188–2192. doi: 10.1523/JNEUROSCI.5086-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus A., Esposito F., Seifritz E., Di Salle F., Ruf M., Valerius G., et al. Amygdala deactivation as a neural correlate of pain processing in patients with borderline personality disorder and co-occurrent posttraumatic stress disorder. Biological Psychiatry. 2009;65:819–822. doi: 10.1016/j.biopsych.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Kroupina M., Gunnar M. R., Johnson D. E. Report on salivary cortisol levels in a Russian baby home. Minneapolis, MN: Institute of Child Development, University of Minnesota; 1997. [Google Scholar]
- Lanius R., Vermetten E., Loewenstein R. J., Brand B., Schmahl C., Bremner J. D., et al. A dissociative subtype of PTSD: Clinical and neurobiological evidence. The American Journal of Psychiatry. 2010;167(6):640–647. doi: 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius R. A., Williamson P. C., Boksman K., Densmore M., Gupta M., Neufeld R. W., et al. Brain activation during script-driven imagery induced dissociative responses in PTSD: A functional MRI investigation. Biological Psychiatry. 2002;52(4):305–311. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- Lanius R. A., Williamson P. C., Densmore M., Boksman K., Gupta M., Neufeld R. W., et al. Neural correlates of traumatic memories in posttraumatic stress disorder: A functional MRI investigation. The American Journal of Psychiatry. 2001;158(11):1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- Lanius R. A., Williamson P. C., Hopper J., Densmore M., Boksman K., Gupta M. A., et al. Recall of emotional states in posttraumatic stress disorder: A function MRI investigation. Biological Psychiatry. 2003;53(3):204–210. doi: 10.1016/s0006-3223(02)01466-x. [DOI] [PubMed] [Google Scholar]
- Le Doux J. The emotional brain: The mysterious underpinnings of emotional life. NY: Simon and Schuster; 1997. [Google Scholar]
- Lemieux A. M., Coe C. L. Abuse-related posttraumatic stress disorder: Evidence for chronic neuroendocrine activation in women. Psychosomatic Medicine. 1995;57(2):105–115. doi: 10.1097/00006842-199503000-00002. [DOI] [PubMed] [Google Scholar]
- Liberzon I., Abelson J. L., Flagel S. B., Raz J., Young E. A. Neuroendocrine and psychophysiologic responses in PTSD: A symptoms provocation study. Neuropsychopharmacology. 1999;21(1):40–50. doi: 10.1016/S0893-133X(98)00128-6. [DOI] [PubMed] [Google Scholar]
- Liberzon I., Martis B. Neuroimaging studies of emotional responses in PTSD. Annals of the New York Academy of Sciences. 2006;1071:87–109. doi: 10.1196/annals.1364.009. [DOI] [PubMed] [Google Scholar]
- Liberzon I., Taylor S. F., Amdur R., Jung T. D., Chamberlain K. R., Minoshima S., et al. Brain activation in PTSD in response to trauma-related stimuli. Biological Psychiatry. 1999;45(7):817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- Litz B. T., Orsillo S. M., Kaloupek D., Weathers F. Emotional processing in posttraumatic stress disorder. Journal of Abnormal Psychology. 2000;109:26–39. doi: 10.1037//0021-843x.109.1.26. [DOI] [PubMed] [Google Scholar]
- Ludascher P., Valerius G., Stiglmayr C., Mauchnik J., Lanius R., Bohus M., et al. Pain sensitivity and neural processing during dissociative states in patients with borderline personality disorder with and without comorbid PTSD – a pilot study. Journal of Psychiatry and Neuroscience. 2010;35(3):177–184. doi: 10.1503/jpn.090022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M., Lin A., Bonaccorso S., van Hunsel F., Van Gastel A., Delmeire L., et al. Increased 24-hour urinary cortisol excretion in patients with post-traumatic stress disorder and patients with major depression, but not in patients with fibromyalgia. Acta Psychiatrica Scandinavica. 1998;98(4):328–335. doi: 10.1111/j.1600-0447.1998.tb10092.x. [DOI] [PubMed] [Google Scholar]
- Marshall R. D., Bianco C., Printz D., Liebowitz M. R., Klein D. F., Coplan J. A pilot study of noradrenergic and HPA axis functioning in PTSD vs panic disorder. Psychiatry Research. 2002;110(3):219–230. doi: 10.1016/s0165-1781(02)00126-9. [DOI] [PubMed] [Google Scholar]
- McCauley J., Kern D. E., Kolodner K., Dill L., Schroeder A. F., DeChant H. K., et al. Clinical characteristics of women with a history of childhood abuse: Unhealed wounds. Journal of the American Medical Association. 1997;277(17):1362–1368. [PubMed] [Google Scholar]
- McFarlane A. C. The long-term costs of traumatic stress: Intertwined physical and psychological consequences. World Psychiatry. 2010;9:3–10. doi: 10.1002/j.2051-5545.2010.tb00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane A. C., Yehuda R., Clark C. R. Biologic models of traumatic memories and post-traumatic stress disorder. The role of neural networks. The Psychiatric Clinics of North America. 2002;25:253–270. doi: 10.1016/s0193-953x(01)00008-9. [DOI] [PubMed] [Google Scholar]
- McTeague L. M., Lang P. J., Laplante M. C., Cuthbert B. N., Shumen J. R., Bradley M. M. Aversive imagery in posttraumatic stress disorder: Trauma recurrence, comorbidity, and physiological reactivity. Biological Psychiatry. 2010;67(4):346–356. doi: 10.1016/j.biopsych.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney M. J., Szyf M. Environmental programming of stress responses through DNA methylation: Life at the interface between a dynamic environment and a fixed genome. Dialogues in Clinical Neuroscience. 2005;7(2):103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M. R., Orr S. P., Lasko N. B., Chang Y., Rauch S. L., Pitman R. K. Presence and acquired origin of reduced recall for fear extinction in PTSD: Results of a twin study. Journal of Psychiatric Research. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M. R., Pitman R. K., Ellis C. B., Gold A. L., Shin L. M., Lasko N. B., et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M. R., Quirk G. J., Pitman R. K., Orr S. P., Fischl B., Rauch S. L. A role for the human dorsal anterior cingulated cortex in fear expression. Biological Psychiatry. 2007;62(10):1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- New A. S., Fan J., Murrough J. W., Liu X., Liebman R. E., Guise K. G., et al. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biological Psychiatry. 2009;66(7):656–664. doi: 10.1016/j.biopsych.2009.05.020. [DOI] [PubMed] [Google Scholar]
- O'Donnell T., Hegadoren K. M., Coupland N. C. Noradrenergic mechanisms in the pathophysiology of post-traumatic stress disorder. Neuropsychobiology. 2004;50(4):273–283. doi: 10.1159/000080952. [DOI] [PubMed] [Google Scholar]
- Ochsner K. N., Bunge S. A., Gross J. J., Gabrieli J. D. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner K. N., Ray R. D., Cooper J. C., Robertson E. R., Chopra S., Gabrieli J. D., et al. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Orr S. P., Metzger L. J., Lasko N. B., Macklin M. L., Peri T., Pitman R. K. De ovo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of Abnormal Psychology. 2000;109:290–298. [PubMed] [Google Scholar]
- Orth U., Wieland E. Anger, hostility, and posttraumatic stress disorder in trauma-exposed adults: A meta-analysis. Journal of Consulting and Clinical Psychology. 2006;74:698–706. doi: 10.1037/0022-006X.74.4.698. [DOI] [PubMed] [Google Scholar]
- Panksepp J. In: Affective Neuroscience: The foundations of human and animal emotions. Davidson R. J., Ekman P., Scherer K., editors. NY: Oxford University Press; 1998. pp. 42–57. [Google Scholar]
- Panksepp J., Northoff G. The trans-species core SELF: The emergence of active cultural and neuro-ecological agents through self-related processing within subcorticol-cortical midline networks. Consciousness and Cognition. 2009;18(1):193–215. doi: 10.1016/j.concog.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Pauli-Pott U., Friedl S., Hinney A., Hebebrand J. Serotonin transporter gene polymorphism (5HTTLPR), environmental conditions, and developing negative emotionality and fear in early childhood. Journal of Neural Transmission. 2009;116(4):503–512. doi: 10.1007/s00702-008-0171-z. [DOI] [PubMed] [Google Scholar]
- Phan K. L., Britton J. C., Taylor S. F., Fig L. M., Liberzon I. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Archives of General Psychiatry. 2006;63:184–192. doi: 10.1001/archpsyc.63.2.184. [DOI] [PubMed] [Google Scholar]
- Phan K. L., Fitzgerald D. A., Nathan P. J., Moore G. J., Uhde T. W., Tancer M. E. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biological Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Pitman R. K. Post-traumatic stress disorder, hormones, and memory. Biological Psychiatry. 1989;26(3):221–223. doi: 10.1016/0006-3223(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Pitman R. K., Orr S. P. Twenty-four hour urinary cortisol and catecholamine excretion in combat-related posttraumatic stress disorder. Biological Psychiatry. 1990;27(2):245–247. doi: 10.1016/0006-3223(90)90654-k. [DOI] [PubMed] [Google Scholar]
- Plotsky P. M., Meaney M. J. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRna, median eminence CRF content and stress-induced release in adult rats. Molecular Brain Research. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Post R. M., Weiss S. R., Smith M., Li H., McCann U. Kindling versus quenching. Implications for the evolution and treatment of posttraumatic stress disorder. Annals of the New York Academy of Sciences. 1997;821:285–295. doi: 10.1111/j.1749-6632.1997.tb48287.x. [DOI] [PubMed] [Google Scholar]
- Quirk G. J. Fear research: Implications for anxiety disorders. Boletín de la Asociación Médica de Puerto Rico. 1998;90(1–3):27–29. [PubMed] [Google Scholar]
- Rasmusson A. M., Lipschitz D. S., Wang S., Hu S., Vojvoda D., Bremner J.D., et al. Increased pituitary and adrenal reactivity in premenopausal women with posttraumatic stress disorder. Biological Psychiatry. 2001;50:965–977. doi: 10.1016/s0006-3223(01)01264-1. [DOI] [PubMed] [Google Scholar]
- Rauch S. L., Shin L. M., Phelps E. A. Neurocircuitry models of post-traumatic stress disorder and extinction: Human neuroimaging research – past, present, and future. Biological Pyschiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Resick P. A., Miller M. W. Posttraumatic stress disorder: Anxiety or traumatic stress disorder? Journal of Traumatic Stress. 2009;22(5):384–390. doi: 10.1002/jts.20437. [DOI] [PubMed] [Google Scholar]
- Rizvi S. L., Kaysen D., Gutner C. A., Griffin M. G., Resick P. A. Beyond fear: The role of peritraumatic responses in posttraumatic stress and depressive symptoms among female crime victims. Journal of Interpersonal Violence. 2008;23(6):853–868. doi: 10.1177/0886260508314851. [DOI] [PubMed] [Google Scholar]
- Schore A. N. Affect dysregulation and disorders of the self. NY: W.W. Norton & Company, Inc; 2003a. [Google Scholar]
- Schore A. N. Affect dysregulation and the repair of the self. NY: W.W. Norton & Company, Inc; 2003b. [Google Scholar]
- Schuder M., Lyons-Ruth K. Hidden Trauma” in infancy: Attachment, fearful arousal, and early dysfunction of the stress response system. In: Osofsky J., editor. Trauma in infancy and early childhood. NY: Guildford Press; 2004. pp. 69–104. [Google Scholar]
- Seckl J. R., Meany M. J. Glucocorticoid “programming” and PTSD risk. Annals of the New York Academy of Sciences. 2006;1071:351–378. doi: 10.1196/annals.1364.027. [DOI] [PubMed] [Google Scholar]
- Selye H. The stress of life. NY: McGraw-Hill; 1956. [Google Scholar]
- Shin L. M., Handwerger K. Is posttraumatic stress disorder a stress-induced fear circuitry disorder? Journal of Traumatic Stress. 2009;22(5):409–415. doi: 10.1002/jts.20442. [DOI] [PubMed] [Google Scholar]
- Shin L. M., Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin L. M., McNally R. J., Kosslyn S. M., Thompson W. L., Rauch S. L., Alpert N. M., et al. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. The American Journal of Psychiatry. 1999;156(4):575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- Shin L. M., McNally R. J., Kosslyn S. M., Thompson W. L., Rauch S. L., Alpert N. M., et al. A positron emission tomographic study of symptom provocation in PTSD. Annals of the New York Academy of Sciences. 1997;821:521–523. doi: 10.1111/j.1749-6632.1997.tb48320.x. [DOI] [PubMed] [Google Scholar]
- Shin L. M., Orr S. P., Carson M. A., Rauch S. L., Macklin M. L., Lasko N. B., et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Archives of General Psychiatry. 2004;61(2):168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin L. M., Wright C. I., Cannistraro P. A., Wedig M. M., McMullin K., Martis B., et al. A functional magnetic resonance imaging study of amygdale and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Southwick S. M., Bremner J. D., Rasmusson A., Morgan C. A., 3rd, Arnsten A., Charney D. S. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biological Psychiatry. 1999;46(9):1192–11204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- Spangler G., Grossman K. E. Individual and physiological correlates of attachment disorganization in infancy. In: Solomon J., George C., editors. Attachment disorganization. NY: Guilford; 1999. pp. 95–124. [Google Scholar]
- Stovall-McClough K. C., Cloitre M. Unresolved attachment, PTSD, and dissociation in women with childhood abuse histories. Journal of Consulting and Clinical Psychiatry. 2006;74:219–228. doi: 10.1037/0022-006X.74.2.219. [DOI] [PubMed] [Google Scholar]
- Suomi S. J. How gene-environment interactions shape the development of impulsive aggression in rhesus monkeys. In: Stoff D. M., Susman E. J., editors. Developmental psychobiology of aggression. NY: Cambridge University Press; 2005. pp. 252–268. [Google Scholar]
- Urry H. L., van Reekum C. M., Johnstone T., Kalin N. H., Thurow M. E., Schaefer H. S., et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hart O., Nijenhuis E. R., Steele K. Dissociation: An insufficiently recognized major feature of complex posttraumatic stress disorder. Journal of Traumatic Stress. 2005;18:413–423. doi: 10.1002/jts.20049. [DOI] [PubMed] [Google Scholar]
- van der Kolk B. A., d'Andrea A. Towards a developmental trauma disorder diagnosis for childhood interpersonal trauma. In: Lanius R. A., Vermetten E., Pain C., editors. The impact of early life trauma on health and disease: The hidden epidemic. Cambridge: Cambridge University Press; 2010. pp. 57–68. [Google Scholar]
- van der Kolk B., Greenberg M., Boyd H., Krystal J. Inescapable shock, neurotransmitters, and addiction to trauma: Toward a psychobiology of post traumatic stress. Biological Psychiatry. 1985;20(3):314–325. doi: 10.1016/0006-3223(85)90061-7. [DOI] [PubMed] [Google Scholar]
- van der Kolk B. A., Roth S., Pelcovitz D., Sunday S., Spinazzola J. Disorders of extreme stress: The empirical foundation of a complex adaptation to trauma. Journal of Traumatic Stress. 2005;18(5):389–399. doi: 10.1002/jts.20047. [DOI] [PubMed] [Google Scholar]
- van Reekum C. M., Johnstone T., Urry H. L., Thurow M. E., Schaefer H. S., Alexander A. L., et al. Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. Neuroimage. 2007;36:1041–1055. doi: 10.1016/j.neuroimage.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Wessa M., Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: Evidence from second-order conditioning. The American Journal of Psychiatry. 2007;164:1684–1692. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
- Wismer Fries A. B., Zigler T., Kurian J., Jacoris S., Pollack S. D. Early experience in humans is associated with changes in neuro-peptides critical for regulating social behaviour. Proceedings of the National Academy of Sciences USA. 2005;102:17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Advances in understanding neuroendocrine alterations in PTSD and their therapeutic implications. Annals of the New York Academy of Sciences. 2006;1071:137–166. doi: 10.1196/annals.1364.012. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Antelman S. M. Criteria for rationally evaluating animal models of posttraumatic stress disorder. Biological Psychiatry. 1993;33(7):479–486. doi: 10.1016/0006-3223(93)90001-t. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Boisoneau D., Mason J. W., Giller E. L. Glucocorticoid receptor number and cortisol excretion in mood, anxiety, and psychotic disorders. Biological Psychiatry. 1993;34(1–2):18–25. doi: 10.1016/0006-3223(93)90252-9. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Flory J., Pratchett L. C., Buxbaum J., Ising M., Holsboer F. Putative biological mechanisms for the association between early life adversity and the subsequent development of PTSD. Psychopharmacology (Berl) doi: 10.1007/s00213-010-1969-6. Manuscript submitted for publication. in press. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Kahana B., Binder-Brynes K., Southwick S. M., Mason J. W., Giller E. L. Low urinary cortisol excretion in Holocaust survivors with posttraumatic stress disorder. The American Journal of Psychiatry. 1995;152:982–986. doi: 10.1176/ajp.152.7.982. [DOI] [PubMed] [Google Scholar]
- Yehuda R., McFarlane A. C. Conflict between current knowledge about posttraumatic stress disorder and its original conceptual basis. American Journal of Psychiatry. 1995;152(12):1705–1713. doi: 10.1176/ajp.152.12.1705. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Siever L. J., Teicher M. H., Levengood R. A., Gerber D. K., Schmeidler J., et al. Plasma norepinephrine and 3-methoxy-4-hydroxyphernylglycol concentrations and severity of depression in combat posttraumatic stress disorder and major depressive disorder. Biological Psychiatry. 1998;44(1):56–63. doi: 10.1016/s0006-3223(98)80007-3. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Southwick S. M., Nussbaum G., Wahby V., Giller E. L. Jr., Mason J. W. Low urinary cortisol excretion in patients with posttraumatic stress disorder. Journal of Nervous and Mental Disorders. 1990;178:366–369. doi: 10.1097/00005053-199006000-00004. [DOI] [PubMed] [Google Scholar]
- Yehuda R., Teicher M. H., Trestman R. L., Levengood R. A., Siever L. J. Coritsol regulation in posttraumatic stress disorder and major depression: A chronobiological analysis. Biological Psychiatry. 1996;40(2):79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]
- Young E. A., Breslau N. Cortisol and catecholamines in posttraumatic stress disorder: An epidemiologic community study. Archives of General Psychiatry. 2004;61(4):394–401. doi: 10.1001/archpsyc.61.4.394. [DOI] [PubMed] [Google Scholar]
- Zlotnick C., Johnson D. M., Yen S., Battle C. L., Sanislow C. A., Skodol A. E., et al. Clinical features and impairment in women with Borderline Personality Disorder (BPD) with Posttraumatic Stress Disorder (PTSD), BPD with PTSD, and other personality disorders with PTSD. Journal of Nervous and Mental Disorder. 2003;191(11):706–713. doi: 10.1097/01.nmd.0000095122.29476.ff. [DOI] [PubMed] [Google Scholar]
- Zlotnick C., Zakriski A. L., Shea M. T., Costello E., Begin A., Pearlstein T., et al. The long-term sequelae of sexual abuse: Support for a complex posttraumatic stress disorder. Journal of Traumatic Stress. 1996;9:195–205. doi: 10.1007/BF02110655. [DOI] [PubMed] [Google Scholar]